Ventilation/Perfusion SPECT: Advancing Pulmonary Diagnostics
Implementation of Ventilation/Perfusion SPECT in Diagnostics beyond Pulmonary Embolism
Marika Bajc, MD, PhD1* and Ari Lindqvist, MD, PhD2
- Marika Bajc, MD, PhD Skåne University Hospital, Department of Clinical Sciences, University Hospital Lund, Lund, Sweden.
- Ari Lindqvist, MD, PhD Research Unit of Pulmonary Diseases, Clinical Research Institute, HUS Helsinki University Hospital and Helsinki University, Helsinki, Finland.
OPEN ACCESS
PUBLISHED: 31 March 2025
CITATION: BAJC, Marika; LINDQVIST, Ari. Implementation of Ventilation/Perfusion SPECT in Diagnostics beyond Pulmonary Embolism. Medical Research Archives, [S.l.], v. 13, n. 3, mar. 2025. Available at: <https://esmed.org/MRA/mra/article/view/6273>.
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.18103/mra.v13i3.6273
ISSN 2375-1924
Abstract
Scintigraphic pulmonary studies are designed to demonstrate patterns of ventilation and perfusion. It is crucial to make an image of both ventilation and perfusion for a proper diagnostic interpretation of any disorder. In pulmonary embolism, there are areas with absent perfusion and preserved ventilation. Pulmonary embolism is diagnosed when there is more than one sub-segment showing a ventilation/perfusion mismatch, representing an anatomic lung unit. Ventilation/perfusion single-photon emission computed tomography (V/P SPECT) is recommended as the first-choice imaging technique for the diagnosis of acute pulmonary embolism and is the golden standard for the diagnosis of chronic pulmonary embolism. Antigravitational perfusion distribution from posterior to anterior region indicates pulmonary congestion (heart failure). As ventilation is usually less affected, the typical pattern is a non-segmental ventilation/perfusion mismatch in dorsal regions of the lung. Some other diseases functional defect. Acute pneumonia causes frequently reverse mismatches. Later in the progress of the pneumonia matched defects may be seen. Using Technegas for the ventilation study over radiolabeled liquid aerosols enable grading of airway obstruction in chronic obstructive pulmonary disease. Areas of absent/decreased ventilation with usually less pronounced perfusion defects (reverse mismatch) are typical for the damage of lung parenchyma (emphysema). Minimizing radiation exposure without sacrificing image quality and diagnostic accuracy are essentials for an imaging test that follows good clinical practice. An optimal combination of radiopharmaceuticals, nuclide activities and acquisition times for ventilation and perfusion, collimators, and imaging matrix yield an adequate ventilation perfusion SPECT study in approximately 20 minutes of imaging time. Ventilation/perfusion SPECT is using a holistic interpretation strategy based on all relevant information about the patient and all ventilation/perfusion patterns. The method allows quantification of lung function and measurement of the extent of functional deteriorations that have impact on the diagnostics and treatment. Following the recent European Association of Nuclear Medicine guideline of ventilation/perfusion SPECT allows diagnostics of comorbidities such as obstructive lung disease, pulmonary congestion, and pneumonia separately or simultaneously with pulmonary embolism.
Keywords
COPD; Functional imaging; Pneumonia; Pulmonary embolism; V/P SPECT
Abbreviations
- EANM – European Association of Nuclear Medicine
- COPD – Chronic obstructive pulmonary disease
- CTPA – Computed tomography pulmonary angiography
- MAA – Human albumin macroaggregates
- mGy – milligray, a measure of absorbed radiation dose
- PE – Pulmonary embolism
- PIOPED – Prospective investigation of pulmonary embolism diagnosis
- MSv – sievert, used for radiation dose quantities
- V/P SPECT – Ventilation/perfusion single-photon emission computed tomography
Introduction
Previously, studies of pulmonary ventilation and perfusion were commonly based on planar imaging that was the primary noninvasive method for diagnosis of pulmonary embolism (PE). However, the technique suffered disrepute since the prospective investigation of pulmonary embolism diagnosis (PIOPED) I study showed that 65% of scans were nondiagnostic. Results from later studies based upon modern imaging techniques and new holistic principles (combining clinical information, pre-test probability, results of chest radiograph, and pattern typical of PE or other diseases) show undeniable advantage of ventilation/perfusion tomography (V/P SPECT) over the planar technique and computed tomography pulmonary angiography (CTPA). V/P SPECT reduces the number of nondiagnostic findings to 4% or less, while sensitivity and specificity are excellent. V/P SPECT was endorsed by the European Association of Nuclear Medicine (EANM) guideline as recommended scintigraphy technique for the diagnosis of PE in 2009. The guideline was updated in 2019 to broaden the diagnostics beyond PE with other cardiopulmonary diseases. Functional imaging by V/P SPECT enables diagnostic conclusions beyond PE, such as pneumonia, pulmonary congestion (heart failure), airway obstruction, and damage to lung parenchyma (emphysema) in chronic obstructive pulmonary disease (COPD). More importantly, it allows estimation of preserved (or lost) overall lung function in the presence of these pathologies. Clinically useful diagnostic capability comparable to V/P SPECT is difficult to achieve with other methods.
Physiological and Methodological Aspects
Physiological basics for functional imaging
Scintigraphic pulmonary studies are designed to demonstrate patterns of ventilation and perfusion. In healthy lungs, there is a balance between regional perfusion and ventilation to achieve optimal gas exchange. Some pulmonary diseases cause changes in either perfusion or ventilation. The mismatch implies an imbalance between perfusion and ventilation. In PE, there are areas with absent perfusion of segmental/sub-segmental character and preserved ventilation complying the pulmonary circulation. The reversal mismatch is a situation where there is a preserved perfusion in non-ventilated lung areas like in acute pneumonia. Some other diseases cause declines in both functional defect. Acute pneumonia causes frequently reversal mismatches. Later, as the disease progresses, matched defects may be seen.
Selection of radiopharmaceuticals
Implementation of V/P SPECT for diagnosis of PE and for making diagnostic conclusions beyond PE in our experience is based on using 99mTc-labeled Technegas® to measure ventilation and 99mTc-labeled human albumin macroaggregates (MAA) to measure perfusion.
Ventilation
Selection of radiopharmaceuticals for ventilation is particularly important for using V/P SPECT in diagnosing pulmonary diseases beyond PE. In clinical practice, Technegas® is an aerosol of choice. Technegas has solid graphite hydrophobic particles with a diameter of about 0.005. The size of the particle is so small that the aerosol behaves like a gas until it arrives at the periphery of the lungs where the particles are deposited in bronchioles and alveoli, mostly by diffusion. Technegas® reduces the problem of central deposition, which facilitates interpretation, particularly in patients with COPD. Hotspots are nevertheless seen in patients with severe airway obstruction. The penetration index for Technegas® may be used for grading COPD severity.
Perfusion
Perfusion follows immediately after ventilation by intravenous injection (I.V.) of 99mTc-labeled human MAA with a diameter of 15 leads to microembolization of pulmonary precapillary arterioles and capillaries in proportion to the local perfusion. In general, in clinical practice about 400,000 labeled particles are injected intravenously. As there are about 300,000 million pre-capillaries and billions of pulmonary capillaries, only a very small fraction of pulmonary vessels will be occluded. Still, for patients with known pulmonary hypertension, right-to-left heart shunt or after single lung transplantation, injection of no more than 100,000-200,000 particles is recommended. The number of particles is also reduced in children and is adjusted according to weight.
Procedure and Signal acquisition
Administration of radio indicators for ventilation and perfusion should be performed with the patient in the supine position to minimize gravitational gradients. The patient is in the supine position during inhalation, I.V. injection and during the acquisition. The study starts with the inhalation of radio-aerosol. During inhalation, activity over the lungs should be monitored to ensure adequate pulmonary deposition of 25-30 MBq in the lungs. Ventilation tomography starts immediately after. Imaging time is about 11 minutes. For the perfusion study 120-160 MBq of MAA is injected without changing the position of the patient. Perfusion tomography starts immediately after. Imaging time is about 5 minutes. The imaging protocol is based on extensive, systematic analysis serving to optimize adequate imaging quality, with low radiation exposure, in a brief time. Relationships between activities, acquisition times, collimators and matrices for SPECT imaging were studied in the context of a dual head gamma camera. Doses for ventilation and perfusion studies were found optimal by using a general-purpose collimator, and 64 × 64 matrices (60-64 steps for each head, 10 s/step for ventilation and 5 s/step for perfusion). Total acquisition time is about 20 min. Higher doses and/or longer acquisition time is not promoted as it did not yield images of significantly higher quality. To follow good medical practice, radiation exposure should be minimized to the lowest level consistent with satisfactory image quality. For this reason, it is also essential to use iterative reconstruction for V/P SPECT. Recommended is Ordered-Subset Expectation Maximization (OSEM) with four iterations and eight subsets. Standard software can be used for image presentation in frontal, sagittal and transversal projections as well as for presentation of rotating 3-D images. For quality control and fast orientation an overview of ventilation and perfusion in coronal and sagittal slices is useful. It is important to present the images so that ventilation and perfusion are carefully aligned to each other. This is facilitated by the one session protocol with the patient in the unchanged position. Proper alignment allows calculating and displaying ventilation/perfusion quotient images using the procedure as described above. Ventilation is normalized to perfusion counts, and then the V/P quotient images are calculated. Using this protocol, attenuation correction is not needed. V/P quotient images facilitate diagnosis and quantification of PE extension. However, quotient images are not a prerequisite for high-quality V/P SPECT.
Interpretation
According to the EANM guideline, all patterns and numbers of ventilation and perfusion defects are described. The clinical probability is considered as well. This holistic principle for reporting gives a clear answer to clinicians, yes or no for PE. This goal was not achieved with previous probabilistic reporting methods according to PIOPED.
Semi-quantification of function
The number of segments and sub-segments indicating typical mismatch in PE are counted and expressed in percentage of the total lung parenchyma. Furthermore, areas with ventilation abnormalities are recognized. In addition, ventilation/perfusion defects are quantified by calculating the total preserved lung function, which equals the percentage of total lung volume exhibiting preserved matching ventilation/perfusion.
Radiation dose
Minimizing radiation exposure without sacrificing image quality and diagnostic accuracy are essentials for an imaging test that follows good clinical practice. The effective dose of the V/P SPECT protocol is 2.1 mSv and lower than the effective dose of CTPA. The breast-absorbed dose of V/P SPECT is estimated to be less than 0.8 mGy (30% of a standard mammography), lung-absorbed dose less than 12 mGy and uterus/embryo/fetal-absorbed dose approximately 0.4-1.0 mGy (corresponding to the natural background radiation during the 9-month gestational period). CTPA has up to a 100-fold breast-absorbed dose, less than a 10-fold lung-absorbed dose and slightly higher uterus/embryo/fetal-absorbed dose compared to V/P SPECT.
Implementation in Diagnostics of PE
Acute PE
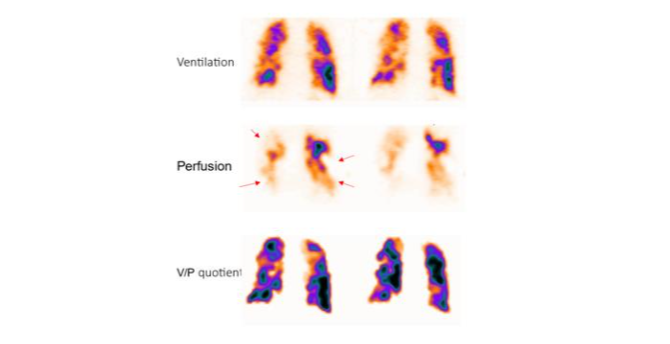
Pulmonary embolism is reported if there is a V/P mismatch of at least one segment or two sub-segments that conform to the pulmonary vascular anatomy. Functional imaging of patients with acute PE by pulmonary scintigraphy usually shows several segmental perfusion defects initially described by Wagner in 1964. Emboli affecting individual pulmonary arteries cause characteristic lobar, segmental, or sub-segmental peripheral wedge-shaped perfusion defects with the base projecting to the lung periphery due to the distinctive pulmonary arterial segmental anatomy. Within a segment or segments affected by PE, ventilation is usually preserved. This pattern of preserved ventilation and absent perfusion, known as V/P mismatch, provides the basis for PE diagnosis.
No PE is reported if there is: (a) normal perfusion pattern conforming to the anatomic boundaries of the lungs (b) matched or reversed mismatch V/P defects of any size, shape, or number in the absence of mismatch. (c) mismatch that does not have a lobar, segmental, or sub-segmental pattern. Non-diagnostic for PE is reported if there are multiple V/P abnormalities not typical of specific diseases. Applying these principles of interpretation, recent V/P SPECT studies amounting to over five thousand cases, reported a negative predictive value of 97-99%, sensitivity of 96-99%, and specificity of 91-98% for PE diagnosis. Rates of non-diagnostic findings were 1-3%.
Importance of quantifying the extent of PE
A crucial step in the diagnostic procedure is to quantify the extent of embolism for selection of a treatment option. In some settings the home treatment decision based on quantification of V/P defects in SPECT has been implemented. The number of segments and sub-segments, indicating mismatch typical for PE, are counted and expressed in percentage of the total lung parenchyma. Furthermore, areas with ventilation abnormalities are recognized and expressed in percentage of the total lung parenchyma. In hemodynamically stable patients with up to 40% PE and not more than 20% of ventilation defect, outpatient management is safe.
Follow up of resolution in an acute PE
The V/P SPECT is the gold standard for following the resolution of an acute PE. V/P SPECT ideally suits for the follow-up of PE because it is applicable to all patients. Obviously, using the same method for diagnosis and follow-up is advantageous. The low radiation exposure enables repeated examinations. V/P SPECT has a high sensitivity and a good repeatability, which allows recognition of both small and large emboli so that regression or progression of thrombotic disease can be studied in detail.
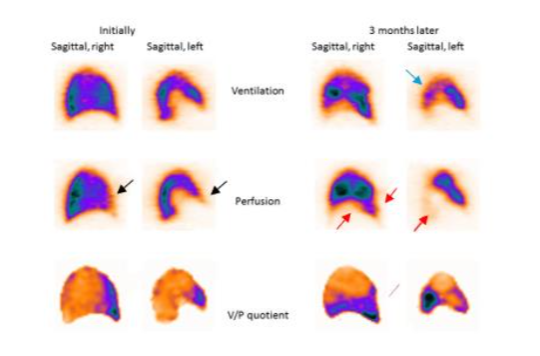
Some patients tend to develop recurrent episodes of PE. The extent of PE has been shown to be an independent risk factor for PE recurrence. A recurring process gives rise to multiple emboli in various stages of resolution. Without initial and follow-up images, it is often impossible to differentiate between old and new PE. Follow-up of PE by using imaging is essential to assess the effect of therapy and to explain physical incapacity after PE. Patients treated with thrombolysis for massive PE suffer risks of bleeding, but also dangers related to unresolved PE. It is valuable to follow up patients after cessation of treatment. Immediate imaging control gives objective information about the need for repeated thrombolysis. Another important group are symptomatic patients with small emboli. The natural history of disease and the value of treatment in this group of patients is unknown. Therefore, it is important to find out if these patients need therapy and if these patients need the same length of treatment. Follow-up is also indicated to adjust the length of individualized therapy.
Chronic PE and chronic thromboembolic pulmonary hypertension (CTEPH)
The V/P SPECT is the gold standard also for diagnosis of chronic PE and CTEPH. Chronic PE (i.e., perfusion defects due to PEs have not resolved) is a progressive disease that develops in about 1-5% after an acute episode of PE, even in treated patients. In non-acute PE, perfusion defects usually do not show clear segmental pattern which might lead to a false negative diagnosis.
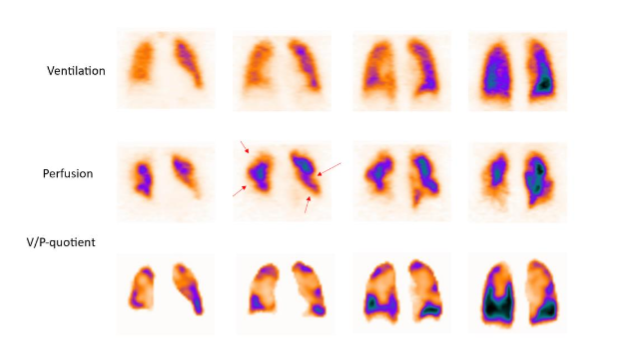
Chronic PE might lead to chronic thromboembolic pulmonary hypertension (CTEPH), right heart failure and arrhythmia. Among patients with pulmonary hypertension, scintigraphy had a sensitivity of 96% and specificity of 90%.
Pneumonia
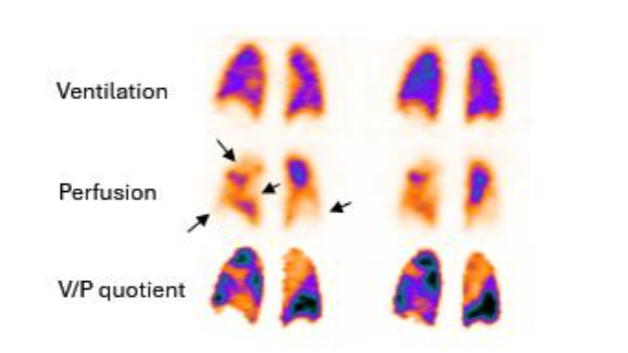
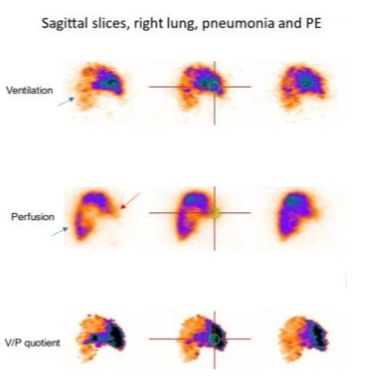
In pneumonia, V/P SPECT shows ventilation defects that usually exceed perfusion defects known as reversed V/P mismatch. PE is a frequent comorbidity in patients with pneumonia or infection that inflames the air sacs in one or both lungs. In these clinical conditions, PE is frequently missed by CT. This is valuable information because in general, current clinical and nuclear medicine practices do not recognize nor use V/P SPECT as a potential imaging method to diagnose or manage pneumonia. Pneumonia is a dynamic process causing also matched V/P defects. Preserved perfusion along the pleural border peripheral to a central matched sign of pneumonia.
Pulmonary Congestion (Heart Failure)
Antigravitational perfusion distribution from posterior to anterior region indicates pulmonary congestion (heart failure). As ventilation is usually less affected, the typical pattern is antigravitational redistribution of perfusion and V/P mismatch in dorsal regions of the lung. This V/P mismatch has a non-segmental pattern (does not conform pulmonary vascular architecture) and should not be misinterpreted as PE. A positive SPECT has been observed.
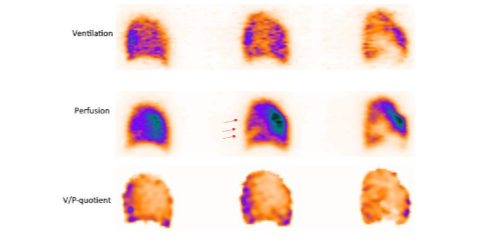
Airway Obstruction and Damage Of Lung Parenchyma In COPD
The characteristic of COPD is a general unevenness of ventilation. Focal deposition of aerosol in central or peripheral airways indicates airway obstruction. The grading of obstruction in ventilation SPECT has been standardized using Technegas. COPD is frequently observed in patients with a suspected PE, because COPD patients are at elevated risk of PE. The rate of PE in patients hospitalized for acute exacerbation of COPD may be as high as 25%. PE can be diagnosed even in the presence of COPD with V/P SPECT.
Conclusion
Ventilation/perfusion single photon emission computed tomography (V/P SPECT) is the recommended scintigraphic technique for the diagnosis and follow-up of PE: it has the highest sensitivity and specificity, and it produces very few non-diagnostic reports. PE is a frequent comorbidity with other pathologies such as broncho-obstructive disease, pneumonia, and heart congestion. With V/P SPECT it is possible to visualize PE and comorbidity simultaneously as well as to quantify the total preserved lung function. V/P SPECT has neither contraindications nor complications. It has a low effective radiation dose and its absorbed radiation doses to many organs, especially to the breast, are lower than those of CTPA. This is particularly important for women in the reproductive period and during pregnancy. To take full advantage of the V/P SPECT potential, it is crucial to apply an optimal protocol for a single session imaging of both ventilation and perfusion using low nuclide activities. Holistic interpretation is the most important diagnostic strategy for V/P SPECT, giving a clear report with respect to PE, its extension as well as other diagnoses based on V/P patterns typical for various diseases. Furthermore, full use should be made of display options, which are integrated into modern camera systems.
Conflict of Interest:
The authors have no conflicts of interest to declare.
Funding Statement:
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Acknowledgements:
None.
ORCID IDs:
Dr Ari Lindqvist: 0000-0002-0062-0117
References:
- 1. The PIOPED Investigators. Value of the ventilation-perfusion scan in the diagnosis of pulmonary embolism (PIOPED). JAMA. 1990;263:2759-2765.
- 2. Bajc M, Olsson B, Palmer J, Jonson B. Ventilation/perfusion SPECT for diagnostics of pulmonary embolism in clinical practice. J Intern Med. 2008;264:379-387.
- 3. Grinning T, Drake BE, Farrell SL, Nooks T. Three-year clinical experience with V/P SPECT for diagnosing pulmonary embolism: Diagnostic performance. Imaging. 2014;38:183-191.
- 4. Lemb M, Pohlabeln H. Pulmonary thromboembolism: A review of the literature. Nuklearmedizin. 2015;54:223-230.
- 5. Friedmann WF, Braunwald E. Alterations in regional pulmonary blood flow in mitral valve disease studied by radiolabeled scanning. A simple noninvasive technique for estimation of left atrial pressure. Circulation. 1975;51:1579-1587.
- 6. Bajc M, Chen Y, Wang J, et al. Identifying heterogeneity of COPD by V/P SPECT. Eur Respir J. 2019;54:1900171.
- 7. Gillett M, Morton J, Jensen CV, et al. Guidelines for lung scintigraphy in children. Eur J Nucl Med Mol Imaging. 2007;34:1518-1525.
- 8. Ciofetta G, Pippa A, Roca I, et al. Guidelines for lung scintigraphy in adults. Eur J Nucl Med Mol Imaging. 2017;44:1518-1525.
- 9. Bajc M, Olsson B, Lindqvist A. Ventilation defect for COPD is frequent among patients suspected for pulmonary embolism but does not prevent the diagnosis of PE by V/P SPECT. EC Pulmonol Respir Med. 2017;4:85-91.
- 10. Bajc M. Value of ventilation-perfusion scintigraphy in the diagnosis of pulmonary embolism. Thromb Haemost. 2005;93:99-104.

