Efficiency of ReLink-C Strategy for Chronic HCV Care
Efficiency of the Relink C strategy: identification and retrieval of chronic HCV patients full and partial diagnosed but unlinked to care
Victoria Aguilera1-3; Rocío González-Grande4-5; María-José Pena6; Ariadna Bono7; Rafael Granados8; Miguel Jiménez-Pérez9; Miriam Serrano10*; Helena Cantero11; Cristina González-de-Adalid11; María Sainz11; Nataly Espinoza-Cámac12; Raquel Domínguez-Hernández12.
OPEN ACCESS
PUBLISHED: 31 Decmeber 2024
CITATION: AGUILERA, Victoria et al. Efficiency of the Relink C strategy: identification and retrieval of chronic HCV patients full and partial diagnosed but unlinked to care. Medical Research Archives, [S.l.], v. 12, n. 12, dec. 2024. Available at: <https://esmed.org/MRA/mra/article/view/6149>.
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.18103/mra.v12i12.6149
ISSN 2375-1924
Abstract
Background: The existence of patients with Hepatitis C virus who were diagnosed but unlinked to care (DBUC) hinders the Hepatitis C elimination. The objectives were to present the results of the ReLink-C strategy in three Spanish hospitals and analyse its efficiency for both patients with positive viral load testing (full diagnosis) and with HCV antibodies, but without positive viral load testing (partial diagnosis); and partial diagnosis only.
Methods: The strategy was divided in two phases: Phase I, DBUC patients with complete or partial diagnosis without follow-up from Microbiology unit were searched and identified; and Phase II, missing patients were contacted by telephone and referred to Hepatology for further treatment. In addition, the characteristics (age, gender, country of origin and fibrosis status) of the lost and treated patients were collected.
Results: The three hospitals identified 3,444 patients to search for retrieval, 1,538 (45%) with full diagnosis and 1,906 (55%) partial diagnosis. Overall, about 35% (1,221/3,444) were DBUC and the ReLink-C strategy successfully localised 903 patients, of which 493 patients were linkage to care and 273 were treated. The ReLink-C strategy (full and partial diagnosis) compared to no intervention showed a reduction in liver and mortality complications (range 22-29%) with an incremental cost-utility ratio of €8,958/patient, and in case of partial diagnosis of €9,396/patient.
Conclusions: The Relink-C active search strategy enabled the retrieval and treatment of a significant number of diagnosed hepatitis C cases that would not have been detected in clinical practice. It was cost-effective for both (full and partial diagnosis) and partial diagnosis only, being in line with the 2030 hepatitis C elimination targets.
Keywords:
diagnosed but unlinked to care, cost-effectiveness, HCV infection
Introduction
Hepatitis C virus (HCV) infection causes acute or chronic hepatitis, with severe clinical manifestations in chronic cases that can have potentially life-threatening consequences. The World Health Organization (WHO) estimated 290,000 deaths due to HCV in 2019 from complications such as cirrhosis and hepatocellular carcinoma, out of an estimated 58 million people with chronic HCV worldwide. The high effectiveness of direct-acting antivirals (DAAs), which have simplified treatment and delayed the onset of serious complications of the disease, led to the WHO’s objective of eliminating liver viruses by 2030. Since the launched of the Spanish National Plan against Hepatitis C in 2015, numerous measures aimed at prevention, detection, diagnosis, and access to treatment have been promoted to reduce the morbidity and mortality caused by HCV, with 165,700 HCV patients being treated by 2023. As diagnostics was the main challenge to achieving hepatitis C elimination by 2030, one-step diagnosis (OSTD) of active HCV infection using the same patient serum sample was implemented. Compared to the standard diagnostic approach, OSTD reduces referral and treatment initiation times helping linkage to treatment care. Nonetheless, the existence of patients with HCV who were diagnosed but unlinked to care (DBUC) for various reasons in the health system is still a barrier to WHO’s HCV elimination strategy. According to the 2017-2018 Spanish survey, approximately 17% of the total individuals with diagnosed active HCV infection were unlinked to care in the National Heaths System (NHS). This loss can lead to delayed diagnosis and treatment of infection, increasing the risk of serious liver complications (hepatic decompensation, hepatocellular carcinoma or the need for liver transplantation) and other comorbidities. In order to work towards the elimination of HCV infection, the ReLink-C strategy was designed to identify DBUC patients link to the NHS for treatment. The strategy aims to search two types of patients (i) patients with HCV-RNA positive (HCV-RNA+) testing (full diagnosis) and (ii) patients with anti-HCV antibodies (anti-HCV+) but without evidence of positive HCV-RNA testing (partial diagnosis). Moreover, in order to ensure the NHS sustainability, economic evaluation of these healthcare interventions is considered relevant to assess their efficiency. The DAAs therapies have proven to be efficient for the treatment of chronic HCV in Spain. The ReLink-C strategy has been shown to be efficient in DBUC patients with chronic HCV, but no evidence was available for the strategy applied to DBUC patients with only anti-HCV+ test. The primary objective of this study was to present the results of the ReLink-C strategy in three Spanish NHS hospitals and analyse its efficiency by pooling both types of DBUC patients: full and partial diagnosis. The secondary objective was to evaluate the efficiency in DBUC patients with partial diagnosis.
Methods
POPULATION STUDY AND CENTRES
This analysis was based on data from three Spanish hospitals: (i) the Regional University Hospital of Malaga (RUHM), with a reference population of 630,000 inhabitants; (ii) the University and Polytechnic Hospital La Fe (UHLF) of Valencia, with a reference population of 290,000 inhabitants; and (iii) the University Hospital Doctor Negrin (UHDN) of Gran Canaria, with a reference population of 350,000 inhabitants. In an informative but not restrictive manner, the protocol for the ReLink-C strategy was submitted to and approved by the respective Ethics Committee. In accordance with Spanish legislation, all data were processed confidentially in an anonymous database accessible only to the researchers.
IDENTIFICATION AND INTERVENTION
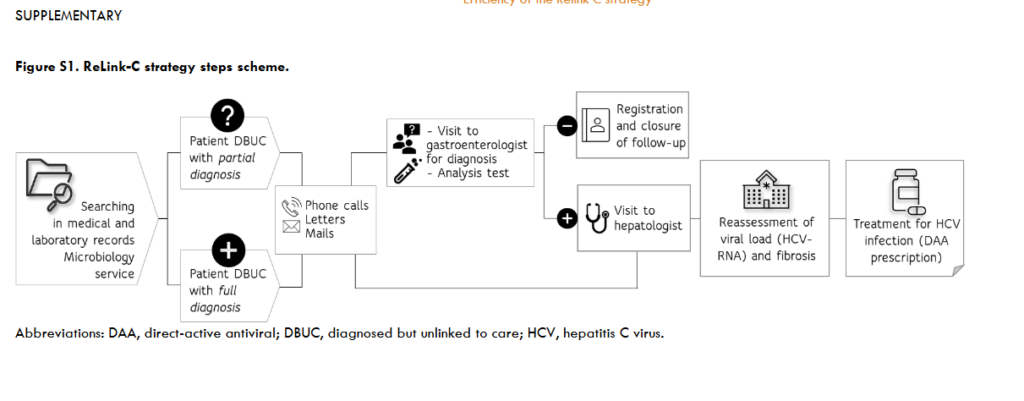
The methodology of the ReLink-C strategy consisted of two successive phases. The first, a retrospective search for patients eligible for treatment based on a review of medical and laboratory records (Phase I: Identification) and the second, the retrieval of these patients to re-evaluate and start treatment when necessary (Phase II: Intervention). In the Phase I, DBUC patients were identified in the Microbiology laboratory database of each hospital, with or without the help of the hospital’s IT services. The DBUC patients were divided into ‘full diagnosis’, defined as anti-HCV+ with HCV-RNA+, and ‘partial diagnosis’, defined as anti-HCV+ without evidence of subsequent HCV-RNA testing. Based on a review of medical records and the laboratory database, an attempt was made to locate all patients identified as potential DBUC patients. Clinical records were used to determine whether patients had been transferred to another healthcare area or had died. The remainder could be on treatment at the time of the search, untreated (due to comorbidity, short life expectancy) or cured. Thus, patients transferred to another healthcare setting, under treatment, untreated, cured or had died were not included. The retrospective data collected cover the period from January 2015 to December 2020 for RUHM, and from January 2010 to December 2020 for UHLF and UHDN. In addition, the patient search also differentiated the implementation of one-step diagnostics introduced in December 2018 (at RUHM), March 2018 (at UHLF), and May 2014 (at UHDN). The phase II, an attempt was made to locate DBUC patients to offer them an appointment with a specialist for assessment and offer treatment when necessary. This was done through telephone calls (range 1-10 per patient), e-mails and/or letters. Contacted cases were categorised into those who accepted the follow-up and those who did not (due to death, refused follow-up, treatment, or appointment). All patients who attended the consultation underwent a series of tests (which included among others, analytical, genotyping, ultrasound, and elastography or FIB-4 score to stage liver fibrosis) to assess their disease condition and offer them treatment if necessary. Patients with partial diagnosis underwent HCV PCR testing to determine whether they had chronic HCV infection and, in the case of a negative HCV-RNA result, they were informed and discharged. The reasons for not starting treatment were failure attended the appointment, refusal of treatment, or comorbidity.
ECONOMIC EVALUATION
To estimate the efficiency of the ReLink-C strategy compared to no intervention, a cost-utility analysis (CUA) was performed for both primary and secondary objectives. The analysis used a previously validated Markov model simulating the evolution of the disease through different health states over the life of the patient. The patients included in the simulation were DBUC patients, excluding those who had been treated or cured and died (once located or accepted for follow-up). The mean age of the patients and the distribution of fibrosis states were derived from hospital-based data. The sustained virologic response (98%) was used as a representative value for efficacy in treated patients. Transition probabilities, utility values, and costs of each health state were the same as those reported in the model. From the perspective of the Spanish NHS, the cost of the ReLink-C strategy included the consumption of direct healthcare resources to HCV diagnosis and dedicated hours to search and location DBUC patients. Unit resource costs were obtained from official regional publications and published literature. The treatment cost was €17,126 according to a Spanish study that provided the average pharmacological cost of antiviral treatment per patient. Costs were expressed in euros for the year 2024 (€). A lifetime time horizon was applied to estimate the number and cost of liver complications, life years (LYs) gained, quality-adjusted life years (QALYs) gained, total cost of intervention (ReLink-C strategy) and total cost of non-intervention. Liver complications included decompensated cirrhosis, hepatocellular carcinoma, liver transplantation and mortality due liver disease. A discount rate of 3.0% was applied for both costs and health outcomes. Sensitivity analysis (SA) was performed varying the cost of treatment (30% and 60% less than the baseline value) to provide different approaches in the healthcare setting and cost of diagnosis (±20%).
Results
IDENTIFICATION AND INTERVENTION
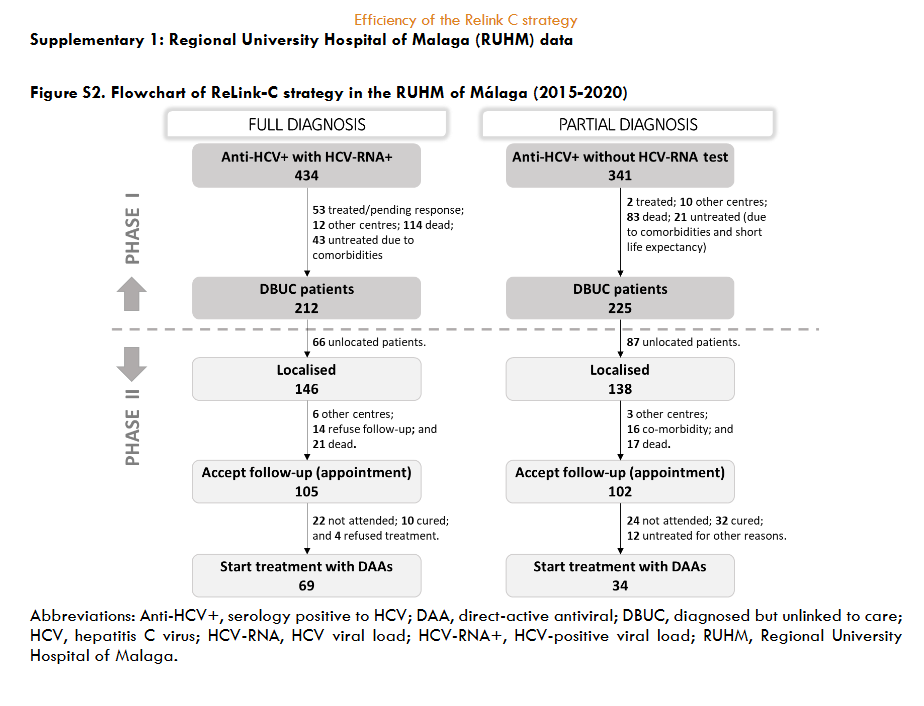
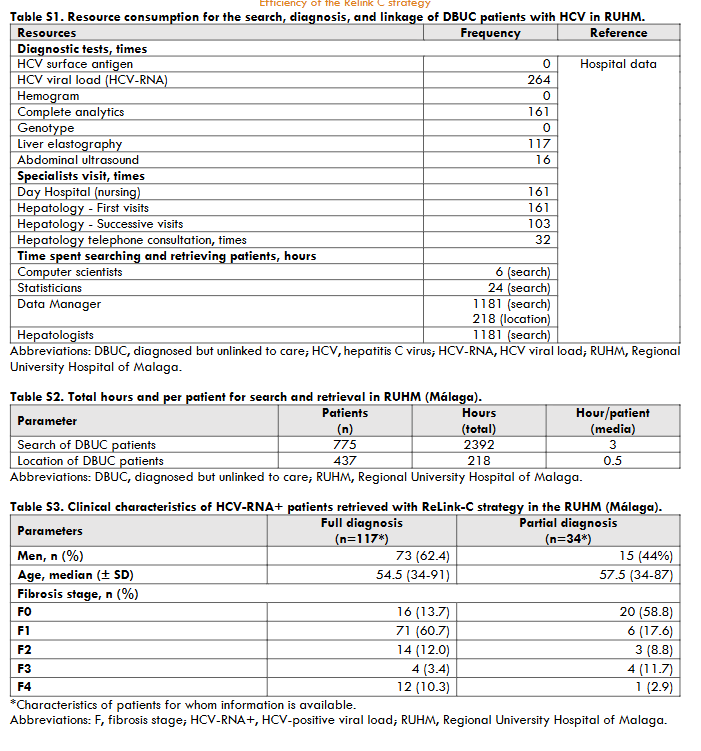
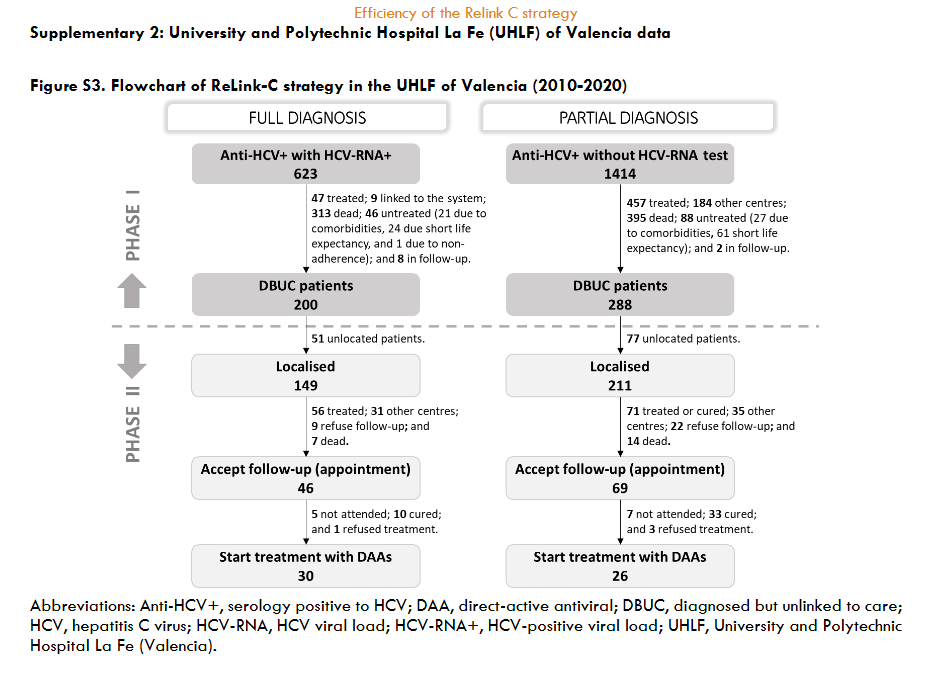
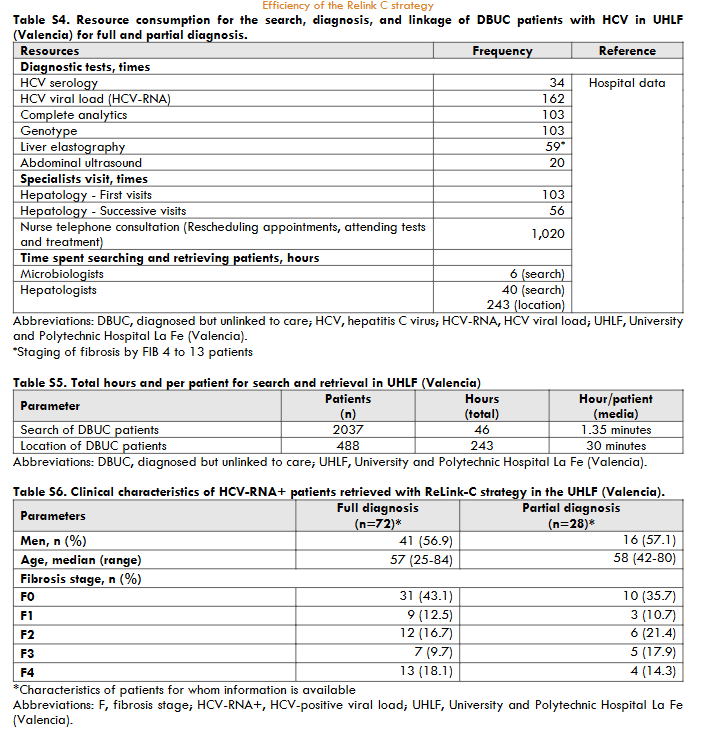
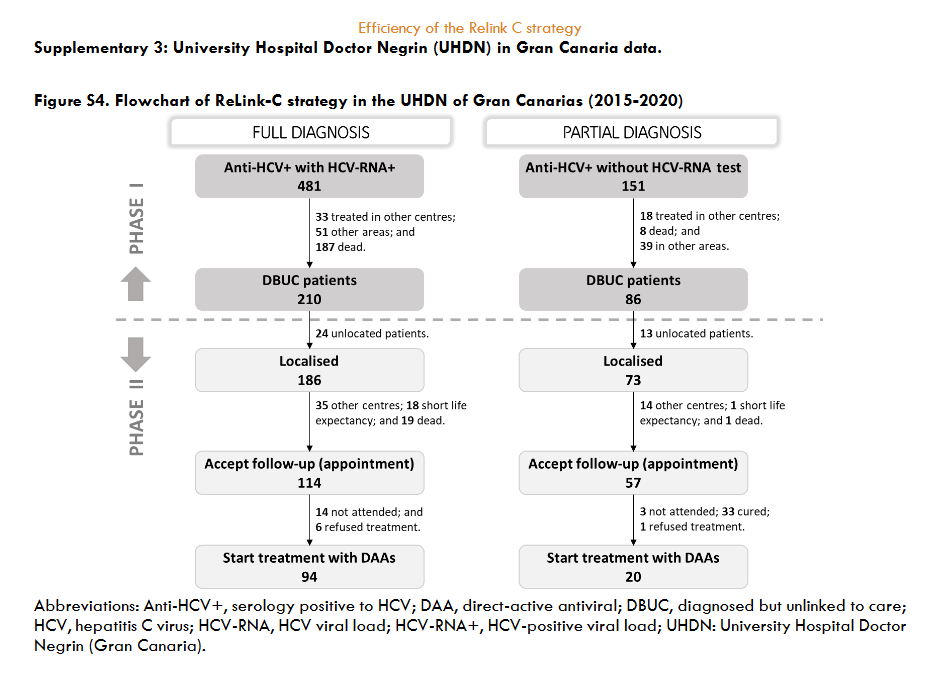
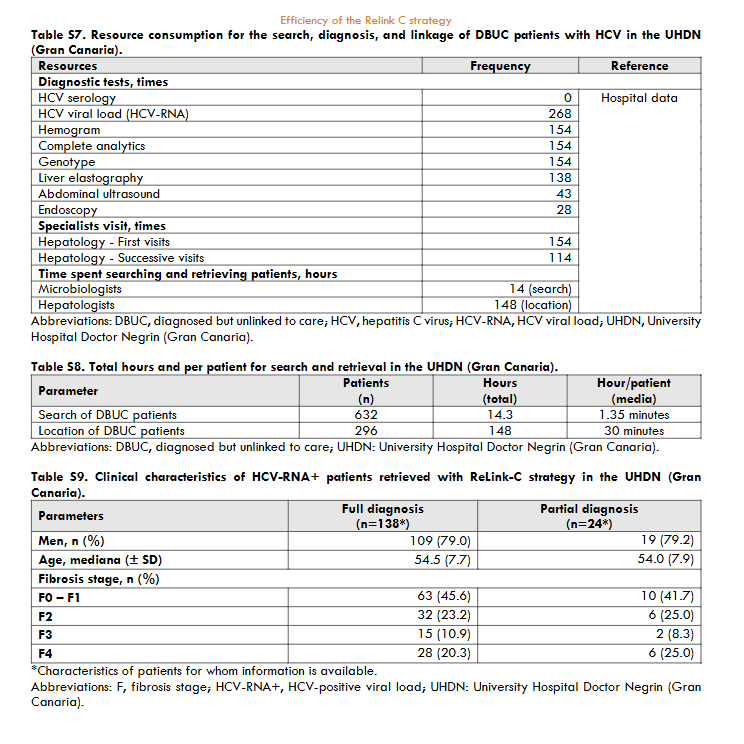
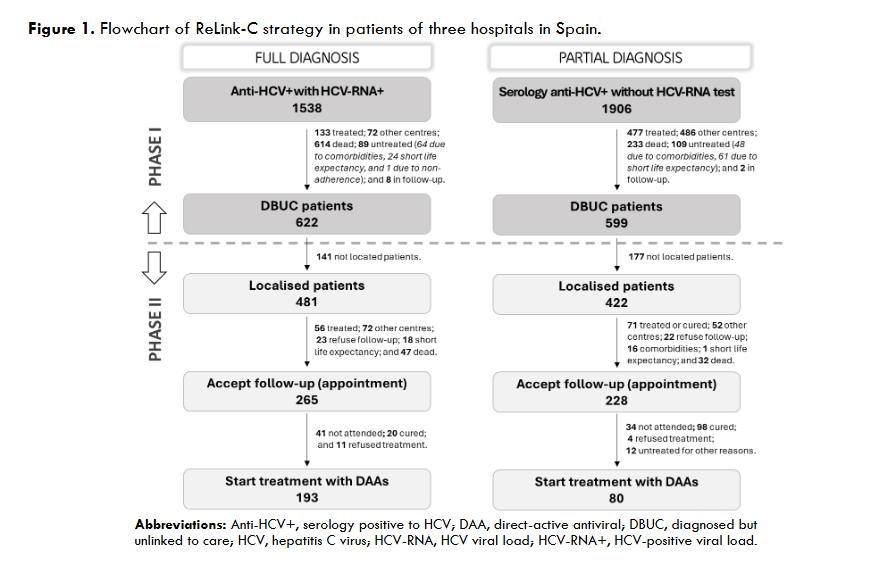
The three hospitals identified a total of 3,444 patients for search and retrieval, 1,538 (45%) with full diagnosis and 1,906 (55%) with partial diagnosis. Of the patients identified as full diagnosis, 622 (40%) were DBUC and selected for recovery in phase II, 133 (9%) were not selected for recovery because they had already been treated, 614 (40%) had died, 72 (5%) were from other centres, 89 (6%) were untreated due to comorbidities or short life expectancy, and 8 (1%) were in follow-up. Out of 622 DBUC patients selected for recovery in phase II, 141 (23%) were not localised. Of the successful contacts (n=481), 265 (55%) accept the hospital appointment and 193 started DAA treatment (73% of those who accepted the appointment). The reasons for non-appointment patients (n=216) were as follows: 128 (27%) were treated or were followed-up in other centres, 23 (5%) declined follow-up, 18 (4%) had comorbidities or short life expectancy, and 47 (10%) died. Of those patients identified as partial diagnosis, of identified patients, 599 (22%) were DBUC and selected for recovery in phase II excluding 477 (25%) already had treated, 233 (12%) had died, 486 (25%) were from other healthcare areas, 109 (6%) were untreated due to comorbidities or short life expectance, and 2 (0.1%) were in follow-up. Out of 599 DBUC patients selected for recovery, 422 (70%) were contacted, of these 228 (54% of contacted) accepted the hospital appointment and finally 80 patients started DAA treatment (representing a 35% of those who accepted the appointment). The patients who were not appointed (n=194) for further treatment were due various reasons: 71 (17%) were treated or cured, 52 (11%) were followed-up in other centres, 22 (5%) refused the follow-up, 17 (4%) had comorbidities or short life expectancy, and 32 (7%) died. The overall, about 35% (1,221/3,444) of hepatitis C patients identified were DBUC and the ReLink-C strategy successfully localised 903 patients of the total of DBUC (1,221 patients with full and partial diagnosis), of which 493 patients were linkage to care and 273 were treated. The clinical characteristics of the DBUC patients were obtained from the patients attending the appointment.
Figure 2. Clinical characteristics of HCV-RNA+ patients retrieved with ReLink-C strategy.
ECONOMIC EVALUATION
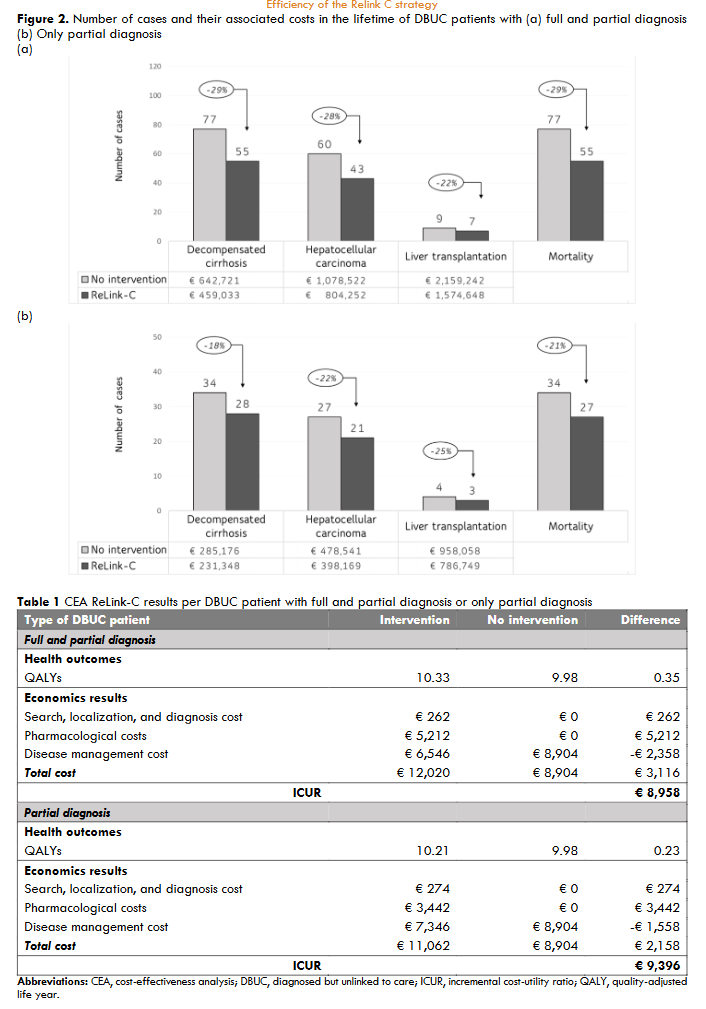
For the primary objective of the ReLink-C strategy, the simulation included a pooled of 897 DBUC patients with full diagnosis and partial diagnosis (out of 1,221 candidates to contact excluding 245 treated or cured, and 79 deaths). Of the pooled DBUC patients, 273 were treated with DAAs. Total cost of the intervention (ReLink-C strategy) was €234,919 corresponding to patient identification, recovery, and diagnostic. The ReLink-C strategy (including full and partial diagnosis patients) compared to no intervention showed a reduction in 22 cases of liver-related mortality and a reduction in the number of liver complications (22 cases of decompensated cirrhosis, 17 cases of hepatocellular carcinoma, and 2 liver transplantation). The total savings generated related to liver complications were €1,042,551. The CEA showed an increase of 0.35 QALYs compared to no intervention with a total incremental cost of €3,116, resulting an incremental cost-utility ratio (ICUR) of €8,958 per patient. In the simulation of the secondary objective, we included 398 DBUC patients with partial diagnosis of which 80 patients were treated. The total cost associated of the ReLink-C strategy for these type patients was €108,883. ReLink-C strategy versus no intervention also decreased a 21% liver-related mortality and between 18-25% liver complications generating total savings of €305,510. The ReLink-C strategy for partial diagnosis patients showed an increase of 0.23 QALYs compared to no intervention with an incremental cost of €2,158, resulting a ICUR of €9,396 per patient. In summary, the model results showed that the ReLink-C intervention was a cost-effective strategy in the search DBUC patients with full and partial diagnosis and with only partial diagnosis patients. At full diagnosis, a 30% decrease in treatment cost decreased the ICUR to €4,462 per patient and 60% made the Relink-C strategy dominant. Variations in the cost of diagnosis varied the ICUR between €8,807 and €9,108. In partial diagnosis, SA showed a variation in the ICUR when changing the cost of treatment or the cost of diagnosis of between €450-€4,900 and €9,158-€9,634, respectively.
Discussion
Since the elimination of hepatitis requires comprehensive interventions in different public health areas, including prevention, diagnosis, and treatment, it is important to increase awareness of the contribution made by the ReLink-C strategy. The collection data obtained provided evidence on the number of diagnosed but untreated patients and demonstrated the importance of searching and contacting these patients in contributing to achieving the HCV elimination targets set by the WHO. Furthermore, the economic evaluation determined the efficiency of this strategy to assist in decision-marking in health systems. The results of the ReLink-C strategy in these hospitals reflect that a significant percentage (about 35%; 1221/3444) of hepatitis C patients were already diagnosed and would remain unidentified if such strategies were not implemented. This is in line with other studies on missing case finding and patient recovery in Spain and other countries. Although it is difficult to compare the results between them due to the diversity in methodology, we can point out that they reaffirm the need to incorporate this strategy in all hospitals given its effectiveness in the recovering lost patients and in achieving their linkage to the health system.
Conclusion
The active search strategy Relink-C, allowed for the retrieval and treated of a significant number of diagnosed hepatitis C cases that would not have been detected by established procedures in the analysis period. The implementation of one-step diagnosis may not guarantee the complete elimination of diagnosed patients from the system, so it is essential to implement automated alerts and periodic active searches. To ensure the success of these strategies, it is essential to provide physicians (multiple specialties and care levels) with the necessary background information and guidelines. The Relink-C strategy proved to be cost-effective for both full and partial diagnosis and partial diagnosis only, being in line with the 2030 hepatitis C elimination targets.
Conflict of interest
V Aguilera has no conflicts of interest regarding this study. R González-Grande MJ Pena has no conflicts of interest regarding this study. A Bono has no conflicts of interest regarding this study. R Granados has no conflicts of interest regarding this study. M Jiménez-Pérez M Serrano has no conflicts of interest regarding this study. H Cantero, M Sainz, and C González-de-Adalid are employees of Gilead Sciences Spain. R Domínguez-Hernández and N Espinoza-Cámac are employees of PORIB and received fees from Gilead Sciences for their consultancy services in relation to the development of this work.
Funding statement
This work has been funded by Gilead Sciences Spain.
Acknowledgments
We would like to thank Sandra Blanco for her support in answering queries to the investigators hospital during data collection.
Author contributions
V Aguilera, R González-Grande, MJ Pena, A Bono, R Granados, M Jiménez-Pérez contributed equally to the data collection and provided information of strategy ReLink-C implementation in Spain. H Cantero, M Sainz, C González-de-Adalid, and R Domínguez-Hernández contributed to the conception, methodology and design of the study. R Domínguez-Hernández and N Espinoza-Cámac contributed to the interpretation of the data, carried out the adaptation of the data analysis, data processing, the writing-draft and writing-reviews of the manuscript. All authors contributed to the critical revision of the intellectual content and approval of the final version of the manuscript.
References
- World Health Organization. Hepatitis C. Accessed December 7, 2023. https://www.who.int/es/news-room/fact-sheets/detail/hepatitis-c
- World Health Organization. Global health sector strategy on viral hepatitis 2016-2021. Towards ending viral hepatitis. Accessed December 7, 2023. https://www.who.int/publications-detail-redirect/WHO-HIV-2016.06
- Ministerio de Sanidad. Strategic plan for tackling Hepatitis C in the Spanish National Health System. May 2015. Accessed November 3, 2023. https://www.sanidad.gob.es/ciudadanos/enfLesiones/enfTransmisibles/hepatitisC/PlanEstrategicoHEPATITISC/docs/PEAHC_eng.pdf
- Ministerio de Sanidad. Plan estratégico para el abordaje de la Hepatitis C en el SNS (PEAHC). October 2020. Accessed November 3, 2023. https://www.sanidad.gob.es/ciudadanos/enfLesiones/enfTransmisibles/hepatitisC/PlanEstrategicoHEPATITISC/docs/Plan_Estrategico_Abordaje_Hepatitis_C_(PEAHC).pdf
- Ministerio de Sanidad. Hepatitis C crónica. Pacientes que inician tratamiento para hepatitis C crónica con antivirales de acción directa. Actualización a 30 de septiembre de 2023. September 30, 2023. Accessed April 18, 2024. https://www.sanidad.gob.es/areas/farmacia/publicaciones/planoptimizacion/tratamientoHepatitisC.htm
- García F, Domínguez-Hernández R, Casado M, et al. The simplification of the diagnosis process of chronic hepatitis C is cost-effective strategy. Enferm Infecc Microbiol Clin (Engl Ed). 2019;37(10):634-641. doi:10.1016/j.eimc.2019.03.001
- Torrecillas M, Gómez-Muñoz N, Ocete MD, et al. One-step diagnosis strategy together with multidisciplinary telematics referral perform an effective approach for identifying and treating patients with active Hepatitis C infection. Ann Hepatol. 2022;27(1):100542. doi:10.1016/j.aohep.2021.100542
- Andaluz García I, Arcos Rueda MDM, Montero Vega MD, et al. Patients with hepatitis C lost to follow-up: ethical-legal aspects and search results. Rev Esp Enferm Dig. 2020;112(7):532-537. doi:10.17235/reed.2020.7077/2020
- Morales-Arraez D, Hernández-Bustabad A, Reygosa Castro C, et al. Reengagement strategies for hepatitis C patients lost to follow-up: A randomized clinical trial. Hepatol Commun. 2023;7(6):e0080. doi:10.1097/HC9.0000000000000080
- Guerra Veloz MF, Del Pino Bellido P, Cordero Ruiz P, et al. HCV microelimination strategies: An interventional study in diagnosed patients without access to the system. Liver Int. 2021;41(5):928-933. doi:10.1111/liv.14824
- Del Pino Bellido P, Guerra Veloz MF, Cordero Ruiz P, et al. Chronic hepatitis C patients lost in the system: predictive factors of non-referral or loss of follow-up in Hepatology units. Rev Esp Enferm Dig. 2021;113(12):833-839. doi:10.17235/reed.2020.7573/2020
- Aleman S, Söderholm J, Büsch K, Kövamees J, Duberg AS. Frequent loss to follow-up after diagnosis of hepatitis C virus infection: A barrier towards the elimination of hepatitis C virus. Liver Int. 2020;40(8):1832-1840. doi:10.1111/liv.14469
- Burgui C, Martín C, Juanbeltz R, et al. Recapture of patients with an incomplete diagnosis of hepatitis C virus infection. Rev Esp Enferm Dig. 2020;112. doi:10.17235/reed.2020.6944/2020
- Vargas-Accarino E, Martínez-Campreciós J, Domínguez-Hernández R, et al. Cost-effectiveness analysis of an active search to retrieve HCV patients lost to follow-up (RELINK-C strategy) and the impact of COVID-19. J Viral Hepat. 2022;29(7):579-583. doi:10.1111/jvh.13686
- Ministry of Health. Prevalence of hepatitis C in Spain: results from a national population based survey in 2017-2018. July 27, 2020. Accessed April 23, 2024. http://www.mscbs.es/ciudadanos/enfLesiones/enfTransmisibles/sida/hepatitis/Prevalence.pdf
- Chang SS, Hu HY, Chen YC, Yen YF, Huang N. Late hepatitis C virus diagnosis among patients with newly diagnosed hepatocellular carcinoma: a case-control study. BMC Gastroenterol. 2022;22(1):425. doi:10.1186/s12876-022-02504-6
- López-Bastida J, Oliva J, Antoñanzas F, et al. Spanish recommendations on economic evaluation of health technologies. Eur J Health Econ. 2010;11(5):513-520. doi:10.1007/s10198-010-0244-4
- Turnes J, Domínguez-Hernández R, Casado MÁ. Cost-effectiveness analysis of two treatment strategies for chronic hepatitis C before and after access to direct-acting antivirals in Spain. Gastroenterol Hepatol. 2017;40(7):433-446. doi:10.1016/j.gastrohep.2017.05.004
- Esteban R, Domínguez-Hernández R, Cantero H, Casado MÁ. Evaluation of the clinical and economic value of sofosbuvir/velpatasvir (SOF/VEL) in patients with chronic hepatitis C in Spain during the last 5 years. Gastroenterología y Hepatología. Published online May 2024:S0210570524001572. doi:10.1016/j.gastrohep.2024.502199
- Retribuciones Andalucía. Resolución 0010/2024. Modificación de la Resolución de Retribuciones del personal de Centros Sanitarios. Ejercicio 2023. Accessed March 13, 2024. https://www.sspa.juntadeandalucia.es/servicioandaluzdesalud/profesionales/guia-laboral/retribuciones
- Retribuciones Canarias. Retribuciones-Personal-estatutarios_Canarias 2023-2022. Accessed March 13, 2024. https://www3.gobiernodecanarias.org/sanidad/scs/content/6d5a6473-9362-11ea-979a-3b49391d2a8b/Retribuciones-estatutarios.pdf
- Retribuciones Comunidad Valenciana. Tablas retributivas del personal al servicio de las instituciones sanitarias de la Conselleria competente en materia de sanidad. 2023. Accessed March 13, 2024. https://www.san.gva.es/es/web/recursos-humanos/retribuciones-personal-iiss
- eSalud Database. Costs and prices of the health sector. Barcelona. Accessed January 31, 2023. http://esalud.oblikue.com/
- García-Herola A, Domínguez-Hernández R, Casado MÁ. Clinical and economic impact of an alert system in primary care for the detection of patients with chronic hepatitis C. PLoS One. 2021;16(12):e0260608. doi:10.1371/journal.pone.0260608
- Morales-Arraez D, Nieto Bujalance Y, Diaz-Flores F, et al. Risk of liver fibrosis progression in patients with suboptimal diagnosis of hepatitis C virus infection. Eur J Gastroenterol Hepatol. 2020;32(4):528-534. doi:10.1097/MEG.0000000000001534
- Morales-Arraez D, Alonso-Larruga A, Diaz-Flores F, et al. Predictive factors for not undergoing RNA testing in patients found to have hepatitis C serology and impact of an automatic alert. J Viral Hepat. 2019;26(9):1117-1123. doi:10.1111/jvh.13122
- Isfordink CJ, van Dijk M, Brakenhoff SM, et al. Hepatitis C Elimination in the Netherlands (CELINE): How nationwide retrieval of lost to follow-up hepatitis C patients contributes to micro-elimination. Eur J Intern Med. 2022;101:93-97. doi:10.1016/j.ejim.2022.04.024
- Isfordink CJ, Brakenhoff SM, van Dijk M, et al. Hepatitis C elimination in the Netherlands (CELINE): study protocol for nationwide retrieval of lost to follow-up patients with chronic hepatitis C. BMJ Open Gastroenterol. 2020;7(1):e000396. doi:10.1136/bmjgast-2020-000396
- Beekmans N, Klemt-Kropp M. Re-evaluation of chronic hepatitis B and hepatitis C patients lost to follow-up: results of the Northern Holland hepatitis retrieval project. Hepatol Med Policy. 2018;3:5. doi:10.1186/s41124-018-0032-9
- Ministerio de Sanidad. Guía de cribado de la infección por el virus de la hepatitis C; 2020. Accessed April 8, 2024. https://mirror.mscbs.gob.es/ciudadanos/enfLesiones/enfTransmisibles/sida/hepatitis/ciudadanosMenu.htm
- Llaneras J, Ruiz-Cobo JC, Rando-Segura A, et al. Integrating viral hepatitis management into the emergency department: A further step towards viral hepatitis elimination. JHEP Reports. 2024;6(1):100932. doi:10.1016/j.jhepr.2023.100932
- Gómez De La Cuesta S, Martín-Arribas MI, Mateos Hernández MI, Oliva Oliva A, Geijo Martínez F. Hepatitis C virus micro-elimination in vulnerable populations before and during a global pandemic. Rev Esp Enferm Dig. Published online 2022. doi:10.17235/reed.2022.9275/2022
- Ortega González E, Ocete Mochón MD, Gimeno Cardona C, et al. Opportunistic population screening as a hepatitis elimination strategy: the CRIVALVIR-FOCUS program. International Journal of Infectious Diseases. 2024;146:107131. doi:10.1016/j.ijid.2024.107131