Gender Variations in Right-Sided Heart Failure
Gender Variations in the Clinical and Phenotypic Presentation of Right-Sided Heart Failure
Abbeer Bashir1, Shehana Bin Shigair2, Abdul Rahman Alghamdi3, Mohammed Meshal Almeshrqi4, Khalid Alshabani5, Majid Abdullah Alsaleh6, Hatem Kheirallah7, Juan Jaime Alfonso8, Ali Khalil Abousa9, Zakaria Bin Mousa10, and Sondos Samargandy11
- Prince Sultan Cardiac Center, Adult Cardiology Department, Riyadh, Saudi Arabia
- King Abdullah Medical City, Adult Cardiology Department, Makkah, Saudi Arabia
- Prince Sultan Cardiac Center, Department of Research, Riyadh, Saudi Arabia
Email: [email protected]
OPEN ACCESS
PUBLISHED: 31 January 2025
CITATION: BAKHSH, Abeer Bakhsh Mohammad et al. Gender Variations in the Clinical and Phenotypic Presentation of Right- Sided Heart Failure. Medical Research Archives, [S.l.], v. 13, n. 1, jan. 2025. Available at: <https://esmed.org/MRA/mra/article/view/6207>. Date accessed: 23 oct. 2025.
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.18103/mra.v13i1.6207
ISSN 2375-1924
ABSTRACT
Right heart failure (RHF) is a complex syndrome and carries a worse prognosis. The studies are limited in identifying gender differences in clinical and imaging findings of RHF. Method: a retrospective review of patients presenting with RHF. Results: 966 patients were reviewed; women accounted for 570 (59%) of patients with RHF. The mean age was 57±16 years. The most common co-morbidity included diabetes 419 (43%), class II obesity 323 (33.5%), and atrial fibrillation 409 (42%). The heart failure with preserved ejection fraction (HFpEF) was the dominant phenotype 601 (62%). Women had more HFpEF 415 (72.8%), while men had more HF with reduced EF (HFrEF) 207 (52.5%). The mean left ventricle (LV) dimension was 5.2±0.8 cm, and the mean right ventricle (RV) dimensions were 4.2±0.8 cm. Men had more RV dilation than women with an RV end-diastolic dimension of 4.4±0.8 cm, while in women, 4.1±0.8 with a p-value <0.0001. The mean tricuspid annular plane systolic excursion (TAPSE) was 1.4±0.5 cm, and the RV lateral wall s’ was 9±3. Men had more RV dysfunction, with TAPSE being 1.3±0.6 cm, while in women, it was 1.5±0.5 cm with a p-value of 0.002. The s’ of the lateral wall in men was 8.4±3 cm/s, while in women, it was 9.7±3.5 cm/s with a p-value of 0.004. Severe tricuspid valve (TV) regurgitation was more common in women 238 (41.7%). The TV intervention was equal for both genders. Conclusion: Women tend to have more HFpEF and a higher grade of TV regurgitation than men. However, men had more RV dilatation and dysfunction. Prospective studies will add to the understanding of the survival outcomes for men and women with RHF.
Keywords
Right heart failure, gender differences, clinical presentation, phenotypic presentation
Introduction
Right heart failure (RHF) carries a high burden of mortality and morbidity. The definition of RHF is “the clinical syndrome of signs and symptoms of heart failure (HF) due to the right ventricle (RV) dysfunction.” The RHF is frequently associated with left ventricle (LV) systolic dysfunction. Variable conditions lead to RHF, including pressure overload, volume overload, and myopathies. High afterload leads to RV dilatation to maintain stroke volume. This will increase the end-diastolic volume and cause RV failure. The RHF can be acute or chronic; acute RHF can develop with a sudden change in the afterload, such as pulmonary embolism, RV myocardial infarction, or an increase in the preload after left ventricle assist devices implantation. Chronic causes of RV failure can be classified into cardiomyopathies, left-sided HF, valvular heart disease, or pulmonary hypertension (PHTN). A meta-analysis of studies involving HF patients with LV systolic dysfunction showed that RHF was present in 47% of these patients, and it was associated with a higher rate of HF hospitalization. The RHF is considered a prognostic marker for adverse outcomes in patients with cardiac disease. The tricuspid valve (TV) regurgitation occurs 80–90% in RHF. The severity of TV regurgitation is correlated with worse survival.
The European Society’s guidelines for valvular disease recommend intervention in TV regurgitation for patients with moderate or severe TV regurgitation who are undergoing left-sided valve surgery. Intervention is recommended for isolated TV regurgitation in the absence of severe RV dysfunction. The intervention for isolated TV regurgitation is underutilized; studies reported 16% mortality in the late stage of RHF. Timely intervention requires a comprehensive evaluation of RV function in addition to the clinical assessment. Non-invasive imaging evaluations of RV function include transthoracic echocardiography (ECHO), cardiac computerized tomography, and cardiac magnetic resonance imaging. Invasive right heart catheterization is the gold standard for assessing right-side filling pressure and pulmonary vascular pressures. ECHO assessment of the Tricuspid annular plane systolic excursion (TAPSE) is recommended for RV function quantification. A TAPSE of <16 mm indicates RV dysfunction and has a strong prognostic implication.
The gender variation in RHF presentation and prognosis is underreported. Women have unique cardiovascular risk factors related to autoimmune inflammatory disease, hormonal cycle, and pregnancy-related adverse outcomes. Obesity and hypertension are prevalent in women with heart failure. Women have more heart failure with preserved ejection fraction (HFpEF) due to arterial stiffness, hormonal changes, and diastolic dysfunction. Women with HFpEF have more HF hospitalization and have more RV dilatation and dysfunction than men. Female gender is a risk factor for PHTN; however, men have worse survival due to reduced RV adaptation to increased afterload. The variability in risk, co-morbidity, and phenotype presentation of RHF between genders needs further understanding.
Objective:
Describe the difference in clinical syndrome and imaging findings of right ventricle failure in both women and men.
Method:
This is a single-center retrospective observational study of patients with RHF as the primary diagnosis at admission and/or who received a TV intervention from 2010 to 2023.
INCLUSION CRITERIA:
-
History of HF admission requiring intravenous diuresis.
-
Presenting with symptoms of right-sided failure: ascites and lower limb edema.
-
And/or echo features of RV dysfunction and TV regurgitation based on American Society of ECHO (ASE) criteria.
-
Had previous TV intervention.
EXCLUSION CRITERIA:
-
Congenital heart disease.
-
Patients with systemic right ventricle or single ventricle.
The Outcome:
The gender variation difference between cardiovascular risk factors, HF phenotypes, and cardiac interventions for patients presenting with right-side heart failure.
Data Collection:
The medical records of all individuals involved in our study will be reviewed to collect the following data:
-
Clinical data: patients’ age, gender, diabetes (DM), hypertension, obesity, coronary artery disease (CAD), heart failure (HF) phenotype; heart failure with reduced ejection fraction (HFrEF) or preserved ejection fraction (HFpEF), atrial fibrillation (AF) and chronic kidney disease (CKD).
-
Echocardiogram parameters: Left ventricle ejection fraction (LVEF), LV end-diastolic volume, LV end-systolic volume, and TAPSE are the most frequently used parameters for RVF quantification.
American Society of Echocardiography (ASE) criteria for RV assessment include: TAPSE of <16 mm indicates RV dysfunction, and tissue Doppler of the free lateral wall (s’) measures the tricuspid annular plane’s longitudinal velocity (base to apex) by tissue Doppler imaging. <0.095 m/s indicates RV dysfunction. Pulmonary hypertension (PHTN) by ECHO criteria is based on the European Society of Cardiology PHTN guidelines. -
Cardiac intervention: coronary bypass surgery, TV surgical or percutaneous repair or replacement, mitral valve (MV) repair or replacement.
Statistical Analysis:
The data analysis was performed using XLSTAT version 2021.2.2 Life-Science.
For quantitative variables, the baseline characteristics for all the patients were reported as median and interquartile range (IQR) or mean ± standard deviation (SD).
Categorical variables were presented as numbers and percentages (%) as appropriate.
The correlation analysis was performed using chi-square, Fisher exact test, and logistic regression as appropriate.
The level of significance is defined as a p-value < 0.05.
Results:
AGE AND CO-MORBIDITIES:
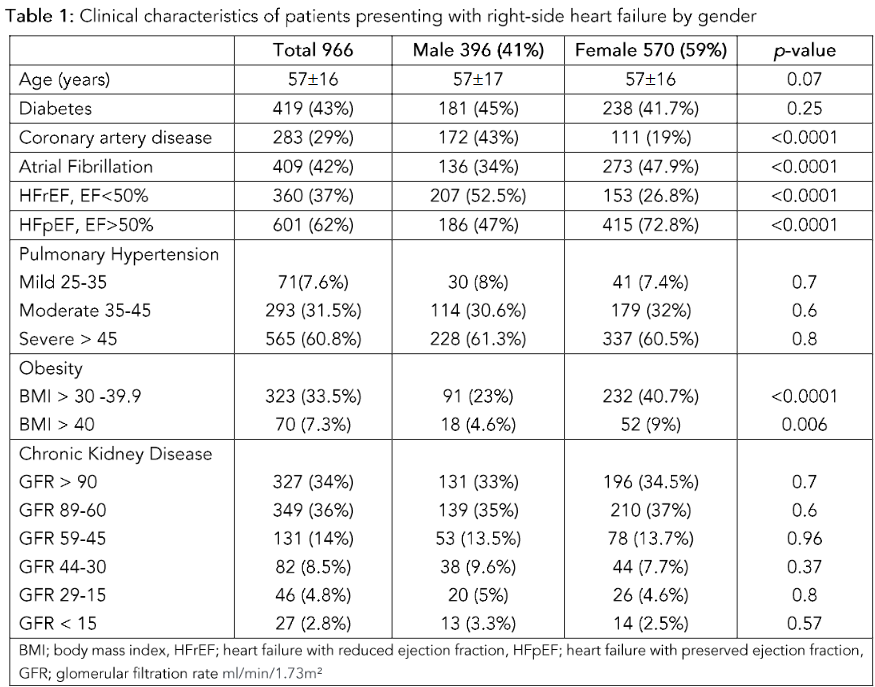
This retrospective study, including 966 patients, showed that more women presented with RHF than men. The women accounted for 570 (59%) of the study cohort. The mean age of the cohort was 57 ± 16 years.
The co-morbidity included:
-
DM in 419 (43%)
-
CAD in 283 (29%)
-
Obesity with BMI > 30 in 323 (33.5%)
-
AF in 409 (42%)
In women:
-
Mean age: 56.8 ± 15.5 years
-
DM: 238 (41.75%)
-
Obesity BMI > 30: 232 (40.7%)
-
BMI > 40: 52 (9%)
-
CAD: 111 (19.5%)
-
AF: 273 (47.8%)
In men:
-
Mean age: 57 ± 17 years
-
CAD: 172 (43%)
Hypertension was underreported and, thus, excluded from the final analysis. The comparative data between men and women in this cohort is presented in Table 1.
ECHOCARDIOGRAPHIC FINDINGS:
Left Ventricle Dysfunction:
Patients with clinical presentation of right-side heart failure had either HFrEF or HFpEF. Women predominantly had HFpEF 415 (72.8%), and HFrEF occurred for 153 (26.8%). In women, the mean LVEF was 50 ± 10% compared to men with LVEF 42 ± 14.7%, p-value < 0.0001. The mean LV end-diastolic dimension in men was 5.5 ± 0.8 cm, while in women, it was 5 ± 0.7 cm with a p-value < 0.0001. The ECHO findings are presented in Table 2.
Right Ventricle Dysfunction:
RV dilatation and dysfunction were evaluated based on the American Society of Echocardiography (ASE) guidelines. The quantitative evaluation of the RV was only available for 363 patients on retrospective review. The mean RV dimension in this cohort was 4.2 ± 0.8 cm. The RV dimension in women was 4.1 ± 0.8 cm, while in men, it was 4.4 ± 0.8 cm.
The RV function was assessed using TAPSE and s’ of the lateral wall. The mean TAPSE was 1.4 ± 0.5 cm, and the mean s’ was 9 ± 3 cm/s. For women, the TAPSE was 1.5 ± 0.5 cm and s’ of the lateral wall 9.7 ± 3.5 cm/s, while in men, the TAPSE was 1.3 ± 0.6 cm and s’ was 8.4 ± 3 cm/s. The ECHO findings are presented in Table 2.
Most patients had PHTN with pulmonary pressure > 45 mmHg 565 (60.8%), measured by a non-invasive method. There was no difference in PHTN between men and women.
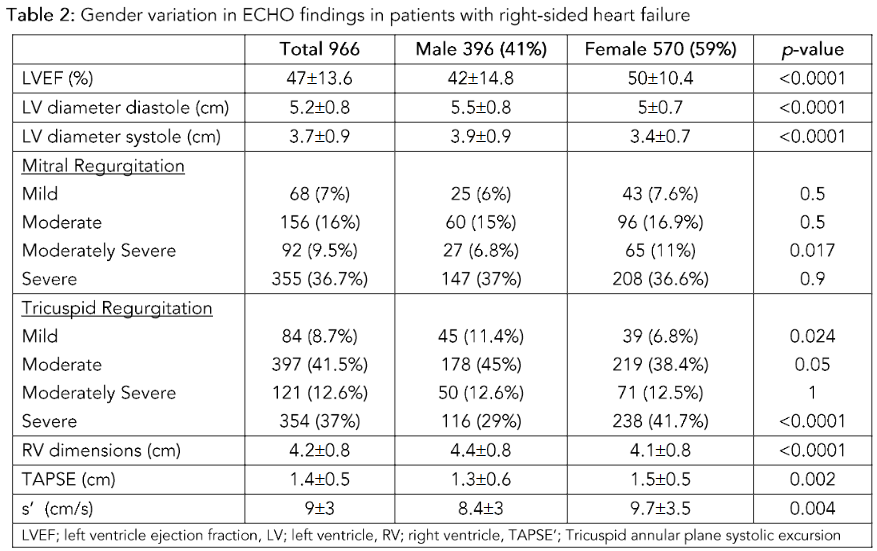
Table 2: Gender variation in ECHO findings in patients with right-sided heart failure
The gender differences in LVEF and LV dimensions were significant in HFrEF. The HFpEF population also had dilated ventricles with preserved LVEF, likely due to concomitant mitral regurgitation. The RV dimension and function were not statistically significant either in HFrEF or HFpEF. However, there was an increase in RV dimension in men compared to women, 4.5 ± 0.9 vs 4.2 ± 0.8 with a p-value of 0.012 (Table 3).
Table 3: Gender differences in the right ventricle in HFpEF and HFrEF
| ECHO parameters | HFrEF Male | HFrEF Female | p-value | HFpEF Male | HFpEF Female | p-value |
|---|---|---|---|---|---|---|
| LVEF (%) | 30.9 ± 10 | 36 ± 9.5 | <0.0001 | 55 ± 5.4 | 55.5 ± 4.0 | 0.6 |
| LV diameter diastole (cm) | 5.9 ± 0.8 | 5.4 ± 0.7 | <0.0001 | 5.3 ± 0.8 | 4.9 ± 0.7 | <0.0001 |
| LV diameter systole (cm) | 4.6 ± 0.8 | 4.0 ± 0.6 | <0.0001 | 3.5 ± 0.7 | 3.2 ± 0.6 | 0.001 |
| RV dimensions (cm) | 4.4 ± 0.8 | 4.0 ± 0.7 | 0.001 | 4.5 ± 0.9 | 4.2 ± 0.8 | 0.012 |
| TAPSE (cm) | 1.3 ± 0.6 | 1.4 ± 0.4 | 0.17 | 1.3 ± 0.5 | 1.59 ± 0.55 | 0.031 |
| s’ (cm/s) | 8.5 ± 2.9 | 8.7 ± 3.0 | 0.9 | 8.3 ± 3.2 | 10.2 ± 3.6 | 0.005 |
LVEF: left ventricle ejection fraction, LV: left ventricle, RV: right ventricle, TAPSE: Tricuspid annular plane systolic excursion
Mitral and tricuspid dysfunction:
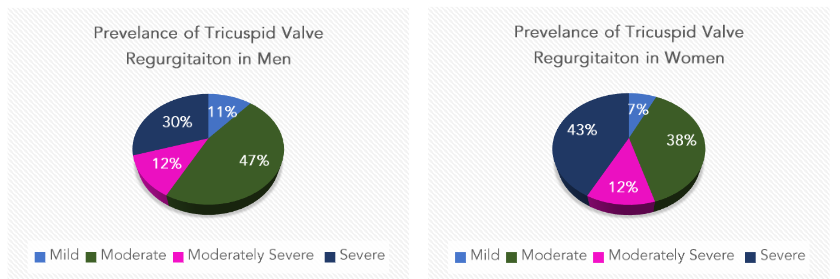
The cohort of patients with right-sided heart failure had variable degrees of tricuspid valve regurgitation. The majority had either moderate TV regurgitation 397 (41.5%) or severe TV regurgitation 354 (37%). The women had TV regurgitation at moderate 219 (38.4%) or severe 238 (41.7%). However, men had a lower rate of severe TV regurgitation 116 (29%), while moderate TV regurgitation was present in 178 (45%).
Mitral valve (MV) regurgitation was present in 355 (81.8%) patients. The majority had severe MV regurgitation (36.7%), while moderate MV regurgitation was present in 156 (16%) and moderately severe in 92 (9.5%). The MV regurgitation was present in 527 (92.6%) of all women in the cohort. Most women had severe MV regurgitation 208 (63.5%) and moderate 96.
(16.9%). However, men had a lower rate of severe MV regurgitation, 147 (37%), and moderate MV regurgitation was present in 60 (15%). These findings are presented in (Figure 1).
Figure 1: Prevalence of Tricuspid Valve Regurgitation in Men and Women.
Cardiac Intervention:
The patients who had coronary artery bypass surgery were 218 (22.5%), and women accounted for 94 (43%) of all cases compared to men 124 (56.9%).
The MV intervention was predominantly surgical; MV replacement was done in 449 (46.5%), while surgical repair was performed in 237 (24.6%).
The TV replacement was done in 533 (55%), while surgical repair occurred in 203 (21%), and percutaneous edge-to-edge repair in 11 (1%).
Isolated TV intervention occurred only in 26 (2.7%), while other cases were combined with left-side valve intervention. There was no significant difference in the TV intervention in severe TR between men and women (Table 4).
Table 4: Comparison of the invasive and surgical therapy rate based on gender
| Total 966 | Male 396 (41%) | Female 570 (59%) | p-value | |
|---|---|---|---|---|
| CABG surgery | 218 (22.5%) | 124 (31%) | 94 (16.5%) | <0.0001 |
| Tricuspid Valve | ||||
| Repair | 203 (21%) | 70 (17.6%) | 133 (23%) | 0.037 |
| Replacement | 533 (55%) | 213 (53.8%) | 320 (56%) | 0.5 |
| TEER | 11 (1%) | 1 (0.25%) | 10 (1.75%) | 0.033 |
| Mitral Valve | ||||
| Repair | 237 (24.6%) | 112 (28.4%) | 125 (21.9%) | 0.031 |
| Replacement | 449 (46.5%) | 152 (38.4%) | 297 (52%) | <0.0001 |
| TEER | 17 (1.8%) | 9 (2.3%) | 8 (1.4%) | 0.46 |
| Aortic Valve Replacement | 153 (21.5%) | 67 (24%) | 86 (19.7%) | 0.5 |
CABG: Coronary artery bypass graft; TEER: Transcatheter Edge to Edge Repair
Discussion:
Women with HF have a high burden of comorbidities. Previous studies reported high prevalence of DM 272 (48.5%), hypertension 267 (47.6%), and AF 74 (13%) in women with HF. This study is a retrospective review of 966 patients admitted with right-sided HF in a tertiary hospital.
This cohort had a high representation of women with right-side HF 570 (59%), and showed prevalence of DM 238 (41.7%) and a higher prevalence of AF 273 (47.9%). Atrial arrhythmias are common in RHF and TV regurgitation. It can impair RV filling and worsen the RHF, and it is a marker of advanced disease and it’s associated with worse outcomes.
poor prognosis. Obesity in women is associated with a fivefold increase in risk of HF compared to men. Class II obesity with a BMI of 30–39.9 was highly prevalent in this cohort for women 232 (40.7%).
Most patients had HFpEF 601 (62%), with a mean LVEF of 47 ± 13.6%. In multiple studies, the RVF in HFpEF was present in 33% to 50% of patients. The HFpEF was higher in women than in men: 415 (72.8%) vs. 186 (47%), respectively. A prospective multicentre study for HFpEF showed that men had more RV end-diastolic diameter and lower TAPSE than women. The HFpEF cohort had a mean RV dimension of 3.4 cm compared to women 3 with a p-value <0.001. This study has shown that RHF in HFpEF is associated with RV remodeling. The RV dilatation was more pronounced in men than in women, 4.5 ± 0.9 vs 4.2 ± 0.8, with a p-value <0.012. PHTN was equally present in both genders in this study.
The TV regurgitation was present at variable degrees in both genders. The women had predominantly severe TV regurgitation at 238 (41.7%), while in men, 116 (29%) had a p-value <0.0001. The TV intervention was common in this cohort, with 533 (55%) undergoing TV replacement and 203 (21%) having repair. Studies looking at TV disease and intervention showed a higher prevalence of TV in women, 67%. In a prospective study evaluating patients undergoing Transcutaneous Edge to Edge Repair, the cohort had a higher degree of RV end-diastolic dimension of 5.3 ± 0.07 cm with severe TV regurgitation in 29% and torrential TV regurgitation in 37%.
Limitation:
This study has limitations related to its retrospective design. Its use of advanced imaging modalities, such as cardiac MRI and RV strain, was limited. Finally, many patients did not have follow-up data, thus, no survival analysis could be performed.
Conclusion:
Patients presenting with RHF have a higher burden of co-morbidities. The risk of developing RHF in HFpEF is higher, especially in women. RHF is commonly associated with moderate to severe TR. This cohort has a high rate of TV surgery; however, the outcome data around HF and mortality are lacking.
Conflict of Interest:
The authors have no conflict of interest related to this project.
Funding Statement:
None.
Acknowledgements:
None.
Ethics Board Review Approval:
Prince Sultan Military Medical City
Institutional Review Board
HP-01-R079/1699
References:
1. Adamo M, Chioncel O, Pagnesi M, et al. Epidemiology, pathophysiology, diagnosis and management of chronic right-sided heart failure and tricuspid regurgitation. A clinical consensus statement of the Heart Failure Association (HFA) and the European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the ESC. Eur J Heart Fail. Jan 2024;26(1):18-33. doi:10.10 02/ejhf.3106
2. Iglesias-Garriz I, Olalla-Gomez C, Garrote C, et al. Contribution of right ventricular dysfunction to heart failure mortality: a meta-analysis. Rev Cardiovasc Med. 2012;13(2-3):e62-9. doi:10.3909/ricm0602
3. Meyer P, Filippatos GS, Ahmed MI, et al. Effects of right ventricular ejection fraction on outcomes in chronic systolic heart failure. Circulation. Jan 19 2010;121(2):252-8. doi:10.1161/CIRCULATIONAH A.109.887570
4. Bozkurt B, Coats AJS, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. European Journal of Heart Failure. 2021;23(3):352-380. doi:https://doi.org/10.1002/ejhf.2115
5. Konstam MA, Kiernan MS, Bernstein D, et al. Evaluation and Management of Right-Sided Heart Failure: A Scientific Statement From the American Heart Association. Circulation. May 15 2018;137(20 ):e578-e622. doi:10.1161/CIR.0000000000000560
6. Sanz J, Sanchez-Quintana D, Bossone E, Bogaard HJ, Naeije R. Anatomy, Function, and Dysfunction of the Right Ventricle: JACC State-of-the-Art Review. J Am Coll Cardiol. Apr 2 2019;73 (12):1463-1482. doi:10.1016/j.jacc.2018.12.076
7. Vieillard-Baron A, Naeije R, Haddad F, et al. Diagnostic workup, etiologies and management of acute right ventricle failure : A state-of-the-art paper. Intensive Care Med. Jun 2018;44(6):774-790. doi:10.1007/s00134-018-5172-2
8. Arrigo M, Huber LC, Winnik S, et al. Right Ventricular Failure: Pathophysiology, Diagnosis and Treatment. Card Fail Rev. Nov 2019;5(3):140-146. doi:10.15420/cfr.2019.15.2
9. Ventetuolo CE, Klinger JR. Management of acute right ventricular failure in the intensive care unit. Ann Am Thorac Soc. Jun 2014;11(5):811-22. doi:10.1513/AnnalsATS.201312-446FR
10. Leeper B. Right Ventricular Failure. AACN Adv Crit Care. Mar 15 2020;31(1):49-56. doi:10.4037/aa cnacc2020172
11. Ashraf H, Rosenthal JL. Right Heart Failure: Causes and Clinical Epidemiology. Cardiol Clin. May 2020;38(2):175-183. doi:10.1016/j.ccl.2020.01.008
12. Pradhan NM, Mullin C, Poor HD. Biomarkers and Right Ventricular Dysfunction. Crit Care Clin. Jan 2020;36(1):141-153. doi:10.1016/j.ccc.2019.08.011
13. Bleasdale RA, Frenneaux MP. Prognostic importance of right ventricular dysfunction. Heart. Oct 2002;88(4):323-4. doi:10.1136/heart.88.4.323
14. Lurz P, Stephan von Bardeleben R, Weber M, et al. Transcatheter Edge-to-Edge Repair for Treatment of Tricuspid Regurgitation. J Am Coll Cardiol. Jan 26 2021;77(3):229-239. doi:10.1016/j. jacc.2020.11.038
15. Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). European Heart Journal. 2021;43 (7):561-632. doi:10.1093/eurheartj/ehab395
16. Sala A, Lorusso R, Zancanaro E, et al. Mid-term outcomes of isolated tricuspid valve surgery according to preoperative clinical and functional staging. European Journal of Cardio-Thoracic Surgery. 2022;62(2)doi:10.1093/ejcts/ezac172
17. Voelkel NF, Quaife RA, Leinwand LA, et al. Right Ventricular Function and Failure. Circulation. 2006;114(17):1883-1891. doi:10.1161/CIRCULATI ONAHA.106.632208
18. Kayali F, Tahhan O, Vecchio G, et al. Left ventricular unloading to facilitate ventricular remodelling in heart failure: A narrative review of mechanical circulatory support. Exp Physiol. Nov 2024;109(11):1826-1836. doi:10.1113/EP091796
19. Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. Jul 2010;23(7):685-713; quiz 786-8. doi:10.1016/j.echo.2010.05.010
20. Dietz MF, Prihadi EA, van der Bijl P, Ajmone Marsan N, Delgado V, Bax JJ. Prognostic Implications of Staging Right Heart Failure in Patients With Significant Secondary Tricuspid Regurgitation. JACC Heart Fail. Aug 2020;8(8):627 -636. doi:10.1016/j.jchf.2020.02.008
21. Cho L, Davis M, Elgendy I, et al. Summary of Updated Recommendations for Primary Prevention of Cardiovascular Disease in Women: JACC State-of-the-Art Review. J Am Coll Cardiol. May 26 2020; 75(20):2602-2618. doi:10.1016/j.jacc.2020.03.060
22. Bakhsh A, AlSayed A, AlTamimi M, et al. The outcome of heart failure in women: a study from a tertiary heart function clinic. Am J Cardiovasc Dis. 2023;13(5):300-308.
23. Kadosh BS, Birs AS, Flattery E, et al. Cardiac allograft vasculopathy in heart transplant recipients from hepatitis C viremic donors. Clin Transplant. Apr 2024;38(4):e15294. doi:10.1111/ctr.15294
24. Robertson J, Lindgren M, Schaufelberger M, et al. Body Mass Index in Young Women and Risk of Cardiomyopathy: A Long-Term Follow-Up Study in Sweden. Circulation. Feb 18 2020;141(7):520-529. doi:10.1161/CIRCULATIONAHA.119.044056
25. Kaur G, Lau E. Sex differences in heart failure with preserved ejection fraction: From traditional risk factors to sex-specific risk factors. Womens Health (Lond). Jan-Dec 2022;18:17455057221140 209. doi:10.1177/17455057221140209
26. Sotomi Y, Hikoso S, Nakatani D, et al. Sex Differences in Heart Failure With Preserved Ejection Fraction. J Am Heart Assoc. Feb 2021;10 (5):e018574. doi:10.1161/JAHA.120.018574
27. DesJardin JT, Kime N, Kolaitis NA, et al. Investigating the “sex paradox” in pulmonary arterial hypertension: Results from the Pulmonary Hypertension Association Registry (PHAR). J Heart Lung Transplant. Jun 2024;43(6):901-910. doi:10.1 016/j.healun.2024.02.004
28. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: Developed by the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by the International Society for Heart and Lung Transplantation (ISHLT) and the European Reference Network on rare respiratory diseases (ERN-LUNG). European Heart Journal. 2022;43(38):3618-3731. doi:10.1093/eur heartj/ehac237
29. Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. Jan 1 2016; 37(1):67-119. doi:10.1093/eurheartj/ehv317
30. Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. Dec 21 2014;35(48):3452-62. doi:10.1093/eurheartj/ehu193
31. Puwanant S, Priester TC, Mookadam F, Bruce CJ, Redfield MM, Chandrasekaran K. Right ventricular function in patients with preserved and reduced ejection fraction heart failure. Eur J Echocardiogr. Aug 2009;10(6):733-7. doi:10.1093/ejechocard/jep052
32. Pahwa S, Saran N, Pochettino A, et al. Outcomes of tricuspid valve surgery in patients with functional tricuspid regurgitation. Eur J Cardiothorac Surg. Apr 13 2021;59(3):577-585. doi:10.1093/ejcts/ezaa350

