Improving Sleep Quality with Botulinum Toxin Treatments
PROSPECTIVE STUDY ON IMPROVING NIGHT’S REST AND QUALITY OF LIFE BY USING BOTULINUM TOXIN
Sistac Palacín, JM Md., Viñas Salas J, Md.Ph., Sistac Ballarin, JM. Md.Ph.
- Arnau de Vilanova University Hospital. Faculty of Medicine. Lleida. Spain
- Faculty of Medicine of the University of Lleida. Spain
- Arnau de Vilanova University Hospital. Faculty of Medicine. Lleida. Spain
OPEN ACCESS
PUBLISHED: 28 Febuary 2025
CITATION: PALACÍN, Sistac; SALAS J, Viñas; BALLARIN, Sistac. PROSPECTIVE STUDY ON IMPROVING NIGHT’S REST AND QUALITY OF LIFE BY USING BOTULINUM TOXIN AT A DOSE OF 25 IU IN HEAD AND FACE PATHOLOGIES REFRACTORY TO ORAL TREATMENT. ON 283 CASES. Medical Research Archives. Available at: <https://esmed.org/MRA/mra/article/view/6228>.
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.18103/mra.v13i2.6228
ISSN 2375-1924
ABSTRACT
The use of Botulinum Toxin is increasingly being extended in different conditions refractory to other treatments.Thus, it is being used in dystonias, trigeminal neuralgia, migraines, headaches and alterations of the temporomandibular joint.
OBJECTIVES
Primary: To assess the improvement of night rest in the treatment with botulinum toxin type A in chronic Migraine. Trigeminal neuralgia (regardless of the affected branch) and involvement of the temporomandibular joint and/or Bruxism, after therapeutic failure maintained over time. Over more than two years of previous oral or topical drug therapies.
Secondary: To assess the effectiveness of the dose used and its average duration over time.
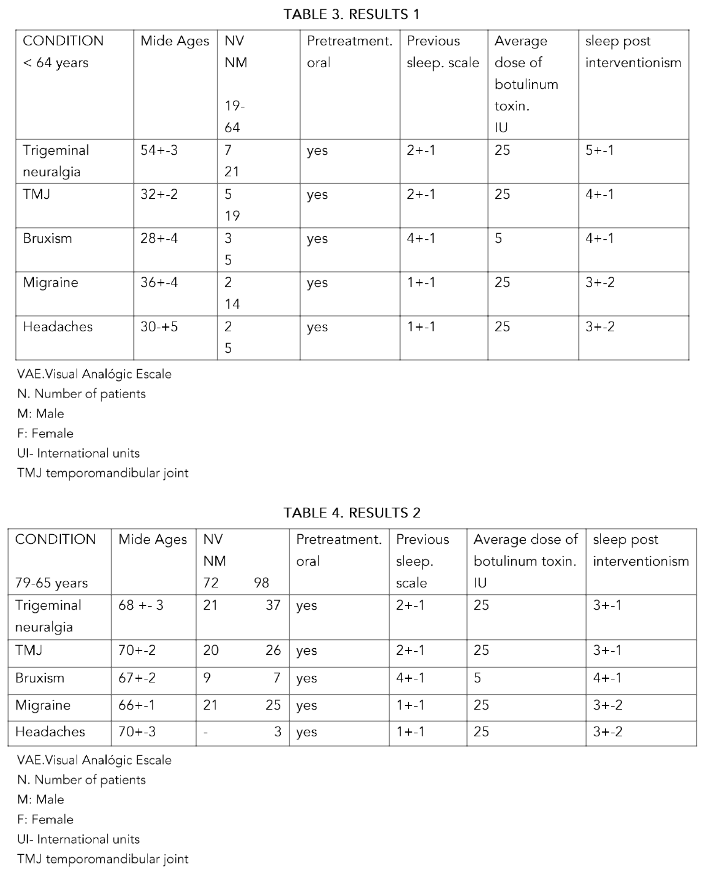
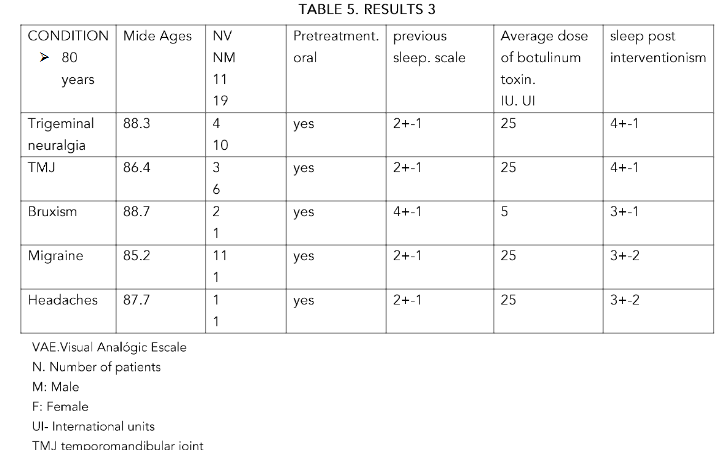
METHODS: 283 patients of both sexes, aged between 85 and 43 years, were included. The patients studied were separated by sex. Group A: over 80 years old. Group B: between 65 and 79 years old and Group C: under 64 years old.25 IU of botulinum toxin type A were administered for trigeminal neuralgia, severe temporomandibular joint (TMJ) pain, migraines and headaches. , refractory for two years or more to medical treatments
RESULTS: In all groups, with doses of 25 IU in a single dose, there was a significant improvement in the rest, from the injection, until the six months of control, especially in the group C, in trigeminal neuralgia. It was not significant in the other conditions and/or age groups
CONCLUSIONS: In all groups the effectiveness was greater in trigeminal neuralgia at the established doses P<0.5 and more in the lower group age (C) with more than 6 months of improvement in the condition. In the rest of the pathologies treated, the results at the established doses are very similar to those of the referenced studies. Its use can be effective at doses of 25 IU.
Keywords
Botulinum Toxin, sleep disorders, refractory conditions, headaches, and other therapeutics.
Introduction
In 1949, the first paper was published indicating how botulinum toxin inhibited the release of acetylcholine in the neuromuscular junctions of skeletal muscles, and its possible application in spasticity. This discovery was carried out by Burgen, Dickens and Zatman, and its first medical application was to treat ocular deviation in strabismus. The use of Botulinum Toxin (BTX-A) was first approved in 1989 by the Food and Drugs Administration (FDA) in the USA, for the treatment of strabismus and blepharospasm in patients over 12 years of age. Nowadays, when we hear about the use of Botulinum toxin or Botox, we almost always associate it with rejuvenation therapies in cosmetic medicine, mainly in the correction of wrinkles on the face, such as those on the forehead, periorbital, nasal and perioral areas, or platysmal bands on the neck. Medicine discovered its application in many areas of treatment, such as ophthalmology, maxillofacial dentistry, physiotherapy, traumatology, neurology, vascular and in the treatment of chronic pain, among others. It was in the 80s when it began to be applied in strabismus, blepharospasm, facial hemispasm, dystonia and in cosmetics itself. In the 90s it was already consolidated as a recognized therapeutic alternative in spasticity and dystonia. Later its application was extended to the treatment of autonomic disorders (sialorrhea, hyperhidrosis), facial asymmetry, tension headache, migraine, myofascial pain, chronic lower back pain, musculoskeletal disorders.
Objectives
OBJECTIVE PRIMARY: To assess the improvement in nighttime rest in treatment with botulinum toxin type A in chronic migraine, trigeminal neuralgia (regardless of the affected branch) and involvement of the temporomandibular joint and/or bruxism, after therapeutic failure maintained for more than two years of previous oral or topical pharmacological therapies.
SECONDARY: To assess the effectiveness of the dose used. To confirm this response after six months of treatment.
Methods
Following approval by the Clinical Research Committee CEIC 2894, the study was conducted from January 2020 to November 2024.
A total of 283 patients of both sexes, aged between 43 and 85 years, were included. The patients studied were separated by sex. Group A: over 80 years of age. Group B: aged between 65 and 79 years. Group C: under 64 years.
The pathologies studied, and also grouped into groups A, B and C, were:
- Trigeminal neuralgia
- Severe temporomandibular joint (TMJ) pain
- Migraines and headaches refractory for two years or more to medical treatments
Exclusion criteria:
- Age under 18 years
- Patient refusal to participate in the study
- Previous infiltrations with corticosteroids or local anesthetics
- Associated psychiatric disorders
The dose of botulinum toxin type A administered in all cases was 25 IU.Prior to the infiltration, the patient was asked about the assessment of his night’s rest using the approved scale, and then he was asked again six months later.This was subsequently processed by the statistical package for processing means and comparing the study groups SPSS 29.0 — a value of P<0.05 being significant in the different variables studied.
References
Table 1. RESULTS 1
| CONDITION | AGE | NV | Pretreatment | Previous sleep intervention | Average dose of botulinum toxin, IU | sleep post intervention |
|---|---|---|---|---|---|---|
| Trigeminal | 34-2 | yes | 2+1 | 5+1 | 25 | 3+2 |
| Bruxism | 34-2 | yes | 2+1 | 3+1 | 25 | 3+2 |
| Headaches | 30+5 | 2 | 1+ | 25 | 3+2 |
Table 2. RESULTS 2
| CONDITION | AGE | NV | Pretreatment | Previous sleep intervention | Average dose of botulinum toxin, IU | sleep post intervention |
|---|---|---|---|---|---|---|
| Trigeminal | 68-3 | yes | 2+1 | 3+1 | 25 | 3+2 |
| TMJ | 70+2 | yes | 2+1 | 3+1 | 25 | 3+2 |
| Headaches | 66+1 | yes | 2+1 | 3+1 | 25 | 3+2 |
Discussion
Regarding the treatment of trigeminal neuralgia (TN) with botulinum toxin type A, the work of Rubis¹⁷ included a bibliographic search in PubMed and the Cochrane library in English from January 2010 to February 2020. In all groups, with a single dose of 25 IU, there was a significant improvement in sleep quality at 6 months with averages on the VAS scale of ±3 points. This was more significant in cases of trigeminal neuralgia in all groups (P<0.05), with a longer duration in the younger age groups (around 7 months). In cases of migraines and headaches we did not obtain significant values with average durations of the effect of around 8 months in groups B and C, but less in group A of older patients. In general, positive responses to the treatments established exceeded 5 months on average, with the dose established at 25 IU: especially in trigeminal neuralgia and bruxism, and/or temporomandibular joint disorders, always in the younger age groups. The review included 4 randomized, double-blind, placebo-controlled trials with a follow-up of 8 to 12 weeks to observe changes in the Visual Analogue Scale (VEA) and in the frequency of TN attacks. The average frequency of TN attacks in 3 studies in the BT-A group decreased by 85%, while in placebo only by 15.9%. Maximum efficacy was observed between 6 weeks and 3 months after the procedure, with clear improvement in nighttime rest. They do not differentiate by age, as in our case. Although in all of them, although at higher doses (50 IU), they suggest a notable improvement in night rest. Serrera-Figallo¹⁸ performed systematic searches in the Medline database looking for research articles published between 2014 and 2019. They found three relevant works on trigeminal neuralgia. This treatment reduced symptoms efficiently enough to satisfy patients, including a notable improvement in rest. These results agree with our study, although. As we pointed out, our doses are much lower (25 IU), although these authors do not discriminate by age. We obtain better results (SEE TABLE) in the younger patient group P<0.05. In this same line we can include the work of Wu¹⁹ on 104 patients where the authors indicated that their study suggests that the success of the treatment was greater in patients aged 50 years or older (OR = 3.66, 95% CI: 1.231–10.885, in our case they are under 64 years old. Muñoz Lora²⁰ carried out a review using the criteria of the American Academy of Neurology on controlled clinical trials in bruxism, temporomandibular disorders and trigeminal neuropathic pain. The use of BTX-A is indicated as effective for the treatment of trigeminal neuralgia (category A), being this more appropriate than in other pathologies. In our case the improvement in the quality of rest improved in all groups, although it was evidently higher in trigeminal neuralgia, in relation to headaches, for example. Moore²¹ Sridharan²² and Meng²³ indicate that, in their work, there were significant differences in the hours of sleep. This is also reflected in the work of Castillo²⁴ although as we can see, they are doses higher than those of all these authors, which are between 50 and 100iu. Türk²⁵ conducted a clinical trial in which a total of 27 patients were injected with 100 units of BTX-A at the maxillary and mandibular level. Doses 50 IU higher than our study. In these patients, the intensity of pain and the frequency of pain attacks were significantly reduced in the first week, second and sixth month after treatment, as well as in the quality of sleep and life. This is also reflected in the work of Morra²⁶ who searched 10 databases and search engines to access relevant publications, as well as Oh HM et al²⁷ who searched the PubMed and OvidSP databases from 1966 to May 2012, the Hu group²⁸ who searched PubMed, EMBASE, Cochrane Library Clinical Trials and Web of Science from January 1966 to March 2013. Five prospective studies and one double-blind, randomized, placebo-controlled study were identified. Response was achieved in approximately 70–100% of patients, and mean pain intensity and frequency were reduced by approximately 60–100% at 4 weeks after treatment in most studies. No major adverse events were reported. And their quality of life and rest were improved, but we insist on higher doses than those we used (see table 6).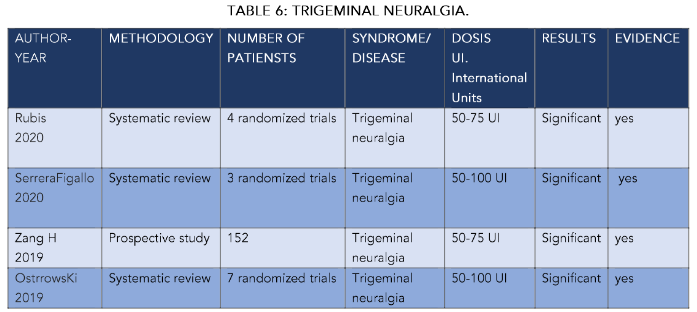
Where the worst results are obtained, and we believe that it is not very effective, is in the treatment of migraines and/or chronic headaches. These are of short duration in time and with many complications associated with their treatment. In table 8 we reflect some of the most significant works in the study of these entities. Among these we will highlight those of Torres³⁸ who carried out a prospective observational study on 395 patients. After 6 months, 49.1% of the patients responded with improvement in headache pain; associating better quality of sleep and rest, figures similar to our study. Although we would like to highlight that their doses were double ours. Bellón³⁹ in 2019 carried out a review of 90 articles that included 28 trials (N: 4190) treated with botulinum toxin. The longest duration of treatment was three rounds of injections over three months. We administered only one dose (which was effective for an average of 5-6 months). The authors indicated that botulinum toxin could reduce the number of days with migraine per month in the population with chronic migraine by 3.1 days (95% confidence interval [CI]: -4.7 to -1.4, with a clear improvement in sleep quality). In one trial, 1384 participants (high evidence), botulinum toxin reduced the number of days with headache per month by 1.9 days (95% CI: -2.7 to -1.0) and improved nighttime rest, although these doses, as can be seen in table 8, respond to doses that may not be very significant and that are closer to what is described in the rest of the consulted literature, and that, as we see, improves the sleep scale by several points, although our scale has been adapted and modified to our reality.
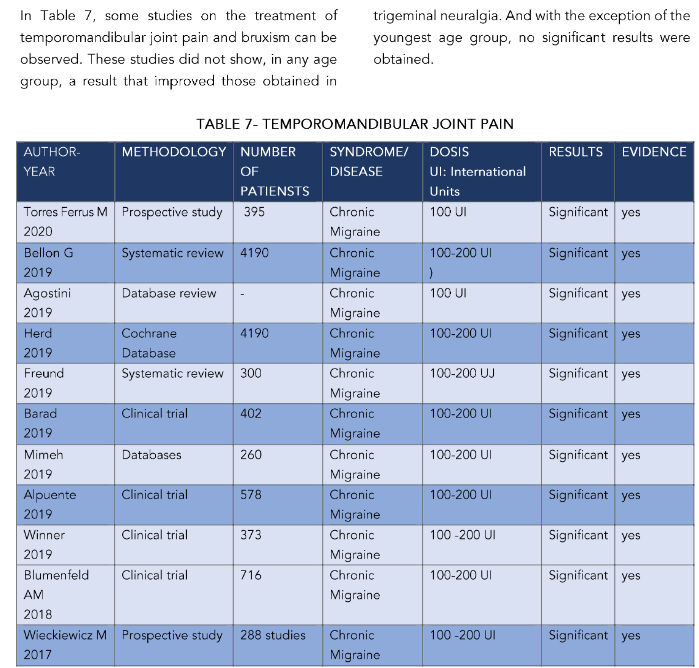
TABLE 8 – MIGRAINES
| AUTHOR-YEAR | METHODOLOGY | NUMBER OF PATIENTS | SYNDROME/DISEASE | DOSIS UI: International Units | RESULTS | EVIDENCE |
|---|---|---|---|---|---|---|
| Torres Ferrus M 2020 | Prospective study | 395 | Chronic Migraine | 100 UI | Significant | yes |
| Bellon G 2019 | Systematic review | 4190 | Chronic Migraine | 100–200 UI | Significant | yes |
| Agostini 2019 | Database review | – | Chronic Migraine | 100 UI | Significant | yes |
| Herd 2019 | Cochrane Database | 4190 | Chronic Migraine | 100–200 UI | Significant | yes |
| Freund 2019 | Systematic review | 300 | Chronic Migraine | 100–200 UI | Significant | yes |
| Barad 2019 | Clinical trial | 402 | Chronic Migraine | 100–200 UI | Significant | yes |
| Mimeh 2019 | Databases | 260 | Chronic Migraine | 100–200 UI | Significant | yes |
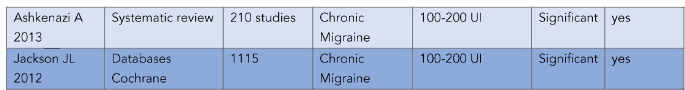
Summary Table of Studies on Botulinum Toxin (BTX-A) for Chronic Migraine
| Author / Year | Study Type | Sample Size | Condition | Dose (UI) | Effect | Significant |
|---|---|---|---|---|---|---|
| Alpuente 2019 | Clinical trial | 578 | Chronic Migraine | 100–200 UI | Significant | Yes |
| Winner 2019 | Clinical trial | 373 | Chronic Migraine | 100–200 UI | Significant | Yes |
| Blumenfeld AM 2018 | Clinical trial | 716 | Chronic Migraine | 100–200 UI | Significant | Yes |
| Wieckiewicz M 2017 | Prospective study | 288 | Chronic Migraine | 100–200 UI | Significant | Yes |
| Ashkenazi A 2013 | Systematic review | 210 studies | Chronic Migraine | 100–200 UI | Significant | Yes |
| Jackson JL 2012 | Databases (Cochrane) | 1115 | Chronic Migraine | 100–200 UI | Significant | Yes |