Haemophilia Gene Therapy: Patient Selection Insights
Haemophilia Gene Therapy: Patient Selection and Experience in the Era of Precision Medicine
Dr Sara Boyce 1MBBS, BSc, MRCP, FRCPath and Dr Rashid Kazmi 2MBBS, FRCPath
- Southampton Haemophilia Centre, University Hospital Southampton, UK
OPEN ACCESS
PUBLISHED: 30 December 2024
CITATION: Boyce, S. and Kazmi, R. 2024. Haemophilia Gene Therapy: Patient Selection and Experience in the Era of Precision Medicine. Medical Research Archives. [online] 12(12). doi:10.18103/mra.v12i12.4051
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.18103/mra.v12i12.6051
ISSN 2375-1924
Abstract
The emergence of gene therapy as a therapeutic modality for haemophilia has generated a spectrum of responses within the patient population, ranging from enthusiastic endorsement to measured scepticism. Following the European Medicines Agency’s approval of two novel gene therapies for haemophilia B, the imperative for sophisticated patient selection protocols has become increasingly apparent to ensure both the safe administration of therapy and optimisation of clinical outcomes. Haemophilia gene therapy trials to date have demonstrated that psychosocial factors are critical components of the gene therapy pathway alongside clinical eligibility to facilitate rigorous follow-up protocols and maximize the probability of sustained transgene expression. Experiences of participants in haemophilia gene therapy clinical trials are valuable for informing prospective recipients during pre-consent counselling to appropriately calibrate treatment expectations and thoroughly comprehend the multifaceted risk-benefit profile, including the inherent uncertainties associated with long-term outcomes.
Keywords
Haemophilia, gene therapy, patient selection, psychosocial impact, clinical criteria
Introduction
Haemophilia, an inherited X-linked disorder, stems from deficiencies in coagulation factors VIII or IX, leading to abnormal bleeding. Affecting over 1.2 million people worldwide, haemophilia A (factor VIII deficiency) occurs in approximately 1 in 5,000 male births, while haemophilia B (factor IX deficiency) affects around 1 in 30,000. Both clotting factors are essential in the coagulation cascade, generating thrombin to secure stable blood clots. Severe haemophilia A is defined by factor VIII levels below 1% (<1 IU/L), and severe haemophilia B by factor IX levels under 1%. People with severe haemophilia endure spontaneous bleeds, particularly within joints and muscles, leading to recurrent “target joint” bleeding and progressive haemophilic arthropathy, resulting in chronic pain, disability, and psychosocial burden. In moderate haemophilia, with factor levels between 1–5%, bleeding tendencies are variable, while those with mild haemophilia (factor levels above 5%) typically experience bleeding only after trauma, with daily activities largely unaffected.
Advancements in haemophilia care have evolved from treating acute bleeds with blood products to preventive home-based regimens using recombinant clotting factors, reducing both bleed frequency and the risk of transfusion-transmitted infections. Modern therapies have elevated the quality of life for people with haemophilia (PwH), aligning aspirations with the goal of enabling PwH to lead lifestyles akin to those of healthy individuals. Over the past decade, gene therapy has brought profound progress and renewed hope to the haemophilia community. Traditional treatment with intravenous clotting factors, often administered several times weekly, imposes significant limitations on PwH. In recent years, the development of extended half-life factors and subcutaneous non-replacement therapies has widened therapeutic options, yet these advancements still carry a risk of breakthrough bleeding. Gene therapy’s potential to normalize factor VIII or IX levels stands as a beacon of optimism, holding the promise of transformative freedom for PwH. However, the gene therapy journey can be arduous and challenges selecting ideal candidates and managing their expectations need to be addressed. We summarise the processes involved in selecting and preparing PwH for gene therapy based on clinical trial experiences.
Gene Therapy in Haemophilia
Haemophilia presents an ideal candidate for gene therapy due to its monogenic nature and the fact that modest increases in factor levels can significantly ameliorate the severe bleeding phenotype. The therapeutic approach utilises adeno-associated virus (AAV) vectors to deliver modified factor VIII or IX genes to hepatocytes. Administration involves a single intravenous infusion, followed by intensive outpatient monitoring to identify potential hepatic immune responses and any associated factor expression decline. Immunosuppression, primarily with corticosteroids, is frequently necessary to maintain factor expression.
Clinical Trials and Outcomes
Recent meta-analyses of AAV-based gene therapies for haemophilia A and B have demonstrated significant reductions in annual bleeding rates, factor infusion frequencies, and overall coagulation factor consumption. Factor IX expression in haemophilia B has shown notable durability, maintaining 95.7% of peak levels at 24 months, while factor VIII levels decreased to 55.8%.
Given the compelling success of recent clinical trials and the high burden of lifelong prophylactic treatments for haemophilia, valoctocogene roxaparvovec (Roctavian®, BioMarin Pharmaceutical) has received FDA approval for haemophilia A, while etranacogene dezaparvovec (Hemgenix®, CSL Behring) and fidanacogene elaparvovec (Beqvez®, Pfizer) have been jointly approved by the FDA and European Medicines Agency for haemophilia B, signalling a transformative shift in the therapeutic landscape.
Patient Selection
While many have eagerly embraced the opportunity for haemophilia gene therapy, others approach it
with understandable caution. The tremendous advancements in this field owe much to the altruism of early trial participants whose commitment has redefined future treatment landscapes; however, for individuals to receive gene therapy, they must meet specific clinical criteria to ensure efficacy and safety, and, if met, psychosocial factors must be thoughtfully evaluated before proceeding.
Clinical Criteria
The pre-existence of neutralising antibodies to wild-type AAV can preclude individuals from receiving gene therapy, as these antibodies may inhibit therapeutic gene expression. Among licensed gene therapy products, only etranacogene dezaparvovec can be administered to individuals with AAV antibodies, though it remains unsuitable for those with high-titre anti-AAV5 antibodies. Early screening for AAV antibodies is essential to manage expectations and explore alternative treatments. Additionally, good liver health is critical for effective transduction and to mitigate potential hepatic immune reactions post-infusion. Comprehensive liver assessments, including ultrasound and elastography, are therefore necessary, though, unfortunately, this requirement excludes some PwH who have developed cirrhosis due to the contaminated blood tragedy. Intense liver monitoring will be required for several months post-treatment. Because the goal is sustained endogenous clotting factor production, a careful consideration of the individual’s overall health and ability to adhere to follow-up demands is essential.
Individuals with inhibitory antibodies against factor VIII or IX — a complication of clotting factor treatments — have traditionally been excluded from gene therapy trials, but promising developments may one day broaden treatment eligibility for this group.
Psychosocial Considerations
Gene therapy’s irreversible nature demands a substantial commitment from recipients, who must be highly motivated to adhere to rigorous monitoring requirements and understand that, while the ultimate goal is a “haemophilia-free mind,” they may initially feel more like a patient than ever before. Thorough consideration of practical aspects — such as the ability to travel to haemophilia centres, work, school, caregiving responsibilities, and financial implications — is essential. Equitable access to care must be prioritised, with healthcare teams and local support services actively seeking ways to address these challenges. For some, the burden of follow-up alone may discourage pursuit of gene therapy, particularly for those whose haemophilia management requires fewer prophylactic injections and may thus view the upheaval as disproportionate to the potential benefit.
Pre-treatment Counselling and Consent
Gene therapy is inherently complex, both in its biological mechanisms and in managing the psychological challenges of navigating significant short- and long-term uncertainty. High-quality information is essential for both the potential recipient and the haemophilia team providing counselling, ensuring a comprehensive understanding of the treatment and related processes before beginning the gene therapy journey. Key practical aspects must be addressed, including the requirement for contraception due to unknown risks associated with vector shedding, which may prompt discussions around family planning. Education on liver health is critical to prevent transaminitis and sustain expression of the factor VIII or IX gene, with clear guidance on abstaining from alcohol and other hepatotoxic substances initially, and adhering to safe alcohol limits over the long term. Emphasis should also be placed on the importance of a healthy diet and regular physical activity to reduce the risk of hepatic steatosis.
Before consenting to gene therapy, individuals must fully understand the physical risks involved, including potential vector infusion reactions such as systemic hypersensitivity and anaphylaxis. Transaminitis occurs frequently, potentially necessitating immunosuppression with associated side effects. There is also a risk of gene overexpression
to supranormal coagulation factor levels and a possible thrombotic risk, though no thrombotic events have been reported to date. Beyond these immediate and short-term effects, recipients must consider potential long-term implications, weighing these against the risks and benefits of alternative haemophilia treatments. Grappling with uncertainties — including viral integration, the theoretical risk of oncogenesis, and potential impacts on liver health — requires comprehensive support and ample time for thoughtful deliberation.
Recently published guidance by the United Kingdom Haemophilia Centre Doctors’ Organization on the implementation of haemophilia gene therapy emphasizes that, along with confirmation of eligibility, education and practical considerations, pre-consent counselling is a key component of the patient pathway. The guideline highlights that feedback from participants in clinical trials demonstrated the need for psychological support throughout the process, and counselling can be provided by nurses, doctors and psychologists with expertise in haemophilia
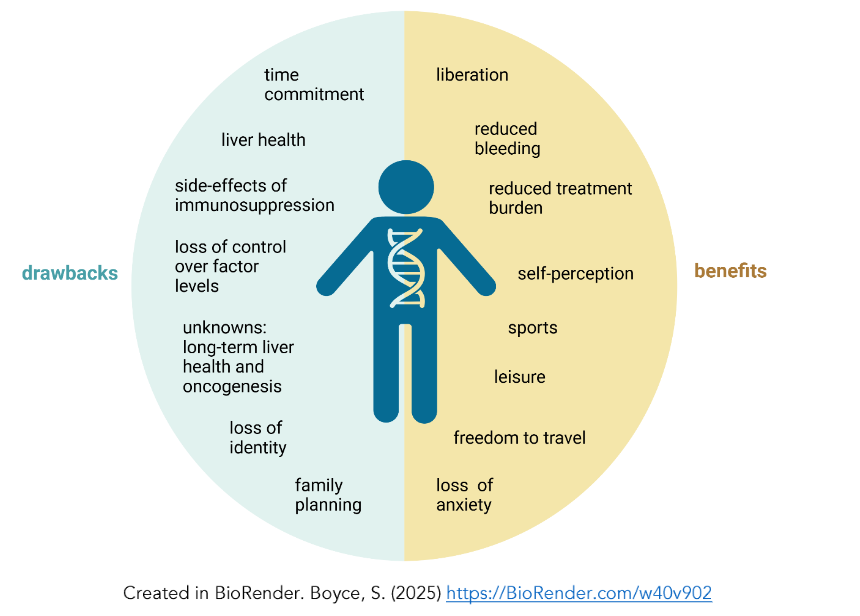
Figure 1: Summary of the potential positive and negative experiences PwH may encounter on the haemophilia gene therapy journey.Table 1: Summary of domains in patient reported outcomes (PROs) utilized in haemophilia gene therapy trials.
Non-disease specifics PROs: EQ-5D-5L, the International Physical Activity Questionnaire (IPAQ) score, the Brief Pain Inventory (BPI) short score and the Work Productivity and Activity Impairment (WPAI) score. Haemophilia-specific PROs: the Haemophilia-Specific Quality of Life Questionnaire for Adults (Haemo-QOL-A) and the Haemophilia Activities List score (HAL).
| PRO score | EQ-5D-5L | IPAQ | BPI short | WPAI | Haem-A-QoL | Haemo-QOL-A | HAL |
|---|---|---|---|---|---|---|---|
| Physical activity | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Mobility | ✔ | ✔ | ✔ | ✔ | |||
| Usual activities | ✔ | ✔ | ✔ | ✔ | |||
| Self-care | ✔ | ||||||
| Pain | ✔ | ✔ | ✔ | ✔ | ✔ | ||
| Emotional impact | ✔ | ✔ | ✔ | ||||
| Employment | ✔ | ✔ | ✔ | ✔ | |||
| Treatment | ✔ | ✔ | |||||
| Future | ✔ | ✔ | |||||
| Self-perception | ✔ |
Experiences of participants in haemophilia gene therapy trials
To provide meaningful counsel to individuals considering gene therapy, healthcare providers need to draw on the experiences and insights from those who have experienced haemophilia gene therapy in clinical trials. Patient-reported outcomes (PROs) have been evaluated in this setting using general and haemophilia-specific tools (Table 1).The HOPE-B study (etranacogene dezaparvovec) utilized six quality of life assessment tools to assess PROs two years after the gene therapy infusion. There were significant improvements in the Hem-A-QoL total score in the first and second years after gene therapy infusion, driven by the Treatment, Feelings, Work/School and Future domains. This demonstrates the positive impact of reduced clotting factor infusions and bleeds. The EQ-5D-5L VAS score, which assesses general health status, improved at the second year mainly due to improvements in pain and mobility. However, IPAQ, HAL and BPI scoring of physical functioning did not improve; this is believed to be due to established severe joint disease. At screening, more than 80% of the HOPE-B participants had at least one chronically damaged joint and over 18% had active target joints.The BioMarin valoctogene roxaparvovec study reported a clinically important difference (CID) in the Haemo-QoL-A Total Score first observed at week 127. The Physical Functioning domain score did not initially meet the CID threshold, but mean HAL scores, which assess self-perceived functioning related to everyday and leisure activities, improved two and four years after gene therapy infusion. A positive impact on work and classroom productivity was suggested through reduced mean impairment scores on WPAI. At year 4, in participants who had not resumed factor VIII prophylaxis, there wasImprovement in Haemo-QOL-A Physical and Role functioning scores and in the Consequences of Bleeding and Worry domains was observed, but the CID was not exceeded for the Emotional Impact domain. Overall, the PROs implied that in those with established joint disease there is ongoing potential for some functional improvement over time, but in those with advanced disease affecting multiple joints there may be minimal gain. Psychosocial benefits were demonstrated in both trial programmes with successful gene therapy expression, but it may not relieve all emotional weight.PROs provide vital information to inform the risk-benefit profile of gene therapy, and there is potential to tailor them further. A multidisciplinary international “My gene therapy experience” advisory board, including seven PwH who had participated in gene therapy trials, used their experience of gene therapy to identify where PROs should align. Overall, the ability to partake in physical activities, treatment burden and future health were considered as the most important aspects. This group describes how PROs specific for haemophilia gene therapy would allow a more holistic measure of gene therapy success that would hold more meaning for PwH.In our experience, similar to the “My gene therapy experience” PwH, most recipients reflect on the journey as positive and worthwhile, yet it is essential to prepare for potential disappointment. Gene expression can be unpredictable, and coagulation factor levels may decline unexpectedly. Prior to these trials, some outcomes were not widely anticipated, such as feelings of identity loss as a PwH and uncertainty about their place within the haemophilia community. Studies, such as the Exigency study and a national survey by the French Haemophilia Society and the French Haemophilia Resource Centre, have explored the experiences of PwH who have received gene therapy. The Exigency study highlighted that altruism motivated many participants to join trials, driven by a desire to advance treatments for the broader haemophilia community and benefit future generations, including their own grandchildren.At our centre, patients have similarly expressed a blend of altruism, a wish to “give back,” and hope to positively impact their descendants’ lives as motivation for participation. The French study revealed a strong desire among patients to reduce the burdens of injections and disease management.A significant drawback reported was the adverse effects of corticosteroids, with some participants noting insufficient information on their potential side effects and extended use post-infusion. High doses of corticosteroids are often required to control transaminitis and sustain gene expression, impacting immunity, blood sugar, weight, sleep, and especially mental health, which was cited as having the most profound impact.For those with severe haemophilia, self-administered clotting factors and routine prophylaxis provide a sense of independence and control due to the predictability of factor levels — a routine that is often disrupted following gene therapy. Alongside mistrust in the therapy’s functionality, individuals may experience anxiety and fixation on blood test results — one of our participants described self-infusing clotting factor for a bleed as “putting out a fire” and relying on the gene-derived factor when potential symptoms of a bleed arose felt like “waiting for the fire to extinguish itself.”Whether gene therapy constitutes a cure is still debated; however, it has the potential to offer a “haemophilia-free mind.” Individuals with chronic pain from haemophilic arthropathy may not entirely feel “haemophilia-free” even with normal coagulation factor levels, but often experience significant psychological relief. Everyday activities that those in good health may take for granted — such as carrying shopping without concern for joint bleeds — can feel wondrous after successful treatment. Recipients frequently report increased confidence, psychological relief, and liberation. The ability to travel worldwide without vulnerability, needles, sharps bins, or medical documentation exemplifies true freedom for many.
Conclusion
There are many components of the gene therapy pathway, and for those receiving approved haemophilia gene therapy in a real-world setting, the journey will be lengthy and complex. Recent UK guidance informed by experiences of gene therapy clinical trials addresses these factors. Alongside favourable data on bleeding events and treatment burden, the experiences of participants from PROs and reflective patient interviews can provide PwH with valuable information to support decision-making before venturing onto the pathway.
Conflicts of Interest
Dr Sara Boyce has received honoraria and speaker fees for CSL Behring and research support for Sangamo Therapeutics Ltd.
Dr Rashid Kazmi has served as a member of Advisory Boards for BioMarin, CSL Behring, Pfizer, LFB, and Novartis.
References
1. Iorio A, Stonebraker JS, Chambost H, et al. Establishing the Prevalence and Prevalence at Birth of Hemophilia in Males: A Meta-analytic Approach Using National Registries. Ann Intern Med. 2019; 171(8):540. doi:10.7326/M19-1208
2. Fornari A, Antonazzo IC, Rocino A, et al. The psychosocial impact of haemophilia from patients’ and caregivers’ point of view: The results of an Italian survey. Haemophilia. 2024;30(2):449-462. doi:10.1111/hae.14926
3. Collins PW, Obaji SG, Roberts H, Gorsani D, Rayment R. Clinical phenotype of severe and moderate haemophilia: Who should receive prophylaxis and what is the target trough level? Haemophilia. 2021;27(2):192-198. doi:10.1111/hae.14201
4. Mannucci PM. Hemophilia treatment innovation: 50 years of progress and more to come. J Thromb Haemost. 2023;21(3):403-412. doi:10.1016/j.jtha.2022.12.029
5. Srivastava A, Santagostino E, Dougall A, et al. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia. 2020;26(S6) :1-158. doi:10.1111/hae.14046
6. Leebeek FWG, Miesbach W. Gene therapy for hemophilia: a review on clinical benefit, limitations, and remaining issues. Blood. 2021;138(11):923-931. doi:10.1182/blood.2019003777
7. Mahlangu J, Oldenburg J, Paz-Priel I, et al. Emicizumab Prophylaxis in Patients Who Have Hemophilia A without Inhibitors. N Engl J Med. 2018;379(9):811-822. doi:10.1056/NEJMoa1803550
8. Matsushita T, Shapiro A, Abraham A, et al. Phase 3 Trial of Concizumab in Hemophilia with Inhibitors. N Engl J Med. 2023;389(9):783-794. doi:10.1056/NEJMoa2216455
9. Matino D, Acharya S, Palladino A, et al. Efficacy and Safety of the Anti-Tissue Factor Pathway Inhibitor Marstacimab in Participants with Severe Hemophilia without Inhibitors: Results from the Phase 3 Basis Trial. Blood. 2023;142(Supplement 1) :285-285. doi:10.1182/blood-2023-181263
10. Pasi KJ. Gene therapy for haemophilia. Br J Haematol. 2001;115(4):744-757.
doi:10.1046/j.1365-2141.2001.03225.x
11. Deshpande SR, Joseph KD, Tong J, Chen Y, Pishko A, Cuker A. Adeno-Associated Virus-based Gene Therapy for Hemophilia A and B: A Systematic Review and Meta-Analysis. Blood Adv. Published online October 7, 2024:bloodadvances.2024014111. doi:10.1182/bloodadvances.2024014111
12. Chowdary P, Duran B, Batty P, et al. UKHCDO gene therapy taskforce: Guidance for implementation of haemophilia gene therapy into routine clinical practice for adults. Haemophilia. Published online November 20, 2024:hae.15125. doi:10.1111/hae.15125
13. Fletcher S, Jenner K, Holland M, Khair K. Barriers to gene therapy, understanding the concerns people with haemophilia have: an exigency sub-study. Orphanet J Rare Dis. 2024;19(1):59. doi:10.1186/s13023-024-03068-2
14. Mingozzi F, High KA. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122(1):23-36. doi:10.1182/blood-2013-01-306647
15. Pipe SW, Leebeek FWG, Recht M, et al. Gene Therapy with Etranacogene Dezaparvovec for Hemophilia B. N Engl J Med. 2023;388(8):706-718. doi:10.1056/NEJMoa2211644
16. Young G. Induction of factor VIII tolerance by hemophilia gene transfer to eradicate factor VIII inhibitors. Blood Adv. Published online October 17, 2024:bloodadvances.2024013000. doi:10.1182/bloodadvances.2024013000
17. Krumb E, Hermans C. Living with a “hemophilia‐free mind” – The new ambition of hemophilia care? Res Pract Thromb Haemost. 2021; 5(5). doi:10.1002/rth2.12567
18. Fletcher S, Jenner K, Pembroke L, Holland M, Khair K. The experiences of people with haemophilia and their families of gene therapy in a clinical trial setting: regaining control, the Exigency study. Orphanet J Rare Dis. 2022;17(1):155. doi:10.1186/s13023-022-02256-2
19. Boyce S, Fletcher S, Jones A, et al. Educational needs of patients, families, and healthcare professionals to support the patient journey in haemophilia gene therapy in the UK. Orphanet J Rare Dis. 2023;18(1):366. doi:10.1186/s13023-023-02977-y
20. Aradom E, Gomez K. The patient gene therapy journey: Findings from qualitative interviews with trial participants at one UK haemophilia centre. J Haemoph Pract. 2021;8(1):32-44.
doi:10.17225/jhp00174
21. Ozelo MC, Mahlangu J, Pasi KJ, et al. Valoctocogene Roxaparvovec Gene Therapy for Hemophilia A. N Engl J Med. 2022;386(11):1013-1025. doi:10.1056/NEJMoa2113708
22. Itzler R, Buckner TW, Leebeek FWG, et al. Effect of etranacogene dezaparvovec on quality of life for severe and moderately severe haemophilia B participants: Results from the phase III HOPE‐B trial 2 years after gene therapy. Haemophilia. 2024; 30(3):709-719. doi:10.1111/hae.14977
23. Quinn J, Delaney KA, Wong WY, Miesbach W, Bullinger M. Psychometric Validation of the Haemo-QOL-A in Participants with Hemophilia A Treated with Gene Therapy. Patient Relat Outcome Meas. 2022;Volume 13:169-180. doi:10.2147/PROM.S357555
24. O’Mahony B, Dunn AL, Leavitt AD, et al. Health-related quality of life following valoctocogene roxaparvovec gene therapy for severe hemophilia A in the phase 3 trial GENEr8-1. J Thromb Haemost. 2023;21(12):3450-3462. doi:10.1016/j.jtha.2023.08.032
25. Leavitt AD, Mahlangu J, Raheja P, et al. Efficacy, safety, and quality of life 4 years after valoctocogene roxaparvovec gene transfer for severe hemophilia A in the phase 3 GENEr8-1 trial. Res Pract Thromb Haemost. 2024;8(8):102615. doi:10.1016/j.rpth.2024.102615
26. Rasul E, Hallock R, Hellmann M, et al. Gene Therapy in Hemophilia: A Transformational Patient Experience. J Patient Exp. 2023;10:2374373523 1193572. doi:10.1177/23743735231193573
27. Baas L, van der Graaf R, Meijer K. Can hemophilia be cured? It depends on the definition. Res Pract Thromb Haemost. 2024;8(6):102559. doi:10.1016/j.rpth.2024.102559

