Music Therapy for PTSD in Neurocognitive Disorder Patients
Music Therapy for Posttraumatic Stress Disorder with Patients Suffering from Neurocognitive Disorder – A Single Case Experimental Design
Estelle Coeur1, Xavier Corveleyn2
OPEN ACCESS
PUBLISHED: 31 December 2024
CITATION: COEUR, Estelle; CORVELEYN, Xavier. Using Music Therapy for Posttraumatic Stress Disorder with Patients suffering from Neurocognitive Disorder – A Single Case Experimental Design. Medical Research Archives, [S.l.], v. 12, n. 12, jan. 2025. Available at: <https://esmed.org/MRA/mra/article/view/6226>.
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.18103/mra.v12i12.6226
ISSN 2375-1924
ABSTRACT
Posttraumatic stress disorder and neurocognitive disorders are highly comorbid. They share similar symptoms such as brain atrophy (hippocampus, amygdala, etc.), cognitive and behavioral disorders (amnesia, executive dysfunction, depression, aggressivity, etc.). A comorbidity implies specificities regarding the symptoms (increase wandering, screaming, sleep disturbances), and there is a lack of evidence-based methods for its treatment. Music therapy is recommended for neuropsychiatric symptoms in neurocognitive disorder. We suggest that it can reduce symptoms of posttraumatic stress disorder.
A single case experimental design with AB multiple baselines was conducted with 4 participants suffering from posttraumatic stress disorder and neurocognitive disorder. Posttraumatic stress disorder, neuropsychiatric symptoms, self-esteem and well-being were assessed. Interventions consisted of 7 music therapy sessions.
The visual and statistical results highlighted an improvement in posttraumatic stress disorder symptoms, neuropsychiatric symptoms and well-being. Self-esteem results are reserved since benefits are clinically observed. Patients mentioned their traumatic event, and posttraumatic stress disorder could not be diagnosed.
Music therapy appears efficient for posttraumatic stress disorder with neurocognitive patients, and its impact should be explored in detail. This clinical study also highlighted the need to improve PTSD diagnosis for elderly individuals with neurocognitive disorders.
Introduction
Neurocognitive disorders (NCD) affect 55 million people¹. They cause brain atrophies in regions such as the temporal lobe and limbic system, leading to cognitive impairment like amnesia or executive dysfunction². Neuropsychiatric symptoms, including irritability, depressive symptoms, and agitation, are commonly observed and often result from unmet needs and because of reduced functioning³. Ageing may negatively impact self-esteem due to changes in physical health, socioeconomic status, and less efficient coping mechanisms⁴˒⁵. Additionally, NCDs affect patients’ autonomy, frequently resulting in lower self-esteem and/or well-being⁶. Non-pharmacological approaches, such as music therapy, are recommended to alleviate symptoms⁷.
Each year, 12 million Americans experience Posttraumatic Stress Disorder (PTSD)⁸. This disorder develops following a traumatic event that disrupts the limbic system’s function⁹. Symptoms include intrusive symptoms (such as recurrent dreams, flashbacks), avoidance behaviour, persistent negative alterations in cognitions and mood arousal and reactivity alterations (such as irritability and hypervigilance)¹⁰. This wide range of symptoms, particularly the negative cognition, often results in self-blaming and low self-esteem¹¹. The well-being of patients with PTSD is diminished, as these symptoms interfere with daily life and lower quality of life¹². While pharmacological treatments are available, their effects are limited¹³. Recommended psychotherapies for both children and adults include Eye Movement Desensitization and Reprocessing and Cognitive Behavior Therapy¹⁴˒¹⁵.
Given the prevalence of both pathologies, a comorbidity is highly likely. Moreover, research indicates posttraumatic stress disorder can increase the risk of developing neurocognitive disorders by up to 70%¹⁶ due to hippocampal atrophy¹⁶˒¹⁷, decreased grey matter, and a lower quality of life¹⁸. Additionally, neurocognitive disorders can elevate the risk of developing or resurfacing PTSD. Although neurocognitive disorders cause memory impairment, traumatic memories often exhibit stronger resistance to deterioration associated with NCD¹⁹˒²⁰. Cognitive impairment may lead to inhibitory dysfunction, causing trauma resurgence²¹, weaker coping mechanisms, and poor environmental understanding, making it seem threatening²². Additionally, amygdala atrophy, which is responsible for trauma resurgence, can occur²³. Finally, an Alzheimer’s disease diagnosis is often perceived as a “death sentence”, with patients described as “empty shell” or “living dead”²⁴. The fear of developing Alzheimer’s disease, losing autonomy, and experiencing neglect in nursing home is prevalent²⁵˒²⁶. Therefore, the diagnosis of an NCD can be traumatic, impacting patients’ moral and physical integrity, and potentially engendering posttraumatic stress disorder.
Given the likelihood of comorbidity between both disorders, it is crucial to provide appropriate care that considers the clinical realities and its characteristics. Symptoms of PTSD often worsen after the onset of neurocognitive disorder²⁰. Patients commonly experience increased sleep disturbances, irritability and a negative emotional state²⁷. Caregivers report significant challenges in managing individuals both disorders²⁸˒²⁹. Therefore, it is essential to provide appropriate for both patients and their caregivers.
As a treatment for posttraumatic disorder, individuals with NCD may engage in Eye Movement Desensitization Therapy and Cognitive Behavior Therapy if their cognitive abilities are relatively preserved³⁰. However, these psychotherapies require significant cognitive resources, including autobiographic memory, attention deployment, and working memory³¹–³³. This poses challenges for patients with severe NCD, limiting their access to treatment. Other non-pharmacological approaches have been studied with older adults and display positive effects on PTSD, such as active music therapy, yoga, and writing therapy³⁴–³⁶. Although their implementation with patients with neurocognitive disorder can be
complex¹⁴˒³⁵, unlike receptive music therapy. This approach consists in listening and spontaneously responding to music³⁷, and can be combined with other techniques such as imagery³⁸˒³⁹. Some authors suggest that receptive music therapy helps regulate the limbic system, particularly the amygdala and hippocampus, which may explain its effectiveness in treating anxiety-related disorders⁴⁰˒⁴¹. In PTSD, the amygdala contributes to negative mood alterations, including emotional numbness and anger, and plays a significant role in traumatic memory¹¹˒⁴². The hippocampus is responsible for the context of a memory and the cues associated with it⁴². In PTSD, the context of the traumatic memory is often inappropriate, causing neutral and unrelated stimuli to trigger symptoms such as flashbacks and re-enactments. Receptive music therapy may alleviate those symptoms by restoring normal functioning in the limbic system. This non-pharmacological approach has also demonstrated beneficial results in veterans⁴³˒⁴⁴, refugees⁴⁵, and Cognitively Behavior Therapy resistant patients⁴⁶. However, no studies have evaluated the impact of receptive music therapy alone on PTSD symptoms, with a sample of patients with neurocognitive disorder.
This research aims to investigate the impact of receptive music therapy on PTSD symptoms in a sample of patients with NCD. It is hypothesized that receptive music therapy will reduce PTSD symptoms and neuropsychiatric symptoms, while also improving self-esteem and well-being.
Method
DESIGN
A single case-experimental design with AB multiple baselines was developed to test the hypothesis⁴⁷˒⁴⁸. This pilot study was conducted in a day centre for people suffering from NCD.
The first phase (phase A) was the baseline period, during which assessments were conducted without interventions to collect control data. Participants underwent a baseline period lasting between 5 to 8 weeks, as shown in Table 1. The multiple baseline design is specific to SCED and helps reduce bias, as increases appearing after the baseline can improve the participants’ general state (e.g., reducing anxiety, improving therapeutic alliance, etc.) By randomizing the baseline starting times for each participant, the observed benefits are more likely attributable to the interventions. The length of the baseline period was randomised.
Table 1. Description of the Participants and their Results
| Sex | Age | Diag | MMSE | Traumatic event(s) | Degree | A | B | Δ Cohen NPI | Δ Glass NPI | Δ Cohen Ros | Δ Glass Ros | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | F | 74 | VaD | 17 | Stroke causing hemiplegia | SC | 5 | 7 | -0.711 | -0.933 | 0.540 | 0.738 |
| P2 | F | 75 | VaD | 12 | Death of two sons | SC | 6 | 7 | -2.780 | -3.750 | 0.278 | 0.749 |
| P3 | F | 88 | VaD | 27 | Death and funerals of husband | ND | 7 | 7 | -2.880 | -4.006 | 3.989 | 3.301 |
| P4 | M | 78 | AD | 9 | Death of twin brother, WWII | MD | 8 | 7 | -1.710 | -3.141 | -0.380 | -0.571 |
Abbreviations: P1: Participant 1; P2: Participant 2; P3: Participant 3; P4: Participant 4; MMSE: Mini Mental Examination Scale; A: Phase A (baseline); B: Phase B (interventions); NPI: Neuropsychiatric inventory; Ros: Rosenberg; F: feminine; M: masculine; VaD: Vascular dementia; AD: Alzheimer’s Disease; WWII: World War II; SC: School Certificate; ND: Nursing diploma; MD: Doctor of Medicine.
The intervention phase (phase B) consisted of weekly sessions of receptive music therapy, totalling 7 sessions⁴⁶. To improve the integrity of the study, the interventions (phase B) and the assessments (phase A and B) were conducted by different individuals, making this a single-blind trial.
Participants
Participants were orally recruited from a day care centre for older adults experiencing autonomy loss. The inclusion criteria required to have both a PTSD and a NCD diagnosis, exhibit neuropsychiatric symptoms, and appreciate classical music. Patients with hearing impairments that would prevent them from listening to music were excluded from the study. Both diagnoses had to be established by appropriate professional (e.g. psychiatrist, neurologist, general practitioner) and validated using appropriate instruments. Posttraumatic stress disorder was confirmed using DSM-5 criteria¹⁰, while the Mini Mental State Examination was employed to confirm NCD diagnosis⁴⁹. Neuropsychiatric symptoms were assessed using the Neuropsychiatric Inventory⁵⁰. Based on these criteria, four participants have been included in the study (mean age = 79.6; SD = 5.86) (see Table 1).Participant 1 was included in the study due to a stroke which left her hemiplegic; she suddenly lost her. Participant 2 experienced the sudden loss of her two sons within two years. Participant 3 experienced significant trauma after losing her husband, from whom she was the primary caregiver. The funeral, during which she had to perform corpse cleansing, was particularly difficult. Participant 4 lost his twin brother under difficult circumstances at the age of 20 and experienced a resurgence of PTSD.
CONTEXT & ETHIC
The entire experiment took place within the day care centre as part of the patients’ routine care. The protocol and participants’ rights were explained to both the patients and their primary caregiver. An information letter detailing the experiment and contact information for the investigators was provided to them. Subsequently, a consent form was administered and signed by both the patient and their primary caregiver. Also, the patients’ consent was asked before every intervention. As outlined in the information letter, this study adhered to the principles of the Declaration of Helsinki. The study was conducted as part of the patients’ standard care at the day care centre.
MEASURES
All hetero assessments were conducted by professional caregivers from the day center. Whenever feasible and appropriate, personal caregivers were also involved in the hetero assessment for participants 1, 2 and 4. Due to living alone, participant 3’s assessments were exclusively conducted with professional caregivers. We aimed to rely on hetero-evaluations exclusively to minimize the cognitive load on participants. Given their neurocognitive disorders, we anticipated that patients may find it challenging to complete multiple scales weekly. Posttraumatic Stress Disorder was assessed qualitatively using DSM-5 criteria¹⁰. This hetero-assessment tool was administered at the beginning (T0) and end of phase A (T1), and at the end of phase B (T2) as shown in Table 2.
Table 2. SPIRIT flow chart for study enrolment, interventions and assessments
| TIMEPOINT | Enrolment | Phase A | Phase B | Follow-up |
|---|---|---|---|---|
| T0 | T1 | T2 | ||
| Eligibility screen | X | |||
| Informed consent | X | |||
| INTERVENTION | X | |||
| ASSESSMENTS: | ||||
| PCL-S | X | X | X | |
| DSM-5 PTSD criteria | X | X | X |
| TIMEPOINT | Enrolment | Phase A | Phase B | Follow-up |
|---|---|---|---|---|
| T0 | T1 | T2 | ||
| NPI | X | X | X | |
| Rosenberg | X | X | X | |
| WEMWBS | X | X | X |
Abbreviations and notes:
T0: Beginning of phase A; T1: end of phase A; T2: end of phase B; T3: 1 month follow-up; T4: 3 month follow-up; T5: 6 month follow-up; PCL-S: PTSD Checklist Scale Specific; DSM-5: Diagnostic and Statistical Manual of Mental Disorder 5th edition; Posttraumatic Stress Disorder; NPI: Neuropsychiatric Inventory; WEMWBS: Warwick-Edinburgh Mental Well-being Scale.
To further support the PTSD assessment, the PTSD Checklist Scale Specific (PCL-S) was also administered⁵¹. This self-assessment instrument allows for a quantitative evaluation of the PTSD symptoms. It consists of 17 questions, with patients rating the severity of their symptoms on a Likert scale from 1 (“Not at all”) to 5 (“Extremely”), resulting in scores ranging from 5 to 85. The pathological threshold is 44; however, patients scoring above 34 should be referred for appropriate psychotherapy, while those scoring 34 or below should be informed about PTSD and monitored regularly⁵². The French version of the PCL-S demonstrates excellent internal consistency (α = 0.94)⁵¹. The PCL-S was administered at the same frequency than the DSM-5 assessments (see Table 2).The neuropsychiatric symptoms were assessed weekly throughout both phases (details in Table 2), using the Neuropsychiatric Inventory (NPI)⁵³. This instrument enables both qualitative and quantitative assessment of the neuropsychiatric symptoms, evaluating 12 different domains for presence, frequency, and severity. The total score ranges from 0 to 144, with each domain scored from 0 to 12; a score above 2 in any domain is considered pathological⁵⁴.The Rosenberg Self-Esteem Scale⁵⁵ was used to assess the patients’ self-esteem weekly during phases A and B (see Table 2). This self-assessment instrument consists of 10 items rated on a scale from 1 (“Totally disagree”) to 4 (“Totally agree”), providing a quantitative measure of self-esteem. The scale includes both positive and negative items related to self-esteem, with global scores ranging from 10 to 40. The instrument demonstrates good internal consistency (α = 0.90)⁵⁶. A score below 25 indicates very low self-esteem; scores between 25 and 31 indicate low self-esteem; scores between 31 and 34 indicate average self-esteem; and scores between 34 and 39 indicate high self-esteem.Patients’ well-being was assessed using the Warwick-Edinburgh Mental Well-being Scale (WEMWBS)⁵⁷. This scale consists of 14 items rated from 1 (“Never”) to 5 (“All the time”), providing a total score range of 14 to 70 to quantify the intensity of patients’ well-being as perceived by them. The instrument demonstrated good psychometric properties across different international samples, with an internal consistency ranging from 0.85 to 0.89⁵⁷. According to the National Health Service Scotland⁵⁸, a score of 40 or below is concerning and indicates a significant risk of depression, warranting professional intervention. Scores between 41 and 45 suggest significant psychological distress. Authors also propose a change of at least 3 points indicates a significative change. Therefore, these cut-off points were used to interpret the data. The WEMWBS assessments were conducted at T0, T1 and T2, as detailed Table 2, to facilitate the assessments for participants with cognitive impairment.Self-assessment scales (WEMWBS, Rosenberg, PCL-S) were administered to patients in presence of the facility psychologist who helped and answered any questions they had. Hetero assessments were also conducted by the facility psychologist, and professional and personal carers when feasible and relevant (P1, P2 and P4). Follow up assessments were conducted at 1 month (T3), 3 months (T4) and 6 months (T5) for all the variables.As previously described, music therapy is a non-pharmacological approach, widely used with patients with NCD to treat various neuropsychiatric symptoms. It is described as the use of musical elements to improve cognitive abilities, neuropsychiatric symptoms, or physical abilities. Receptive music therapy, also qualified as passive, consists of passively listening to structured music, specifically selected to achieve specific goals. In this study, a U sequence was used, which consists of three phases. The first is characterized by a descending sequence where beats per minute of the songs progressively decrease, inducing relaxation. The second phase features songs with a consistently low beats per minute to maintain a state of relaxation. Finally, the third phase gradually increases the beats per minute to re-energize patients while keeping them calm. During the interventions, participants were encouraged to express their feelings, memories, and perceptions related to the music. They interacted with each other and with the experimenter, making each session unique based on the participants’ feelings and comments. After all the songs were played, a debriefing was conducted to discuss how they felt during the session and their current state. Each session was planned to last one hour.The interventions (phase B) were conducted in small groups, consisting of participants and randomly selected patients, totalling 2 to 6 individuals. The random patients were chosen based on their neuropsychiatric symptoms to also benefit from the interventions. Participants listened to preselected music, which comprised non-vocal piano pieces to create an enveloping and warm continuum. Each session featured the same 33-minute playlist. The playlist used for this study is not published yet, as it is part of an ongoing study, but it can be requested to the corresponding author. The sessions were conducted by a psychologist trained in receptive music-therapy. Each session began with a debriefing about participants’ general condition and a check-up of their basic needs (thirst, hunger, bathroom needs). Following this, the session outline was discussed, including a reference to the previous session. Participants were seated comfortably during the session, and the music was then played.
DATA ANALYSIS
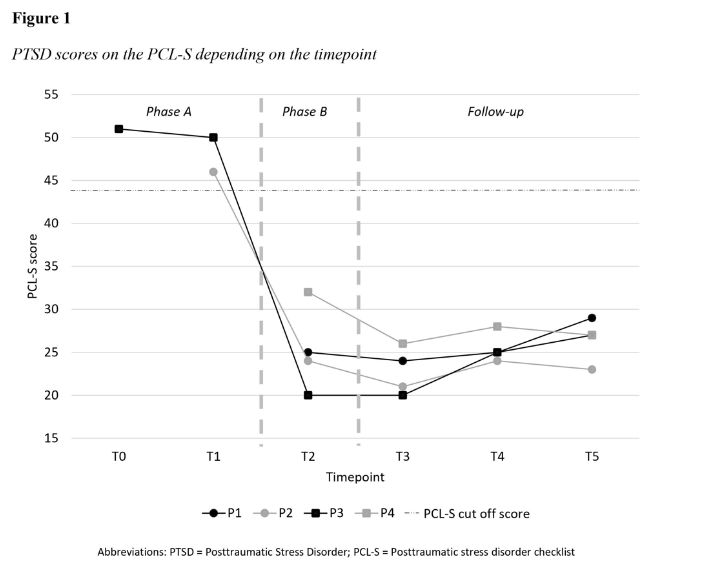
Posttraumatic stress disorder, assessed using DSM-5 criteria, were analysed by comparing the presence or absence of symptoms for each patient across the two phases. The PCL-S was also administered at the same frequency as possible, as described earlier. A cut-off score of 44 or above on the PCL-S was considered pathological. Results are depicted on participants’ graphs.
Qualitative analyses for neuropsychiatric symptoms (NPI) and self-esteem (Rosenberg scale) were conducted using the split-middle method to highlight the linear trend of each phase. As recommended by guidelines, quantitative analyses included Cohen’s delta and Glass’s delta to evaluate the general effect size, and Tau-U determine the non-overlap between phases A and B.Mental well-being, measured by the WEMWBS, was assessed at the beginning and end of phase A (T0 and T1, see Table 2), then at the end of phase B. Following NHS recommendations, we used the cut-off (score between 41 and 45 suggest psychological distress) and reported significant change to analyse data.
It is important to note that personal negative events occurred for some participants during the protocol, which adversely affected the results. These events are respectively indicated by symbols on the participants’ graphs.
Results
POSTTRAUMATIC STRESS DISORDER
At T0 and T1, all participants met full DSM-5 PTSD criteria as assessed by professional caregivers (Table 3). However, participants 1 and 4 did not complete the PCL-S assessment during phase A due to psychological distress. Participant 2 initially stated not having experienced a traumatic event, despite spontaneously mentioning it with caregivers. She completed the PCL-S at T1. For participants he completed PCL-S at T0 and T1 indicated scores above the pathological threshold (Figure 1). Following the interventions (T2), all participants no longer met the full DSM-5 PTSD criteria (Table 3). The PCL-S scores for participants 2 and 3 decreased below pathological threshold (Figure 1). While PTSD symptoms fluctuated between T2 and T5, none of the participants met the full diagnostic criteria again or scored above pathological threshold (Figure 1).
Table 3 PTSD Criteria from DSM-5 met by each Participants Depending on the Time of the Protocol.
| Participants | T0 | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|---|
| P1 | A, B, C, D, E | A, B, C, D, E | A, B, D | A | A, B, D | A |
| P2 | A, B, C, D, E | A, B, C, D, E | A, B | A, B | A, B | A, E |
| P3 | A, B, C, D, E | A, B, C, D, E | A | A, D | A | A |
| P4 | A, B, C, D, E | A, B, C, D, E | A, B | A, B, E | A, B, E | A, B, D |
Abbreviations and notes: NA: Non assessable; PTSD: Posttraumatic stress disorder; DSM-5: Diagnostic and Statistical Manual of Mental Disorders, fifth edition. Grey cells indicate patient meeting full DSM-5 criteria.
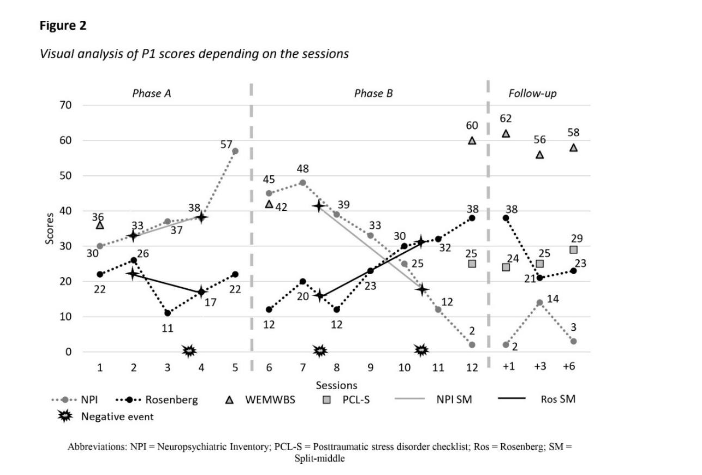
Post-intervention, participant 1 met psychological distress related to trauma stimuli (criterion B) and negative beliefs about herself and persistent negative emotional state of shame (criterion D). By T3, she reported some negative and persistent expectations about herself due to her hemiplegia.
By T4, she met the same symptoms as at T2. At T5, participant 1 exhibited negative beliefs about herself (criterion D) and sleep disturbances (criterion E). From T2 to T5, her PCL-S scores remained below the pathological threshold (24–29; Figure 2).
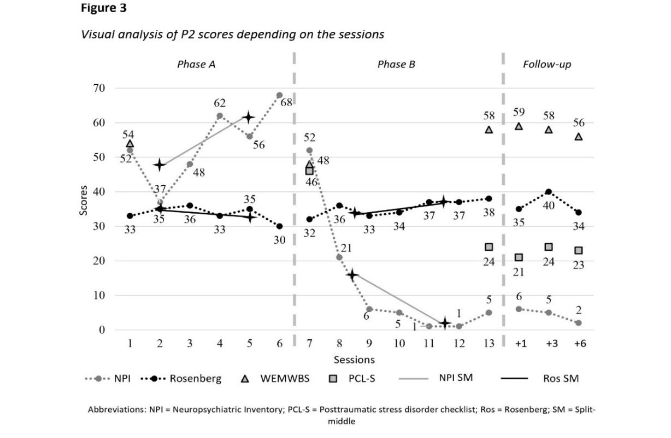
At T2, participant 2 displayed psychological distress when exposed to trauma-related stimuli (criterion B) and some persistent negative emotional state. During T3 assessment, she reported nightmares (criterion B) and some hypervigilance. At T4, the nightmares remained (criterion B), with some persistent negative mood alteration and sleep disturbances. By T5, irritable and reckless behaviours were observed (criterion E). Despite these symptoms, full PTSD criteria were not met, and PCL-S scores ranged from 21 to 24 (Figure 3).
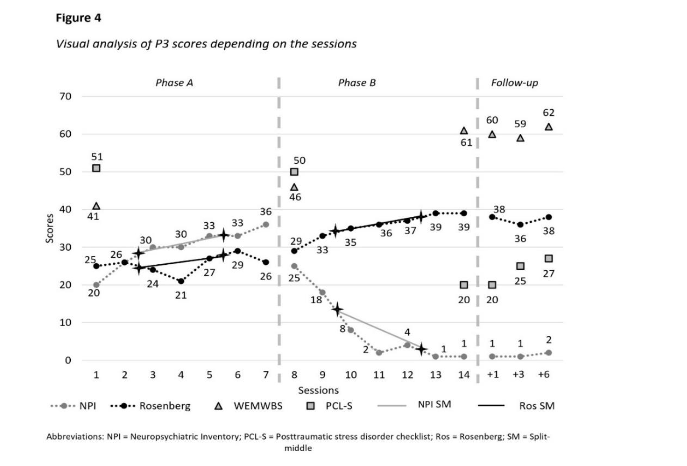
Following intervention, participant 3 exhibited signs of negative mood alteration and sleep disturbances. At T3 she met criterion D indicating negative mood alteration and cognitive, while still having sleep disturbances. By T4, only negative mood alterations persisted. At T5, caregivers noted startle reactions. Post-interventions, PTSD diagnostic criteria were not met, and the PCL-S scores of participant 3 remained non-pathological (21–27; Figure 4).
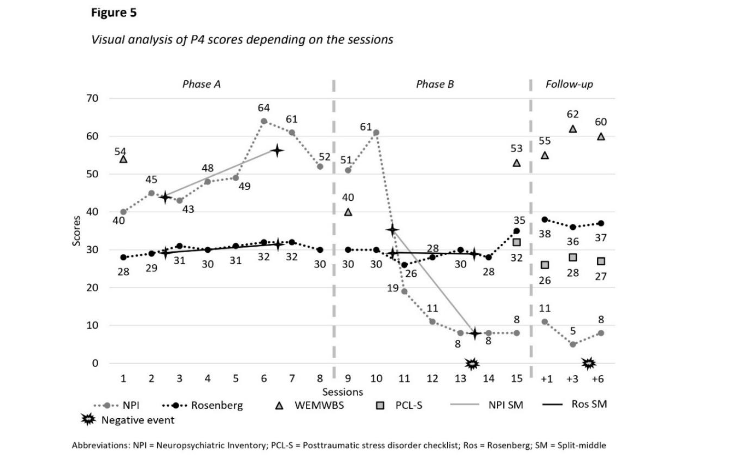
The war in Ukraine exacerbated participant 4’s symptoms during phase B (Figure 5), but post-intervention he only displayed distressing dreams and psychological distress (criterion B). By T3, he also experienced sleeping disorders and concentration disorders (criterion E). Despite ongoing PTSD symptoms, their intensity had lessened. During the PCL-S assessment, participant 4 discussed improvement in his psychological state, expressing ongoing sadness but acknowledging overall improvement.
At T4, he exhibited physiological reactions to trauma-related cues (criterion B) and difficulties concentrating and sleeping (criterion E). While he also experienced some physiological reactions, it was not met as it was neither persistent nor intense.
At T5, participant 4 continued having distressing memories (criterion B), reduced interest in activities and detachment from loved ones (criterion D), while continuing displaying sleep disturbances. Post-intervention and throughout the follow-up period, PTSD criteria were not met, and PCL-S scores remained below pathological threshold (26–32; Figure 5).
NEUROPSYCHIATRIC SYMPTOMS
Overall, receptive music therapy significantly reduced neuropsychiatric symptoms for all participants during phase B, with stable results throughout the 6-month follow-up period. The intervention effectively reversed the upward trend observed during phase A, providing a clinically meaningful benefit across various symptoms.
During phase A, participant 1 exhibited various neuropsychiatric symptoms: delusions, hallucinations, agitation and aggressivity, depressive symptoms, anxiety, and sleeping disorders (median = 37; split 1 = 33; split 2 = 38) with fluctuating severity and frequency as shown in Figure 2. Phase B scores indicated significant decrease of symptoms (median = 33; split 1 = 42; split 2 = 18.5), corroborated by results in Table 1. At T3, P1 reported depressive symptoms (score = 1) and sleeping disorders (score = 1). A peak occurred at T4, characterized by depressive symptoms (score = 12) and sleep disturbance (score = 2); the latter persisted at T5 (score = 3).
Participant 2 exhibited a wide range of neuropsychiatric symptoms during phase A, including delusions, agitation and aggressivity, depressive symptoms, anxiety, apathy, euphoria and elation, disinhibition, irritability, motor disturbances and sleeping disorders (Figure 3). Symptoms fluctuated during baseline while remaining severe (median = 54; split 1 = 48; split 2 = 62). In phase B, neuropsychiatric symptoms showed a marked decrease (median = 5; split 1 = 16.5; split 2 = 3) until she only displayed depressive symptoms (score = 4) and anxiety (score = 1). Results in Table 1 indicate significant reduction in overall neuropsychiatric symptoms. At T3, participant 2 experienced depressive symptoms and anxiety (scores = 1), along with irritability and aggressivity (scores = 2). By T4, she exhibited sleep disturbances (score = 1), anxiety and depressive symptoms (scores = 2). At T5, only anxiety and depressive symptoms remained (scores = 2).
Participant 3 exhibited depressive symptoms, anxiety, and sleeping disorders with varying severity and frequency (Figure 4). Scores increased during phase A (median = 30; split 1 = 28; split 2 = 33) and decreased in phase B (median = 4; split 1 = 13; split 2 = 2.5) until reaching a floor effect from the 4th intervention onward. These results indicated a significant impact of the interventions (details in Table 1). From T2 to T4, only depressive symptoms persisted (scores = 1). At T5, participant 3 exhibited signs of anxiety and irritability (scores = 1).
At T0, participant 4 exhibited various neuropsychiatric symptoms with a score above pathological threshold (>2): agitation and aggressivity, depressive symptoms, anxiety, euphoria and elation, apathy, motor disturbances, disinhibition, delusions, and irritability. Symptoms fluctuated during phase A (Figure 5), with an upward trend (median = 48.5; split 1 = 44; split 2 = 56.5) and a decreasing trend during phase B (median = 11; split 1 = 35; split 2 = 8) with a significant effect size (details in Table 1). However, Tau-U analysis was not significant despite being close to the threshold (Table 1). At T2, participant 4 displayed sleep disturbance (score = 2) and depressive symptoms (score = 6). At T3, similar symptoms persisted, along with elation behaviours (score = 1). By T4, he exhibited depressive symptoms (score = 2), elation disorders, disinhibition behaviours, and sleep disturbances (scores = 1). At T5, participant 4 displayed depressive symptoms (score = 4), anxiety, apathy, and sleep disturbances (scores = 1).
SELF-ESTEEM
For all participants, self-esteem scores were higher at T2 compared to T0. However, analysis did not reveal significant differences across the study (Table 1). Although participants showed small improvements, receptive music therapy did not significantly enhance participants’ self-esteem.
For participant 1, results decreased during phase A and increased in phase B (Figure 2). Her scores fluctuated between low and very low levels during phase A (median = 22; split 1 = 22; split 2 = 17), while fluctuating between very low and strong in phase B (median = 23; split 1 = 16; split 2 = 31).
Her self-esteem decreased after T2, as follow-ups scores ranged from 21 to 38.
Throughout phase A, participant 2 displayed a small reduction of her self-esteem, followed by a small increase in phase B (Figure 3). During phase A, her score fluctuated between low and strong (median = 34; split 1 = 35; split 2 = 33) and it increased to vary between average and strong during phase B (median = 36; split 1 = 33.5; split 2 = 37). All follow-ups’ scores indicated strong self-esteem (range 34–40).
Participant 3 displayed a small improvement of her self-esteem before the interventions, which intensified during the interventions (Figure 4). During phase A, scores indicated low self-esteem category (median = 26; split 1 = 24.5; split 2 = 26.5). It increased in phase B, until reaching the high self-esteem category (median = 36; split 1 = 34; split 2 = 38) and indicating significant improvement, as shown in Table 1. Results during the follow-ups remained stable (range 36–38).
Participant 4 exhibited decreasing self-esteem in phase A, followed by increasing scores in phase B (Figure 5). During phase A, scores oscillated between low and average (median = 30.5; split 1 = 29.5; split 2 = 31.5), while in phase B they varied between average and strong (median = 30; split 1 = 29; split 2 = 29). Results remained stable during follow-ups (range 36–38).
WELL-BEING
Well-being of both participants 1 and 3 followed the same pattern, characterized by a small increase between T0 and T1, and a significant improvement between T1 and T2. Participant 1 exhibited significant psychological distress at T0 (Figure 2), with her score rising slightly at T1 but still indicated distress. During phase B, her score increased further, reaching a non-concerning score at T2. These results were stable through all follow-ups. Similarly, participant 3 showed significant psychological distress at T0 (Figure 4), with a slight increase during phase A reaching a non-concerning score. Her score improved further during phase B and remained stable throughout follow-ups.
For participants 2 and 4, their well-being was initially satisfying at T0 (Figures 3 and 5), but declined during phase A, indicating psychological distress for participant 2, while remaining satisfying for participant 4. During phase B, their well-being significantly increased, reaching similar scores to those at T0. Scores remained stable for participant 2, and participant 4 displayed improvement in his well-being through follow-ups.
Discussion
The aim of this study was to investigate the effects of receptive music therapy on PTSD symptoms in patients with NCD and exhibiting neuropsychiatric symptoms. An AB single case experimental design with multiple baselines was implemented with four patients with PTSD and NCD (Table 1). Results showed a significant reduction in PTSD symptoms following the intervention, with nearly all symptoms disappearing (Table 3), and DSM-5 criteria for PTSD were no longer met. These effects remained stable for up to 6 months post-intervention.
Regarding neuropsychiatric symptoms (Table 1), participants showed significant improvement, consistent with findings from previous studies. Notably, the most substantial reduction occurred after the second session of music therapy. Clinically, patients’ self-esteem improved by T2, as indicated by clinical observations and scale cut-off scores. However, statistical analysis did not reveal a significant benefit of the interventions on self-esteem.
For participants 1 and 3, well-being increased during phase A and then improved further during phase B. For participants 2 and 4, who had the most severe cognitive impairment and pronounced neuropsychiatric symptoms (Table 1), they initially experienced a decline in well-being due to the severity of their cognitive impairment and neuropsychiatric symptoms. They often result from unmet needs and directly affect quality of life and exacerbate NCD. Thus, severe cognitive impairment and neuropsychiatric symptoms had a negative impact on patients’ well-being.
In conclusion, receptive music therapy interventions had benefits on
participants’ PTSD, neuropsychiatric symptoms, and overall well-being. Additionally, the protocol appeared to improve patients’ self-esteem even if it is not corroborated by analysis (Table 1).
Although all patients exhibited stronger self-esteem after the interventions, the analysis did not reveal a statistically significant difference, likely because self-esteem increased during the baseline period, potentially due to the clinician’s interventions. In prioritizing patient well-being and ethical considerations, the clinician employed clinical techniques such as reassurance and validation to prevent psychological distress.
The impact of music therapy on various neuropsychiatric symptoms has been well-documented in numerous studies. Therefore, results concerning neuropsychiatric symptoms are in line with existing literature and confirm the relevance of our protocol. The innovative aspect lies in the PTSD outcomes. The reduction of the symptoms (Table 3) to the point where they could no longer be confirmed by assessment tools may be attributed to music’s influence on the limbic system. The limbic system, including the amygdala, hippocampus and prefrontal cortex, is known to be dysfunctional in PTSD. These dysfunctions prevent the anterior cingulate cortex from inhibiting the amygdala, thus triggering a fear response. Some authors have proposed that music could be used to treat anxiety-related disorders, such as PTSD, by helping regulate the amygdala and hippocampus. This hypothesis is supported by our findings, which suggest that the regulation of the limbic system reduced fear responses and subsequently decreased PTSD symptoms. Investigating brain activity during receptive music therapy sessions could provide further insights into the underlying mechanisms.
An identified limitation of this study is the limited number of PTSD assessments throughout the protocol. Initially, the PCL-S was not administered weekly because frequent repetition of the questions could have increased participants’ psychological distress. To minimize psychological distress for patients, we chose to limit the use of PTSD scales, which can be distressful for patients. In our effort to prevent any risk to participants, it was not always possible to complete the PCL-S. Some patients denied having experienced traumatic events (participants 1 and 2), and for one patient (participant 4), recalling the traumatic event was too painful, leading to interrupted assessments at T0 and T1.
These challenges with self-assessment tools during T0 and T1 led us to explore potential reasons for patients’ difficulties in completing the PCL-S. We hypothesized that this may be due to behavioural avoidance, possibly stemming from an avoidant coping strategy. This strategy, although used to manage psychological distress, is correlated with increased PTSD symptoms. A component of this strategy is a reduced ability to retrieve autobiographical memories, which can make recalling specific memories difficult. This may explain why our participants were not always able to spontaneously mention their traumatic events, even when completing the PCL-S.
This limitation underscores the lack of appropriate instruments for screening PTSD in older adults with neurocognitive disorder. Moreover, literature highlights specific challenges related to comorbidity between posttraumatic stress disorder and NCD, noting that some items are relevant for adults but not for older adults. For instance, older adults are more likely to exhibit agitation, wandering, screaming and sleep disorders, while displaying less avoidance behaviors. This reduced avoidance may be attributed to their limited ability to avoid external trauma-related stimuli.
Finally, the self-assessments of PTSD were conducted with participants based on their most distressing event, aligning with the DSM-5 criterion A. As is common with older adults, participants have experienced several stressful events throughout their lives, some of which also met criterion A. Research suggests that individuals exposed to several stressful events, including one traumatic event, may develop PTSD following a stressful event that does not meet criterion A. These findings are particularly important for older adults, as their higher lifetime exposure to stressful
Events significantly increase their risk for PTSD, since cumulative exposure is a major factor risk for PTSD. Therefore, this population may require a specialized PTSD instrument that accommodates their unique comorbidity characteristics. Future research should focus on the development of screening tools specifically designed to assess PTSD in this population. This tool will prevent PTSD misdiagnosis in populations with neurocognitive disorder, as mistakes in identifying PTSD could have significant impact in both research and clinical practice.
In addition to the accumulation of stressful events, several other factors contribute to the increased PTSD risk among older adults. These include the lack of social support following a traumatic event, history of medical condition, low perceived control after trauma, and low resilience. Since these characteristics are commonly observed in older adults, research should focus on strategies or therapeutic approaches to enhance resilience, both in older populations and at earlier stages of life, to help prevent PTSD and other mental health issues across the lifespan.
As a pilot study, this research aimed to explore the feasibility and relevance of receptive music therapy for individuals with NCD. While the results are promising in addressing posttraumatic stress disorder, their generalizability is limited due to the small sample size. Further research is required to replicate these findings using a randomized controlled trial to expand understanding of this non-pharmacological approach. Moreover, future studies should investigate the neurobiological mechanisms involved by evaluating the limbic system activity before and after music therapy interventions. Exploring the effects of personalized music selection across various musical genres is also recommended.
Conclusion
Receptive music therapy using a U-protocol showed a significant reduction of PTSD symptoms in patients with NCD, with effects lasting up to six months post-intervention. Neuropsychiatric symptoms also improved markedly, and the interventions had a positive impact on patients’ overall well-being and, to a lesser extent, on self-esteem. These results suggest that receptive music therapy is a promising intervention to enhance quality of life in this vulnerable population by reducing PTSD symptoms. Finally, the study underscores the need for continued exploration into tailored interventions and assessment for patients with different levels of cognitive impairment and neuropsychiatric symptoms.
Conflict of Interest:
The authors have no conflicts of interest to declare.
Funding Statement:
This work was supported by the Jean-Louis Noisiez Foundation, in the context of a thesis research. Additional support came from the University Côte d’Azur and the Innovation Alzheimer Association.
Acknowledgements:
We thank our partner, Jean-Louis Noisiez Foundation, who allowed us to recruit participants. We thank all the patients, the participants’ families and the professional caregivers, who all participated in this study.
ORCID ID:
Estelle Coeur — 0000-0001-8186-3921
Xavier Corveleyn — 0000-0001-5181-0597
Author Note:
This study was not preregistered. Data and study materials are available by asking the corresponding authors.
Author Contributions:
Conceptualization: E.C. and X.C.;
Study Design: E.C. and X.C.;
Data Collection: E.C.;
Data Analysis: E.C. and X.C.;
Interpretation of Results: E.C. and X.C.;
Manuscript Drafting: E.C.;
Manuscript Review and Editing: X.C. and E.C.;
Supervision: X.C.
References
1. Alzheimer’s Disease International. World Alzheimer Report 2021 – Journey through the Diagnosis of Dementia. McGill University; 2021. https://www.alzint.org/u/World-Alzheimer-Report-2021.pdf
2. Alzheimer’s Society. Dementia and Brain.; 2019. https://www.alzheimers.org.uk/sites/default/files/2019-05/456lp-dementia-and-the-brain-190521.pdf
3. Cohen-Mansfield J, Dakheel-Ali M, Marx MS, Thein K, Regier NG. Which unmet needs contribute to behavior problems in persons with advanced dementia? Psychiatry Res. 2015;228(1): 59-64. doi:10.1016/j.psychres.2015.03.043
4. Orth U, Trzesniewski KH, Robins RW. Self-esteem development from young adulthood to old age: A cohort-sequential longitudinal study. J Pers Soc Psychol. 2010;98(4):645-658. doi:10.1037/a0018769
5. Orth U, Robins RW. The Development of Self-Esteem. Curr Dir Psychol Sci. 2014;23(5):381-387. doi:10.1177/0963721414547414
6. Kloos N, Trompetter HR, Bohlmeijer ET, Westerhof GJ. Longitudinal Associations of Autonomy, Relatedness, and Competence With the Well-being of Nursing Home Residents. The Gerontologist. 2019;59(4):635-643. doi:10.1093/geront/gny005
7. Moreno-Morales C, Calero R, Moreno-Morales P, Pintado C. Music Therapy in the Treatment of Dementia: A Systematic Review and Meta-Analysis. Front Med. 2020;7:160. doi:10.3389/fmed.2020.00160
8. U.S. Department of Veterans Affairs. How Common is PTSD in Adults? – PTSD: National Center for PTSD. August 4, 2022. Accessed July 18, 2022. https://www.ptsd.va.gov/understand/common/common_adults.asp
9. Karl A, Schaefer M, Malta L, Dorfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006; 30(7):1004-1031. doi:10.1016/j.neubiorev.2006.03.004
10. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association; 2013.
11. Yehuda R, Hoge CW, McFarlane AC, et al. Post-traumatic stress disorder. Nat Rev Dis Primer. 2015;1(1):15057. doi:10.1038/nrdp.2015.57
12. Harnett PH, Kelly MC, Gullo MJ. The impact of posttraumatic stress disorder on the psychological distress, positivity, and well-being of Australian police officers. Psychol Trauma Theory Res Pract Policy. 2023;15(2):340-348. doi:10.1037/tra0001136
13. Institut National de la Santé et de la Recherche Médicale (INSERM). Troubles Du Stress Post-Traumatique : Quand Un Souvenir Stressant Altère Les Mécanismes de Mémorisation.; 2020. https://www.inserm.fr/dossier/troubles-stress-post-traumatique/
14. Bisson JI, van Gelderen M, Roberts NP, Lewis C. Non-pharmacological and non-psychological approaches to the treatment of PTSD: results of a systematic review and meta-analyses. Eur J Psychotraumatology. 2020;11(1):1795361. doi:10. 1080/20008198.2020.1795361
15. Martin A, Naunton M, Kosari S, Peterson G, Thomas J, Christenson JK. Treatment Guidelines for PTSD: A Systematic Review. J Clin Med. 2021;10(18):4175. doi:10.3390/jcm10184175
16. Flatt JD, Gilsanz P, Quesenberry CP, Albers KB, Whitmer RA. Post‐traumatic stress disorder and risk of dementia among members of a health care delivery system. Alzheimers Dement. 2018;14(1): 28-34. doi:10.1016/j.jalz.2017.04.014
17. Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic Stress Disorder and Risk of Dementia Among US Veterans. Arch Gen Psychiatry. 2010;67 (6):608. doi:10.1001/archgenpsychiatry.2010.61
18. Gradus JL, Horváth-Puhó E, Lash TL, et al. Stress Disorders and Dementia in the Danish Population. Am J Epidemiol. 2019;188(3):493-499. doi:10.1093/aje/kwy269
19. Mittal D, Torres R, Abashidze A, Jimerson N. Worsening of Post-Traumatic Stress Disorder Symptoms with Cognitive Decline: Case Series. J Geriatr Psychiatry Neurol. 2001;14(1):17-20. doi: 10.1177/089198870101400105
20. Van Achterberg ME, Rohrbaugh RM, Southwick SM. Emergence of PTSD in Trauma Survivors With Dementia. J Clin Psychiatry. 2001;62(3):206-207. doi:10.4088/JCP.v62n0312c
21. Grossman AB, Levin BE, Katzen HL, Lechner S. PTSD Symptoms and Onset of Neurologic Disease in Elderly Trauma Survivors. J Clin Exp Neuropsychol. 2004;26(5):698-705. doi:10.1080/1 3803390409609793
22. Zeisel J, Bennett K, Fleming R. World Alzheimer Report 2020: Design, dignity, dementia: Dementia-related design and the built environment. Published online September 21, 2020. Accessed December 11, 2022. https://www.alzint.org/resource/world-alzheimer-report-2020/
23. Bruneau M, Desmarais P, Pokrzywko K. Post‐traumatic stress disorder mistaken for behavioural and psychological symptoms of dementia: case series and recommendations of care. Psychogeriatrics. 2020;20(5):754-759. doi:10.1111/psyg.12549
24. Rosin ER, Blasco D, Pilozzi AR, Yang LH, Huang X. A Narrative Review of Alzheimer’s Disease Stigma. J Alzheimers Dis. 2020;78(2):515-528. doi: 10.3233/JAD-200932
25. Cantegreil-Kallen I, Pin S. Fear of Alzheimer’s disease in the French population: impact of age and proximity to the disease. Int Psychogeriatr. 2012;24(1):108-116. doi:10.1017/S1041610211001529
26. Kirkman AM. Dementia in the news: the media coverage of Alzheimer’s disease. Australas J Ageing. 2006;25(2):74-79. doi:10.1111/j.1741-66 12.2006.00153.x
27. Van Dongen DHE, Havermans D, Deckers K, Olff M, Verhey F, Sobczak S. A first insight into the clinical manifestation of posttraumatic stress disorder in dementia: a systematic literature review. Psychogeriatrics. 2022;22(4):509-520. doi: 10.1111/psyg.12830
28. Craftman ÅG, Swall A, Båkman K, Grundberg Å, Hagelin CL. Caring for older people with dementia reliving past trauma. Nurs Ethics. 2020; 27(2):621-633. doi:10.1177/0969733019864152
29. Pinciotti CM, Bass DM, McCarthy CA, et al. Negative Consequences of Family Caregiving for Veterans With PTSD and Dementia. J Nerv Ment Dis. 2017; 205(2):106-111. doi:10.1097/NMD.0000 000000000560
30. Tay K, Subramaniam P, Oei TP. Cognitive behavioural therapy can be effective in treating anxiety and depression in persons with dementia: a systematic review. Psychogeriatrics. 2019;19(3) :264-275. doi:10.1111/psyg.12391
31. Cottraux J, Dattilio F, eds. Thérapie cognitive et émotions: la troisième vague. Elservier Masson; 2007.
32. Haour F, Dobbelaere E, de Beaurepaire C. Scientific Evaluation of EMDR Psychotherapy for the Treatment of Psychological Trauma Summary: Scientific evaluation of EMDR psychotherapy. J Neurol Neuromedicine. 2019;4(2):5-14. doi:10.292 45/2572.942X/2019/2.1234
33. Hofmann SG, Asmundson GJG, Beck AT. The Science of Cognitive Therapy. Behav Ther. 2013; 44(2):199-212. doi:10.1016/j.beth.2009.01.007
34. Ma YM, Yuan MD, Zhong BL. Efficacy and acceptability of music therapy for post-traumatic stress disorder: a systematic review and meta-analysis of randomized controlled trials. Eur J Psychotraumatology. 2024;15(1):2342739. doi:10. 1080/20008066.2024.2342739
35. Nascimento JCP, Santos KVG dos, Dantas JK dos S, Dantas DV, Dantas RAN. Non-pharmacological therapies for the treatment of post-traumatic stress disorder among emergency responders: a scoping review. Rev Esc Enferm USP. 2021;55:e03724. doi:10.1590/s1980-220×2020011603724
36. Wang CC, Emrich M, Rives H, et al. Music interventions for posttraumatic stress disorder: A systematic review. J Mood Anxiety Disord. 2024;6: 100053. doi:10.1016/j.xjmad.2024.100053
37. De Witte M, Pinho ADS, Stams GJ, Moonen X, Bos AER, Van Hooren S. Music therapy for stress reduction: a systematic review and meta-analysis. Health Psychol Rev. 2022;16(1):134-159. doi:10.10 80/17437199.2020.1846580
38. Tang Q, Huang Z, Zhou H, Ye P. Effects of music therapy on depression: A meta-analysis of randomized controlled trials. Torun S, ed. PLOS ONE. 2020;15(11):e0240862. doi:10.1371/journal. pone.0240862
39. Wigram T, Pedersen IN, Bonde LO. A Comprehensive Guide to Music Therapy: Theory, Clinical Practice, Research and Training. Jessica Kingsley; 2002.
40. Dalla Bella S, McGill University, Fondazione Pierfranco e Luisa Mariani, eds. The Neurosciences and Music III: Disorders and Plasticity, Volume 1169. Blackwell; 2009.
41. Koelsch S. Brain correlates of music-evoked emotions. Nat Rev Neurosci. 2014;15(3):170-180. doi:10.1038/nrn3666
42. Lambert HK, McLaughlin KA. Impaired hippocampus-dependent associative learning as a mechanism underlying PTSD: A meta-analysis. Neurosci Biobehav Rev. 2019;107:729-749. doi:10. 1016/j.neubiorev.2019.09.024
43. Bensimon M, Amir D, Wolf Y. Drumming through trauma: Music therapy with post-traumatic soldiers. Arts Psychother. 2008;35(1):34-48. doi:10. 1016/j.aip.2007.09.002
44. Story KM, Beck BD. Guided Imagery and Music with female military veterans: An intervention development study. Arts Psychother. 2017;55:93-102. doi:10.1016/j.aip.2017.05.003
45. Beck BD, Lund ST, Søgaard U, et al. Music therapy versus treatment as usual for refugees diagnosed with posttraumatic stress disorder (PTSD): study protocol for a randomized controlled trial. Trials. 2018;19(1):301. doi:10.1186/s13063-018-2662-z
46. Carr C, d’Ardenne P, Sloboda A, Scott C, Wang D, Priebe S. Group music therapy for patients with persistent post-traumatic stress disorder – an exploratory randomized controlled trial with mixed methods evaluation: Group music therapy for post-traumatic stress disorder. Psychol Psychother Theory Res Pract. 2012;85(2):179-202. doi:10.1111/j.2044-8341.2011.02026.x
47. Gana K, Gallé-Tessonneau M, Broc G. Le protocole individuel en psychologie : tutoriel à l’usage des psychologues praticiens. Prat Psychol. 2019;25(2): 153-167. doi:10.1016/j.prps.2018.11.002
48. Tate RL, Perdices M, Rosenkoetter U, et al. The Single-Case Reporting Guideline In BEhavioural Interventions (SCRIBE) 2016: Explanation and elaboration. Arch Sci Psychol. 2016;4(1):10-31. doi: 10.1037/arc0000027
49. Kalafat M, Hugonot-Diener L, Poitrenaud J. The Mini Mental State (MMS): French standardization and normative data [Standardisation et étalonnage français du “Mini Mental State” (MMS) version GRÉCO]. Rev Neuropsychol. 2003;13(2):209-236.
50. Cummings J. The Neuropsychiatric Inventory: Development and Applications. J Geriatr Psychiatry Neurol. 2020;33(2):73-84. doi:10.1177/089198871 9882102
51. Ashbaugh AR, Houle-Johnson S, Herbert C, El-Hage W, Brunet A. Psychometric Validation of the English and French Versions of the Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5). Mazza M, ed. PLOS ONE. 2016;11(10):e0161645. doi:10. 1371/journal.pone.0161645
52. Paul F, Marimoutou C, Pommier de Santi V, Clervoy P. Post-Traumatic Stress Disorder among French Armed Forces Members in Afghanistan: A New Approach. In: Tatu L, Bogousslavsky J, eds. Frontiers of Neurology and Neuroscience. Vol 38. S. Karger AG; 2016:228-236. doi:10.1159/000443221
53. de Medeiros K, Robert P, Gauthier S, et al. The Neuropsychiatric Inventory-Clinician rating scale (NPI-C): reliability and validity of a revised assessment of neuropsychiatric symptoms in dementia. Int Psychogeriatr. 2010;22(6):984-994. doi:10.1017/S1041610210000876
54. Sisco F, Taurel M, Lafont V, et al. Les troubles du comportement chez le sujet dément en institution : évaluation à partir de l’inventaire neuropsychiatrique pour les équipes soignantes. In: L’année Gérontologique. Vol 14. Serdi; 2000:151-173.
55. Tinakon W, Nahathai W. A Comparison of Reliability and Construct Validity between the Original and Revised Versions of the Rosenberg Self-Esteem Scale. Psychiatry Investig. 2012;9(1): 54. doi:10.4306/pi.2012.9.1.54
56. Vallieres EF, Vallerand RJ. Traduction et Validation Canadienne-Française de L’échelle de L’estime de Soi de Rosenberg. Int J Psychol. 1990 ;25(2):305-316. doi:10.1080/00207599008247865
57. Trousselard M, Steiler D, Dutheil F, et al. Validation of the Warwick-Edinburgh Mental Well-Being Scale (WEMWBS) in French psychiatric and general populations. Psychiatry Res. 2016;245:282-290. doi:10.1016/j.psychres.2016.08.050
58. NHS Health Scotland, Taggart F, Stewart-Brown S, Parkinson J. Warwick-Edinburgh Mental Well-Being Scale (WEMWBS) User Guide – Version 2.; 2015. http://helpscout.net.s3.amazonaws.com/docs/assets/5f97128852faff0016af3a34/attachments/5fe10a9eb624c71b7985b8f3/WEMWBS-Scale.pdf
59. Lin TH, Liao YC, Tam KW, Chan L, Hsu TH. Effects of music therapy on cognition, quality of life, and neuropsychiatric symptoms of patients with dementia: A systematic review and meta-analysis of randomized controlled trials. Psychiatry Res. 2023;329:115498. doi:10.1016/j.psychres.20 23.115498
60. Guétin S, Portet F, Picot MC, et al. Effect of Music Therapy on Anxiety and Depression in Patients with Alzheimer’q Type Dementia: Randomised, Controlled Study. Dement Geriatr Cogn Disord. 2009;28(1):36-46. doi:10.1159/000229024
61. Guétin S, Giniès P. EP40 – Une technique de musicothérapie : la méthode en U. Douleurs Eval – Diagn-Trait. 2004;5:49. doi:10.1016/S1624-5687 (04)94635-5
62. Vink AC, Bruinsma MS, Scholten RJ. Music therapy for people with dementia. In: The Cochrane Collaboration, ed. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd; 2003:CD003477 .pub2. doi:10.1002/14651858.CD003477.pub2
63. Palazzolo J, Baudu C, Quaderi A. Psychothérapies du sujet âgé : prise en charge des pathologies du vieillissement.; 2016.
64. Krasny-Pacini A, Evans J. Single-case experimental designs to assess intervention effectiveness in rehabilitation: A practical guide. Ann Phys Rehabil Med. 2018;61(3):164-179. doi: 10.1016/j.rehab.2017.12.002
65. Lane JD, Gast DL. Visual analysis in single case experimental design studies: Brief review and guidelines. Neuropsychol Rehabil. 2014;24(3-4): 445-463. doi:10.1080/09602011.2013.815636
66. Lee JB, Cherney LR. Tau-U: A Quantitative Approach for Analysis of Single-Case Experimental Data in Aphasia. Am J Speech Lang Pathol. 2018; 27(1S):495-503. doi:10.1044/2017_AJSLP-16-0197
67. Parker RI, Vannest KJ, Davis JL, Sauber SB. Combining Nonoverlap and Trend for Single-Case Research: Tau-U. Behav Ther. 2011;42(2):284-299. doi:10.1016/j.beth.2010.08.006
68. Aalbers S, Fusar-Poli L, Freeman RE, et al. Music therapy for depression. Cochrane Common Mental Disorders Group, ed. Cochrane Database Syst Rev. 2017;2017(11). doi:10.1002/14651858. CD004517.pub3
69. Haute Autorité de Santé. Maladie d’Alzheimer et maladies apparentées : prise en charge des troubles du comportement perturbateurs. May 27, 2009. Accessed November 22, 2021. https://www.has-sante.fr/jcms/c_819667/fr/maladie-d-alzheimer-et-maladies-apparentees-prise-en-charge-des-troubles-du-comportement-perturbateurs
70. Dyer SM, Harrison SL, Laver K, Whitehead C, Crotty M. An overview of systematic reviews of pharmacological and non-pharmacological interventions for the treatment of behavioral and psychological symptoms of dementia. Int Psychogeriatr. 2018;30(3):295-309. doi:10.1017/S1 041610217002344
71. Martyr A, Nelis SM, Quinn C, et al. Living well with dementia: a systematic review and correlational meta-analysis of factors associated with quality of life, well-being and life satisfaction in people with dementia. Psychol Med. 2018;48 (13):2130-2139. doi:10.1017/S0033291718000405
72. Akyirem S, Salifu Y, Bayuo J, Duodu PA, Bossman IF, Abboah‐Offei M. An integrative review of the use of the concept of reassurance in clinical practice. Nurs Open. 2022;9(3):1515-1535. doi:10. 1002/nop2.1102
73. Etkin A, Wager TD. Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. Am J Psychiatry. 2007;164(10): 1476-1488. doi:10.1176/appi.ajp.2007.07030504
74. Shin LM. Amygdala, Medial Prefrontal Cortex, and Hippocampal Function in PTSD. Ann N Y Acad Sci. 2006;1071(1):67-79. doi:10.1196/annals.1364.007
75. Havermans DCD, van Alphen SPJ, Olff M, et al. The Need for a Diagnostic Instrument to Assess Post-Traumatic Stress Disorder in People with Dementia: Findings from a Delphi Study. J Geriatr Psychiatry Neurol. Published online June 17, 2022: 089198872211035. doi:10.1177/08919887221103583
76. Thompson NJ, Fiorillo D, Rothbaum BO, Ressler KJ, Michopoulos V. Coping strategies as mediators in relation to resilience and posttraumatic stress disorder. J Affect Disord. 2018;225:153-159. doi:10.1016/j.jad.2017.08.049
77. Pineles SL, Mostoufi SM, Ready CB, Street AE, Griffin MG, Resick PA. Trauma reactivity, avoidant coping, and PTSD symptoms: A moderating relationship? J Abnorm Psychol. 2011;120(1):240-246. doi:10.1037/a0022123
78. Hermans D, Defranc A, Raes F, Williams JMG, Eelen P. Reduced autobiographical memory specificity as an avoidant coping style. Br J Clin Psychol. 2005;44(4):583-589. doi:10.1348/014466 505X53461
79. Bardeen JR, Benfer N. Methodological considerations for assessing trauma history via self-report. Psychol Trauma Theory Res Pract Policy. 2019; 11(5):505-512. doi:10.1037/tra0000398
80. Ogle CM, Rubin DC, Siegler IC. Cumulative exposure to traumatic events in older adults. Aging Ment Health. 2014;18(3):316-325. doi:10.1080/136 07863.2013.832730
81. Tortella-Feliu M, Fullana MA, Pérez-Vigil A, et al. Risk factors for posttraumatic stress disorder: An umbrella review of systematic reviews and meta-analyses. Neurosci Biobehav Rev. 2019;107:154-165. doi:10.1016/j.neubiorev.2019.09.013
82. Vogt D, Smith B, Elwy R, et al. Predeployment, deployment, and postdeployment risk factors for posttraumatic stress symptomatology in female and male OEF/OIF veterans. J Abnorm Psychol. 2011;120(4):819-831. doi:10.1037/a0024457
83. Kushner MG, Riggs DS, Foa EB, Miller SM. Perceived controllability and the development of posttraumatic stress disorder (PTSD) in crime victims. Behav Res Ther. 1993;31(1):105-110. doi: 10.1016/0005-7967(93)90048-Y
84. Frazier P, Steward J, Mortensen H. Perceived Control and Adjustment to Trauma: A Comparison Across Events. J Soc Clin Psychol. 2004;23(3):303-324. doi:10.1521/jscp.23.3.303.35452
85. Norcross JC, Lambert MJ. Evidence-Based Psychotherapy Relationship: The Third Task Force. In: Psychotherapy Relationships That Work. Oxford University Press; 2019:1-23. doi:10.1093/med-psych/9780190843953.003.0001


 http://orcid.org/0000-0001-8186-3921
http://orcid.org/0000-0001-8186-3921