Current Status of Bovine Tuberculosis in Brazil
Current Status of Bovine Tuberculosis in Brazil
José Soares Ferreira Neto1
- Faculty of Veterinary Medicine and Animal Science, University of São Paulo, São Paulo, Brazil. WOAH Collaborating Center for Economics of Animal Health in the Americas Region.
Correspondence: [email protected]
OPEN ACCESS
PUBLISHED: 30 December 2024
CITATION: NETO, José Soares Ferreira. Current Status of Bovine Tuberculosis in Brazil. Medical Research Archives, [S.l.], v. 12, n. 12, dec. 2024. Available at: <https://esmed.org/MRA/mra/article/view/6154>. Date accessed: 15 oct. 2025.
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.18103/mra.v12i12.6154.
ISSN 2375-1924
Abstract
Bovine tuberculosis is a major zoonotic disease, and cattle are the main source of infection for humans. The disease also causes economic losses in the dairy and beef industries, primarily due to reduced milk and meat production, condemned carcasses at slaughterhouses, and, in rare cases, cattle mortality. As a result, many countries have implemented measures to control or eradicate it. Brazil is a major player in the international beef market, but bovine tuberculosis remains a significant animal health issue in search of an effective solution. This review provides data on the epidemiological situation of the disease in the country, the initiatives being implemented, and the prospects for the future. Brazil only established a rational program to combat the disease in 2001. For 90% of Brazil’s cattle population, high-quality epidemiological data show that the prevalence of tuberculosis-infected herds in Brazilian states ranges from 0.16% to 9.0%, with some states exhibiting significant internal heterogeneity. The disease in Brazil is most commonly associated with high-production dairy farms. Its spread is primarily attributed to the introduction of animals without proper precautions against the disease, although specific factors may vary depending on the state. The accreditation of tuberculosis-free herds has yielded very poor results in Brazil. Currently, there are only 5,472 tuberculosis-free accredited farms in the entire country, 97% of which are in the two southern states: Santa Catarina (3,305) and Rio Grande do Sul (1,981). The remaining 197 tuberculosis-free herds are distributed across 11 other states. Santa Catarina and Mato Grosso have recently implemented surveillance systems with the aim of eradicating the disease. It is essential that these systems achieve adequate levels of efficiency, as they will serve as a model for other Brazilian states. This situation raises concerns about the zoonotic transmission of Mycobacterium bovis in Brazil, both occupationally and among populations that consume unpasteurized dairy products. Special attention should be given to dairy products made with unpasteurized milk, which can be authorized only if the properties are accredited as free of tuberculosis and brucellosis. Otherwise, the milk must be pasteurized to ensure health guarantees for dairy consumers.
Keywords
Bovine tuberculosis, zoonotic tuberculosis, Brazil, control, eradication, epidemiological situation
Introduction
Although the zoonotic transmission of various species of the Mycobacterium tuberculosis complex has been reported, suggesting the need for a broader definition of the term zoonotic tuberculosis, cattle infected with M. bovis are still the main problem in most countries. According to the World Organization for Animal Health, bovine tuberculosis (bTB) is a chronic bacterial disease of animals caused by members of the Mycobacterium tuberculosis complex, primarily by M. bovis. It is a major zoonotic disease, and cattle are the main source of infection for humans. The annual estimate of human cases of zoonotic tuberculosis is 140,000, with 11,400 deaths. The disease also causes economic losses in the dairy and beef industries, primarily due to reduced milk and meat production, condemned carcasses at slaughterhouses, and, in rare cases, cattle mortality. As a result, many countries have implemented measures to control or eradicate bTB, primarily consisting of detecting infected herds and animals through surveillance systems, culling test-positive animals, and imposing movement restrictions on infected herds.
Bovine tuberculosis is found worldwide, and a country can declare itself free of bTB by demonstrating through a surveillance system that, in the last three years, 99.8% of the premises were tested and found to be uninfected. While some countries have never detected it, many developed nations have achieved good levels of control or eradicated it from their cattle populations. Australia, the Netherlands, Sweden, Denmark, Finland, Estonia, the Czech Republic, Latvia, Lithuania, Luxembourg, Slovakia, and Canada are considered officially bTB-free (OTF) with no cases reported in the last three years. In contrast, France, Germany, Austria, Poland, Belgium, Hungary, and Slovenia are also OTF but report a few cases annually.
In 2021, Japan declared itself free of M. tuberculosis complex infections in cattle, while Egypt declared the same for its dairy cattle compartment. In 2023, Namibia declared its Foot-and-Mouth Disease-free zone also free of M. tuberculosis complex infections in cattle. Wild animals have been identified as reservoirs of bTB for domestic cattle in some countries, complicating efforts to eradicate it: Elk (Cervus canadensis) in Canada, Wild boar (Sus scrofa) and some cervids in the Iberian Peninsula, Badger (Meles meles) in Ireland and United Kingdom, Brushtail possum (Trichosurus vulpecula) in New Zealand, African buffalo (Syncerus caffer) in South Africa, and White-tailed deer (Odocoileus virginianus) in certain regions of the United States.
Bovine tuberculosis remains endemic and uncontrolled in much of Africa, Asia, Latin America, and most Middle Eastern countries. The highest prevalence is in Africa and parts of Asia, but the bTB is also present in countries across Europe and the Americas. In South America, only Uruguay is running eradication strategies, with well-documented, available information.
This review provides data on the epidemiological situation of bovine tuberculosis in Brazil, the initiatives being implemented to combat the disease, and the prospects for the future.
Bovine tuberculosis in Brazil
In Brazil, bTB is a major health problem for the dairy and beef industries. The country has not yet achieved self-sufficiency in milk production and depends on imports. On the other hand, thanks to the favorable disease status regarding Foot-and-Mouth Disease and Bovine Spongiform Encephalopathy, it has recently become the largest beef exporter in the world. Brazil has approximately 235 million head of cattle and exports around 2.5 million tons of beef a year, mainly to China, the United Arab Emirates, the United States of America, and Chile.
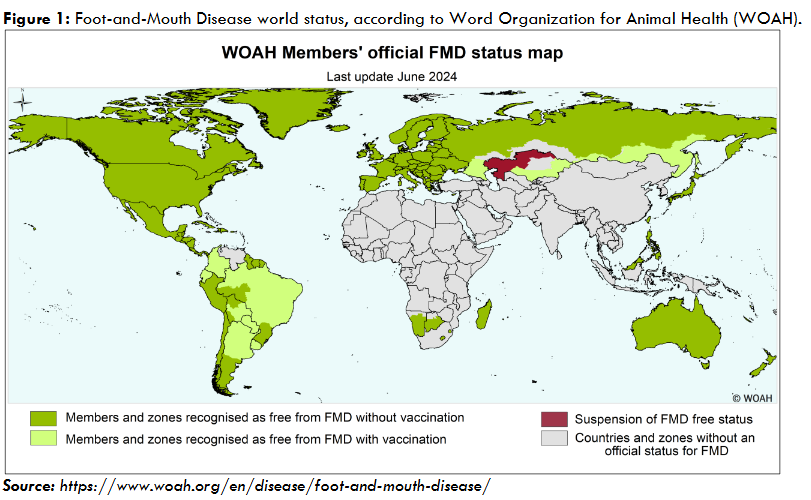
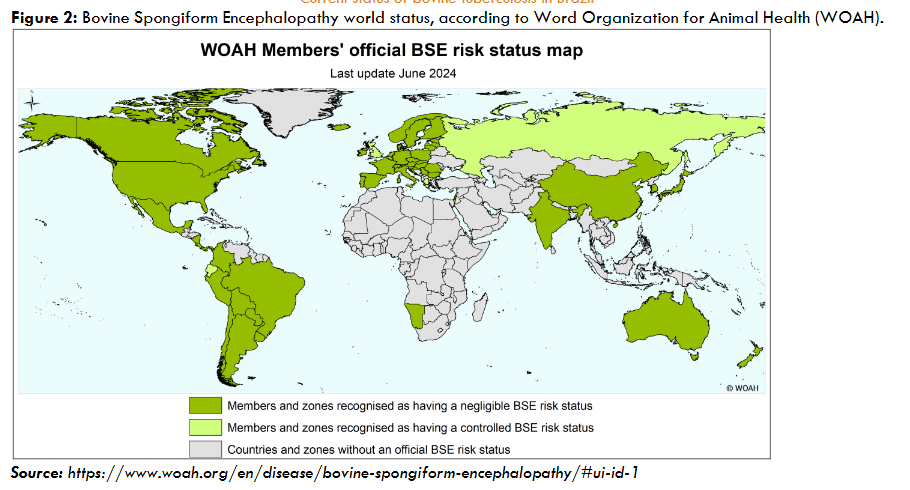
The fight against bTB in Brazil was largely ineffective until 2001, when the National Program for the Control and Eradication of Brucellosis and Tuberculosis (PNCEBT) was launched. Until 2001, the disease spread uncontrollably throughout the country, likely reaching endemic equilibrium in its various regions. Although it was known that bTB occurred throughout Brazil, there was a lack of detailed information on the epidemiological situation of the disease in the different Brazilian states. Thus, a major effort was initiated to characterize the disease in all 27 Brazilian states, involving the Ministry of Agriculture and Livestock (Ministério da Agricultura e Pecuária – MAPA), the state Official Veterinary Services (OVSs), and the scientific support of our Collaborating Center for Animal Health at the University of São Paulo (USP).
This initiative made it possible to conduct standardized studies that generated high-quality epidemiological data to support decision-making in selecting the best strategies for each state and to evaluate the effectiveness of actions based on the initial conditions.
To capture internal heterogeneities in these studies, the states were divided into regions. Within each region, a predetermined number of properties were randomly selected, and within each property, a set number of animals were drawn and tested using the comparative cervical tuberculin test. Additionally, a questionnaire on health and management practices potentially associated with the disease was administered to each sampled property. This approach allowed for the prevalence estimation of bTB-infected herds and tuberculin-positive animals, as well as the identification of bTB risk factors specific to each state.
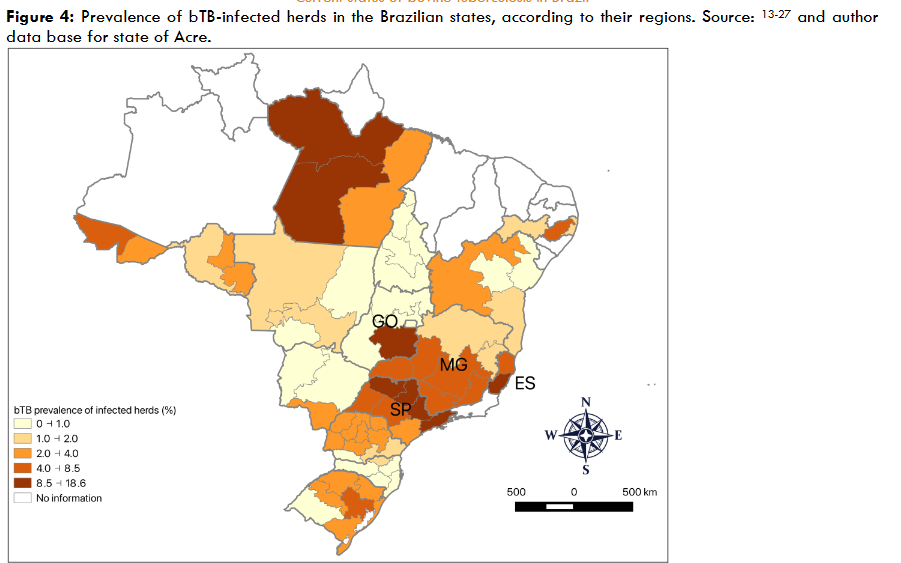
Prevalence and risk factors for bovine tuberculosis in Brazil
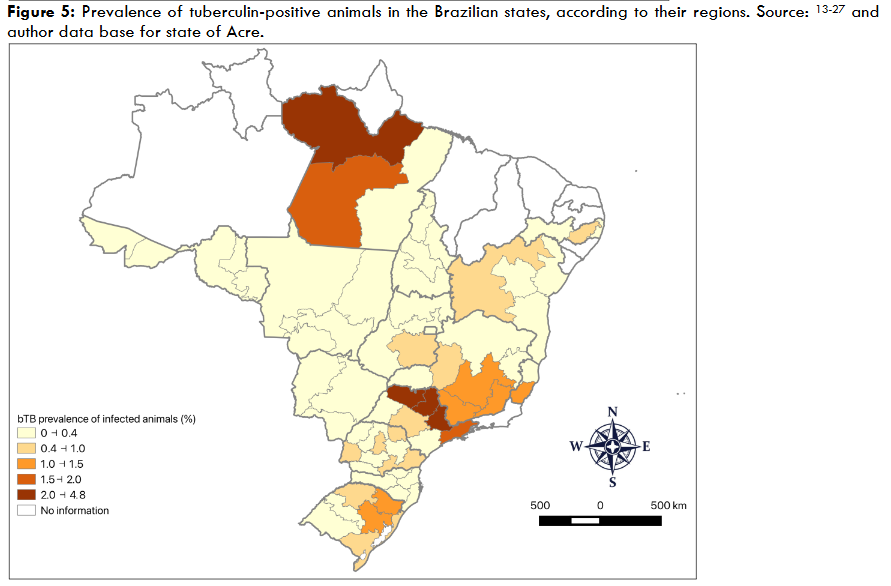
To date, 16 states, comprising 90% of Brazil’s bovid population, have completed the study. They are: Distrito Federal, Bahia, Paraná, Mato Grosso do Sul, Mato Grosso, Rondônia, São Paulo, Santa Catarina, Rio Grande do Sul, Minas Gerais, Pernambuco, Espírito Santo, Goiás, Tocantins, Pará, and Acre. The results for the states of Acre have not yet been published.
| State | Number of Bovids | Year of the Fieldwork | Prevalence (P) (%) | Infected Herds | Positive Animals | P 95% CI | P 95% CI |
|---|---|---|---|---|---|---|---|
| Distrito Federal | 85,544 | 2003 | 0.36 | 0-2.0 | 0.05 | 0-0.4 | |
| Bahia | 12,526,243 | 2004 | 1.6 | 1.0-2.6 | 0.21 | 0.07-0.60 | |
| Paraná | 7,922,486 | 2005-2007 | 2.1 | 1.3-3.0 | 0.42 | 0.04-0.81 | |
| Mato Grosso do Sul | 18,433,728 | 2009 | 1.3 | 0.72-2.4 | 0.04 | 0.02-0.07 | |
| Mato Grosso | 34,246,313 | 2009 | 1.3 | 0.7-2.4 | 0.12 | 0.03-0.44 | |
| Rondônia | 17,688,225 | 2009-2010 | 2.3 | 1.5-3.5 | 0.12 | 0.06-0.25 | |
| São Paulo | 11,071,603 | 2011 | 9.0 | 7.8-10.5 | 1.3 | 0.9-1.7 | |
| Santa Catarina | 4,481,625 | 2012 | 0.5 | 0.07-0.93 | 0.06 | 0-0.12 | |
| Rio Grande do Sul | 11,932,838 | 2013 | 2.8 | 1.8-4.0 | 0.70 | 0.4-1.0 | |
| Minas Gerais | 22,993,105 | 2013 | 4.2 | 3.4-5.1 | 0.56 | 0.46-0.66 | |
| Pernambuco | 2,280,130 | 2014 | 2.9 | 1.8-4.5 | 0.62 | 0.3-1.3 | |
| Espírito Santo | 2,231,036 | 2012-2014 | 7.6 | 5.7-9.9 | 0.70 | 0.3-1.1 | |
| Goiás | 24,410,182 | 2013-2014 | 3.4 | 2.2-4.7 | 0.30 | 0.10-0.49 | |
| Tocantins | 10,772,509 | 2014-2015 | 0.16 | 0.023-1.1 | 0.009 | 0.001-0.063 | |
| Pará | 24,791,060 | 2018-2020 | 8.6 | 7.1-10.0 | 0.91 | 0.68-1.0 | |
| Acre | 4,635,381 | 2018-2020 | 4.3 | 2.9-6.2 | 0.36 | 0.14-0.89 |
The Federal District (DF) and Tocantins (TO) each had only one bTB-infected herd, making it impossible to study risk factors. In Mato Grosso (MT), due to the small number of bTB-infected herds (12), only a univariate analysis was conducted. This analysis revealed that the disease was associated with dairy farms raising specialized European breeds under a regime of 2 to 3 daily mechanical milkings with milk cooling. The total number of animals on these farms ranged from 142 (2nd quartile) to 486 (3rd quartile).
The most frequently identified risk factor among the states studied was the size of the property, as measured by the number of cows. Farms with a larger number of breeding adult females have often been found to be at greater risk for slow-spreading endemic diseases. These large farms require more complex management practices, which can facilitate the spread of diseases among animals. Additionally, they have a higher demand for replacement animals, leading to more frequent introductions of new animals onto the property – a factor associated with increased vulnerability to tuberculosis. Thus, this variable indirectly suggests that the true risk factor for tuberculosis is the introduction of animals, of course without proper precautions regarding bTB. The purchase and introduction of animals is a well-known risk factor for bTB.
Being a dairy farm emerged as the second most significant risk factor for the states studied. The association between bTB and dairy production has been reported by several authors and can be attributed to characteristics of this type of production that predispose bovines to the disease, such as high animal density and a long production cycle. The results for Mato Grosso also show that bTB is more commonly associated with highly specialized dairy farms that use European breeds and operate with a regimen of 2-3 mechanical milkings per day along with milk cooling, i.e., high production dairy farms. In Brazil, these farms are generally of the confined or semi-confined type, meaning they have a high density of animals.
Pasture sharing was identified as the third most frequent herd-level risk factor for bTB in Brazilian states. The various forms of pasture sharing allow contact between animals from different properties with varying health statuses, facilitating the transmission of not only bTB but also other diseases.
Some variables emerged as risk factors in only a single state, indicating regional peculiarities. For example, feeding calves with whey was identified as a risk factor in Paraná, while the exclusive breeding of buffaloes was a risk factor in Pará.
In summary, bTB in Brazil is most commonly associated with high-production dairy farms, and its spread is primarily attributed to the introduction of animals without adequate precautions against the disease, although there are specificities depending on the state.
Fighting bovine tuberculosis in Brazil
The Official Veterinary Service model adopted by Brazil includes a central level, represented by MAPA, and local levels, represented by the OVS of each state. The central level defines national programs and works collaboratively with the state levels. However, there is significant variation in the quality of state OVS, leading to substantial differences in operational capacity. OVS have considerable autonomy in adopting actions proposed by the central level. MAPA encourages state OVS to take action but does not mandate it.
Based on the results of these studies, a set of actions was recommended for each state to more effectively address bTB. Regarding the studies of risk factors, it was suggested that educational initiatives be implemented to inform farmers about steps they should take to reduce the vulnerability of their herds to bTB. Logically, this depended on the results obtained for each state; however, the most frequent and important measure was to take precautions against the disease when introducing new animals onto the property.
For all states, it was suggested that they choose between adopting control or eradication strategies. To control the disease, it was recommended that states promote the accreditation program for bTB-free properties, as outlined in the PNCEBT, by offering better prices for the milk and beef produced by farms accredited as bTB-free.
To eradicate bTB, the states must implement a surveillance system to detect infected farms and make it compulsory to convert them into bTB-free ones, in accordance with the PNCEBT rules. It is crucial to select appropriate strategies for detecting infected farms, considering the characteristics of the state’s beef and dairy industries. In the Brazilian context, the possible strategies for detecting bTB-infected farms are: 1) tracing properties based on carcass condemnations for tuberculosis in slaughterhouses, tests conducted for animal movement, and diagnoses of human tuberculosis in rural residents, and 2) actively searching for infection on dairy farms, those with epidemiological links to infected farms, and through cross-sectional studies on properties not covered by the above components.
Given the complexity of a surveillance system, the most rational approach for the Brazilian context would be to start with a few components aimed at detecting bTB-infected farms and, after consolidating them, add more complex ones. Based on the results of the previously mentioned studies and the characteristics of Brazilian states, an initial strategy could include tracking properties based on the condemnation of bTB-infected carcasses in slaughterhouses, actively searching for infection on dairy farms – especially those with high production – and conducting active searches on farms with epidemiological links to detected bTB-infected farms.
For the control or eradication strategies to be successful, a constructive dialogue between the OVSs and the state’s beef and dairy industries is absolutely necessary. Fundamentally, the private sector must identify in these programs a clever opportunity to efficiently exchange bTB-infected animals for healthy ones.
Therefore, each state used the results of these studies to implement bTB mitigation measures tailored to its animal health policies and operational capacity.
Only the state of Paraná conducted a second study to verify the effectiveness of the implemented actions. The fieldwork was carried out in 2018-2019, resulting in a prevalence of bTB-infected herds of 2.5% and a prevalence of tuberculin-positive animals of 0.35%, showing no change compared to the 2005-2007 study.
The accreditation of bTB-free herds has yielded very poor results in Brazil. Currently, there are only 5,472 bTB-free accredited farms in the entire country, 97% of which are in the two southern states: Santa Catarina has 3,305, and Rio Grande do Sul has 1,981. The remaining 197 bTB-free herds are distributed across 11 other states.
In 2018, Santa Catarina’s dairy industry began a program to encourage the accreditation of bTB-free herds by offering higher payments for milk from these farms. By 2020, the state started implementing surveillance systems aimed at eradicating bTB. Detection of infected properties is conducted through tracking those with carcasses condemned for bTB at slaughter and those with animals testing positive for tuberculin, which is required for the movement of breeding animals within the state. These infected farms are converted to bTB-free status through a routine of tuberculin tests, as outlined by the PNCEBT. Additionally, the state recently made the tuberculin test mandatory every three years for dairy herds. Despite all these initiatives, of the approximately 170,000 properties in the state, only 3,411 (2%) are accredited as bTB-free.
Rio Grande do Sul has chosen to mitigate the risk of bTB only in the dairy industry by encouraging an increase in the volume and frequency of tuberculin tests on milk-producing farms through tax breaks. As a result, some dairies have promoted regular tuberculin testing among their network of supplier farms using two incentives: higher milk prices and covering the cost of the tests. Infected properties must undergo routine tuberculin testing, with positive animals removed until all remaining animals test negative. Although there has been a significant increase in the number of tuberculin tests conducted in the state, only 1,981 farms currently meet the requirements for bTB-free accreditation.
In 2019, Mato Grosso began implementing a surveillance system to detect bTB-infected properties and convert them into bTB-free farms using tuberculin tests followed by the removal of positive animals. The state’s efforts have focused exclusively on the dairy industry, identifying infected herds based on carcass condemnation for bTB in slaughterhouses and requiring tuberculin tests for the movement of breeding animals. Although 310 bTB-infected properties have been detected and converted to bTB-free, the state currently has only one property that meets the requirements for bTB-free accreditation. However, the state has faced challenges in tracing infected properties based on tuberculosis lesions detected at slaughter because these infected animals are often sold to slaughterhouses by cattle traders, whose business model is purchasing cows at the end of their reproductive life from dairy farms, quickly fattening them, and selling them in larger batches to slaughterhouses. As these cattle traders do not pose a risk of spreading bTB, an efficient individual tracking system would be necessary to identify the original bTB-infected farm that needs to be converted to bTB-free.
Conclusions
To date, the Brazilian states have shown very little interest in effectively combating bTB, even though they have high-quality epidemiological information to better define their strategies and manage the process. Only the two Southern states have made some progress, but very little considering the scale of the challenge. In Santa Catarina, with around 4.5 million cattle, only 2.0% of its properties are accredited as bTB-free, and in Rio Grande do Sul, with approximately 12 million cattle, only about 7% of dairy farms or 1% of cattle farms in the state.
Combating bTB through a surveillance system seems to be the best way forward for Brazilian states because it focuses tuberculin testing where it is most needed – on bTB-infected farms. This approach involves implementing systematic actions to detect infected farms and subsequently transform them into bTB-free farms through tuberculin testing followed by the removal of positive animals.
So, it is crucial that the surveillance systems operated by Santa Catarina and Mato Grosso achieve adequate levels of efficiency, as they will serve as a model for other Brazilian states.
The bTB situation in Brazil raises concerns about the zoonotic transmission of the disease, both occupationally and among populations that consume unpasteurized dairy products. Special attention should be given to dairy products made with unpasteurized milk, which can be authorized only if the properties are accredited as free of tuberculosis and brucellosis. Otherwise, the milk must be pasteurized to ensure health guarantees for dairy consumers.
Conflicts of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
Author José Soares Ferreira Neto has received research fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant number 302899/2022-7). He also thanks CAPES for paying the publication fees (CAPES PROEX 3368/2024).
Acknowledgements
We thank the Brazilian Official Veterinary Services for the information provided on bovine tuberculosis.
References
- Duffy SC, Marais B, Kapur V, Behr MA. Zoonotic tuberculosis in the 21st century. Lancet Infect Dis. 2024; 24(4): 339-341. https://doi.org/10.1016/S1473-3099(24)00059-8
- WOAH (World Organization for Animal Health). Bovine tuberculosis. Animal Diseases. 2024a. https://www.woah.org/en/disease/bovine-tuberculosis/ [Accessed March 20, 2024].
- WHO (World Health Organization). Global tuberculosis report 2020. World Health Organization. 2020. https://reliefweb.int/report/world/global-tuberculosis-report-2020?gad_source=1&gclid=CjwKCAjw8fu1BhBsEiwAwDrsjG3jCkMQblTxjLtz32EXbaBzFQvd43I0WCIDnK3O020z1TC41sGusxoCDnAQAvD_BwE [Accessed January 11, 2024].
- Homem VSF, Higa ZMM, Ferreira Neto JS. Proposed model to study the economic impact of bovine brucellosis and tuberculosis: Case study of Pirassununga, SP, Brazil. Semina: Ciênc Agrár. 2016; 37(5): 3793-3802. https://doi.org/10.5433/1679-0359.2016v37n5Supl2p3793
- WOAH (World Organization for Animal Health). Infection with Mycobacterium tuberculosis complex. Terrestrial Animal Health Code. 2024b. https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/?id=169&L=1&htmfile=chapitre_bovine_tuberculosis.htm [Accessed March 20, 2024].
- Rodrigues DL, Amorim EA, Ferreira F et al. Apparent prevalence and risk factors for bovine tuberculosis in the state of Paraná, Brazil: an assessment after 18 years since the beginning of the Brazilian program. Tropical Animal Health and Production. 2022; 54(6):360. https://doi.org/10.1007/s11250-022-03350-0
- WHO (World Health Organization). Self-declared disease-free status. World Health Organization. 2024c. https://www.woah.org/en/what-we-offer/self-declared-disease-status/ [Accessed January 11, 2024].
- WOAH (World Organization for Animal Health). Mammalian tuberculosis: Infection with Mycobacterium tuberculosis complex. Terrestrial Manual. 2022. https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.01.13_Mammalian_tuberculosis.pdf [Accessed March 15, 2024].
- Ferreira Neto JS. Brucellosis and tuberculosis in cattle in South America. Brazilian Journal of Veterinary Research and Animal Science. 2018; 55(2):1–23. https://doi:10.11606/issn.1678-4456.bjvras.2018.141139
- More SJ, Radunz B, Glanville RJ. Lessons learned during the successful eradication of bovine tuberculosis from Australia. Vet Rec. 2015: 177(9): 224-232. https://doi.org/10.1136/vr.103163
- Brasil, Ministério da Agricultura Pecuária e Abastecimento, Secretaria de Defesa Agropecuária. 2001. Instrução normativa n. 2, de 10 de janeiro de 2001. https://www.gov.br/agricultura/pt-br/assuntos/sanidade-animal-e-vegetal/saude-animal/programas-de-saude-animal/pncebt/principais-normas-pncebt/in-2-de-10-de-janeiro-de-2001-institui-o-pncebt.pdf/view [Accessed January 11, 2024].
- Lage AP, Roxo E, Müller EE et al. Programa Nacional de Controle e Erradicação da Brucelose e da Tuberculose Animal (PNCEBT). Manual Técnico. Brasília, Ministério da Agricultura e Pecuária. 2006. p.184.
- Ribeiro LA, Gonçalves VSP, Francisco PFC et al. Epidemiological status of bovine tuberculosis in the Federal District of Brazil. Semina: Ciênc Agrár. 2016; 37(5): 3561–3566. https://doi.org/10.5433/1679-0359.2016v37n5Supl2p3561
- Bahiense L, Ávila LN, Bavia ME et al. Prevalence and risk factors for bovine tuberculosis in the State of Bahia, Brazil. Semina: Ciênc Agrár. 2016; 37(5): 3549–3560. https://doi.org/10.5433/1679-0359.2016v37n5Supl2p3549
- Silva MCP, Gonçalves VSP, Mota ALAA et al. Prevalence and herd-level risk factors for bovine tuberculosis in the State of Paraná, Brazil. Semina: Ciênc Agrár. 2016; 37(5): 3611–3624. https://doi.org/10.5433/1679-0359.2016v37n5Supl2p3611
- Guedes IB, Bottene IFN, Monteiro LARC et al. Prevalence and risk factors for bovine tuberculosis in the State of Mato Grosso do Sul, Brazil. Semina: Ciênc Agrár. 2016; 37(5): 3579–3588. https://doi.org/10.5433/1679-0359.2016v37n5Supl2p3579
- Néspoli JMB, Negreiros RL, Amaku M et al. Epidemiological situation of bovine tuberculosis in the state of Mato Grosso, Brazil. Semina: Ciênc Agrár. 2016; 37(5): 3589–3600. https://doi.org/10.5433/1679-0359.2016v37n5Supl2p3589
- Vendrame FB, Amaku M, Ferreira F et al. Epidemiologic characterization of bovine tuberculosis in the State of Rondônia, Brazil. Semina: Ciênc Agrár. 2016; 37(5): 3639–3646. https://doi.org/10.5433/1679-0359.2016v37n5Supl2p3639
- Dias RA, Stanojlovic FMU, Belchior APC et al. Prevalence and risk factors for bovine tuberculosis in the state of São Paulo, Brazil. Semina: Ciênc Agrár. 2016; 37(5): 3673–3684. https://doi.org/10.5433/1679-0359.2016v37n5Supl2p3673
- Veloso FP, Baumgartem KD, Mota ALAA et al. Prevalence and herd-level risk factors of bovine tuberculosis in the State of Santa Catarina. Semina: Ciênc Agrár. 2016; 37(5): 3659–3672. https://doi.org/10.5433/1679-0359.2016v37n5Supl2p3659
- Queiroz MR, Groff ACM, Silva NDS et al. Epidemiological status of bovine tuberculosis in the state of Rio Grande do Sul, Brazil. Semina: Ciênc Agrár. 2016; 37(5): 3647–3658. https://doi.org/10.5433/1679-0359.2016v37n5Supl2p3647
- Barbieri JDM, Oliveira LF, Dorneles SEM et al. Epidemiological status of bovine tuberculosis in the state of Minas Gerais, Brazil. Semina: Ciênc Agrár. 2016; 37(5): 3531–3548. https://doi.org/10.5433/1679-0359.2016v37n5Supl2p3531
- Lima PRB, Nascimento DL, Almeida EC et al. Epidemiological situation of bovine tuberculosis in the state of Pernambuco, Brazil. Semina: Ciênc Agrár. 2016; 37(5): 3601–3610. https://doi.org/10.5433/1679-0359.2016v37n5Supl2p3601
- Galvis JOA, Grisi Filho JHH, Costa D et al. Epidemiologic characterization of bovine tuberculosis in the state of Espírito Santo, Brazil. Semina: Ciênc Agrár. 2016; 37(5): 3567–3578. https://doi.org/10.5433/1679-0359.2016v37n5Supl2p3567
- Rocha WV, Jayme VS, Mota ALAA et al. Prevalence and herd-level risk factors of bovine tuberculosis in the State of Goiás, Brazil. Semina: Ciênc Agrár. 2016; 37(5): 3625–3638. https://doi.org/10.5433/1679-0359.2016v37n5Supl2p3625
- Ferreira Neto JS, Barbosa RG, Ferreira F et al. Epidemiological situation of bovine tuberculosis in the State of Tocantins, Brazil. Semina: Ciênc Agrár. 2021; 42(3): 1673-1684. https://doi.org/10.5433/1679-0359.2021v42n3Supl1p1673
- Oliveira, BCRS, Oliveira, JP, Pinho, APVB et al. Epidemiological situation of bovine and bubaline tuberculosis in the State of Pará, Amazon region of Brazil. Front Vet Sci. 2024; 11, 1466199. https://doi.org/10.3389/fvets.2024.1466199
- Gilbert M, Mitchell A, Bourn D, Mawdsley J, Clifton-Hadley R, Wint W. Cattle movements and bovine tuberculosis in Great Britain. Nature. 2005; 435:491–496. https://doi:10.1038/nature03548
- Skuce RA, Allen AR, McDowell SWJ. Herd-level risk factors for bovine tuberculosis: a literature review. Vet Med Int. 2012; 621210. https://doi/10.1155/2012/621210
- Porphyre T, Stevenson MA, Mckenzie J. Risk factors for bovine tuberculosis in New Zealand cattle farms and their relationship with possum control strategies. Prev Vet Med. 2008; 86(1-2): 93-106, 2008. https://doi.org/10.1016/j.prevetmed.2008.03.008
- Ramírez-Villaescusa AM, Medley GF, Mason SA, Green LE. Risk factors for herd breakdown with bovine tuberculosis in 148 cattle herds in the South West of England. Prev Vet Med. 2010; 95(3-4): 224-230. https://doi.org/10.1016/j.prevetmed.2010.03.009
- Callefe JLR, Ferreira Neto JS. Sistemas de vigilância em saúde animal. São Paulo: Universidade de São Paulo. 2020. p.103. https://doi/10.11606/9786587778044

