Role of IL-18 in Asthma: Pathogenesis and Therapy Insights
Role of IL-18 in Asthma
Junling Wang1,2, Mengmeng Zhan1, Shihao Liu1, Siqin Wang1, Shaoheng He2- Department of Allergy, Henan Provincial People’s Hospital, Zhengzhou University People’s Hospital, No. 7, Weiwu Street, Jinshui District, Zhengzhou, Henan 450003, China.
- Allergy and Clinical Immunology Research Centre, the First Affiliated Hospital of Jinzhou Medical University, No. 2, Section 5, Renmin Street, Guta District, Jinzhou, Liaoning, 121001 Chin
OPEN ACCESS
PUBLISHED 31 December 2024
CITATION Wang, J., Zhan, M., et al., 2024. Role of IL-18 in Asthma. Medical Research Archives, [online] 12(12). https://doi.org/10.18103/mra.v12i12.6123
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI https://doi.org/10.18103/mra.v12i12.6123
ISSN 2375-1924
ABSTRACT
Asthma is a common heterogeneous disorder characterized by chronic airway inflammation, remolding and hyperresponsiveness. Although substantial advance has been achieved in understanding its complex pathogenesis, which involves genetic factors, environmental exposures, and immune system imbalances, the effective clinical therapeutics of asthma remains challenging. IL-18, a pleiotropic cytokine of IL-1 superfamily produced mainly by monocyte-macrophage system, is an imperative participant in the pathology of asthma through interacting with IL-18R, and is likely to be the potential targets for the diagnosis and therapeutics of asthma. In this review, we addressed briefly the phenotypes and endotypes of asthma. Then we introduced the regulation mechanisms of the IL-18 production, and summarized the roles of IL-18 in the pathogenesis of different asthma endotypes, in particular emphasizing the roles of IL-18 alone and IL-18 in synergy with other cytokines. Furthermore, we highlighted the effects of IL-18 on airway pathological characteristics and disorder severity of asthma. Finally, we discussed the future research directions and challenges based on IL-18 therapy, which is expected to provide novel ideas for the precise treatment of asthma.Keywords: IL-18; asthma; pathogenesis; diagnosis; therapy
1. Introduction
Asthma is a chronic respiratory disorder affecting over 300 million people worldwide, and its prevalence is increasing in economically developing counties. The main pathologic characteristics of asthma are chronic airway inflammation, airway remodeling and hyperresponsiveness, which lead to the recurrent episodes of wheezing, breath shortness, chest tightness, cough, and even life-threatening acute respiratory distress. The pathogenesis of asthma involves the interplay of genetics, environment and microbiota of host gut and airway. Additionally, imbalanced immune responses induced by various cells such as macrophages, innate lymphoid cells (ILC), helper T cells, regulatory T cells (Treg) and natural killer (NK) T cells, lead to the release of inflammatory cytokines and aggravation of inflammation in asthma. Therefore, the complex pathological mechanisms of asthma bring challenges to its clinical treatment. IL-18, a pleiotropic cytokine of IL-1 superfamily, exerts its biological activity by interacting with IL-18 receptor α (IL-18Rα)/IL-18Rβ complex, and thus activating downstream signaling pathways. Recently, the role of IL-18 in asthma has attracted much attention. This review aims to analyze the role of IL-18 in the pathogenesis of asthma, and systematically explore the effect of IL-18 on airway pathology in asthma. Meanwhile, we will also evaluate the possibility of IL-18 as a potential therapeutic target, particularly its application prospects in alleviating asthma symptoms and blocking inflammatory signaling. This review hopes to provide novel ideas for future research, reveal the potential of IL-18 in the treatment of asthma, and promote the development of precise therapeutics.2. Asthma phenotypes and endotypes
2.1 ASTHMA PHENOTYPES
Asthma is a heterogeneous respiratory condition, and asthma phenotype refers to observable characteristics resulting from the interaction between individual genetics and environment. Asthma is classified into two major phenotypes, allergic asthma and non-allergic asthma, based on observable clinical characteristics such as onset age, triggers and symptoms. In general, early-onset allergic asthma is most prevalent during childhood and into young adulthood, after which the non-allergic form predominates among adults.2.2 ASTHMA ENDOTYPES
Asthma endotypes are defined based on underlying molecular and immunological mechanisms. Understanding asthma endotypes is crucial for the development of targeted therapies and personalized treatment strategies. As suggested by Hammad H and Lambrecht BN, the chronic airway inflammation in approximately a half of asthmatics is driven by IL-4-, IL-5- and IL-13-producing Th2 cells or ILC2, which is defined as type 2-high asthma. These type 2 cytokines promote pathological features of the asthma such as eosinophilia, mucus hypersecretion, airway hyperresponsiveness, IgE production and susceptibility to exacerbations. Type 2-low asthma is dominated by non-type 2 immune responses such as Th1 and Th17 pathways, and involves IL-1β, IL-6 and neutrophilic inflammation. Usually, patients with type 2-low asthma are characterized by unresponsiveness to corticosteroids and resistance to asthma therapy.3. Biological functions of IL-18
3.1 IL-18 PRODUCTION AND REGULATION
IL-18 is constitutively expressed by monocytes, macrophages and epithelial cells. In addition, blood CD4+ T cells, basophils, mast cells, neutrophils and B cells also express IL-18. IL-18 is synthesized as an inactive precursor in the cytoplasma, while the activation and release of IL-18 depends on caspase-1, the central component of the inflammasome, which cleaves IL-18 precursor into biologically mature IL-18. The production of IL-18 is regulated by multiple signaling pathways. On the one hand, extracellular pathogen invasion and endogenous damage factors such as heat shock proteins and uric acid crystals, activate Toll-like receptors (TLR) and NOD-like receptors (NLR), which subsequently induces the activation of caspase-1 via downstream signaling cascades, and directly promotes the activation and release of IL-18. Indeed, TLR and NLR also act as a signal amplifier in inflammatory response by regulating the secretions of other proinflammation cytokine such as IL-1β, TNFα and IL-6. On the other hand, the excessive activation of TLR and NLR induces the secretion of IL-18, and further exacerbates airway chronic inflammation and hyperresponsiveness in asthma, which seems to be ameliorated by blocking TLR- and NLR-related pathways. Therefore, an in-depth understanding of the regulatory mechanism of IL-18 is helpful to reveal its role in asthma and provide potential therapeutic targets for asthma.3.2 INTERACTION AND COMPARISON OF IL-18 WITH OTHER CYTOKINES
As presented in Table 1, IL-18 closely interacts with various cytokines, and particularly exhibits its proinflammatory properties in synergy with IL-12, which induces the production of IFNγ, even Th2-type cytokine IL-9 and IL-13 from Th1 cells. IFNγ is a classical Th1-type cytokine that helps defend against pathogens such as bacteria and viruses, also exacerbates the chronic inflammation in type 2-low asthma by recruiting neutrophils.| Cytokines | Cell reactions | Involvement in asthma endotypes |
|---|---|---|
| IL-18/IL-18+IL-2/IL-18+IL-3 | Induction of IFNγ production from Th1 cells and IL-4, IL-5, IL-9 and IL-13 from Th2 cells, mast cells and eosinophils. | Involved in type 2-high asthma |
| IL-18+IL-4 | Induction of IgE production from B cells. | Involved in type 2-high asthma |
| IL-18+IL-5 | Induction of eosinophil development, transformation and maturation. | Involved in type 2-high asthma |
| IL-18+IL-12 | Induction of IFNγ, IL-9 and IL-13 production. | Involved in both type 2-high and -low asthma |
| IL-18+IL-15 | Induction of NK cell proliferation. | Involved in both type 2-high and -low asthma |
| IL-18+IL-12+IL-15 | Induction of NK cell death. | Involved in both type 2-high and -low asthma |
| IL-18+IL-23 | Induction of Th17 cell differentiation, and IL-17A and IL-22 production. | Involved in type 2-low asthma |
4. The role of IL-18 in the pathological characteristics of asthma
4.1 IL-18 AND AIRWAY INFLAMMATION
Airway inflammation in asthma results from various cellular inflammation. It has been demonstrated that IL-18 alone activates neutrophils to release CXCL8, activates airway epithelial cells to release MCP-1α, activates eosinophils to release IL-6, CXCL8 and CCL2, and in synergy with anti-CD3 induces Th1 cells to release CXCL8 and IL-13. These cytokines can induce substantial neutrophil and eosinophil recruitment in the airway, and thereby exacerbating the local inflammatory response. In fact, IL-18 also enhances eosinophil survival though without modulation effects on neutrophil survival. Additionally, elevated level of IL-18 in asthma patients induces mast cells and basophils to release a variety of mediators such as histamine and prostaglandins, leukotrienes, consequently leading to increased vascular permeability, mucosal edema and mucus secretion, and thereby exacerbating airway constriction and hyperresponsiveness. More importantly, IL-18-deficient mice exhibit diminished chronic inflammation and airway remodeling in ovalbumin (OVA)-induced asthma model. Furthermore, the inflammasome activation in asthma also contributes to the release of another proinflammatory cytokine IL-1β, which induces airway epithelial cells release plentiful chemokines such as CXCL8, CCL5, MCP-1α and MCP-1β. Therefore, the proinflammatory effect of IL-18 plays an important role in airway inflammation exacerbation of asthma, which makes IL-18 a key factor that maintains the chronic inflammation.4.2 IL-18 AND AIRWAY REMODELING
Airway remodeling is one of the common chronic pathological changes in asthma, which is characterized by the thickening of the airway induced by airway epithelial cell proliferation, matrix protein deposition and smooth muscle cell hypertrophy, thus resulting in persistent, irreversible airflow obstruction. Indeed, IL-18 has been demonstrated to induce bronchial epithelial cell activation and differentiation, airway fibrosis and mucus metaplasia via exacerbating chronic inflammation in the airways. Additionally, IL-18 also promotes the production of matrix metalloproteinases (MMP) such as MMP1, MMP2 and MMP9, which contribute to the degradation and deposition of the extracellular matrix and angiogenesis, and thus inducing airway wall thickness, airway constriction and airflow limitation. Notably, IL-18-deficient mice exhibit diminished airway remodeling in OVA-induced asthma model. These suggest IL-18-mediated pathological changes affect airway structure.4.3 IL-18 AND AIRWAY HYPERRESPONSIVENESS
Airway hyperresponsiveness is the core pathological feature of asthma, which refers to the abnormal sensitivity of the airway to environmental stimuli such as allergens and cold air. Actually, airway hyperresponsiveness results from airway remodeling in asthma. IL-18 plays an important role in airway hyperresponsiveness via various mechanisms. On the one hand, IL-18 seems to promote the contraction of airway smooth muscle cells, thereby triggering bronchospasm and leading to the symptoms of wheezing and dyspnea in asthmatic patients. On the other hand, IL-18 induces the production of IL-13 and IL-4 from activated Th2 cells, basophils and mast cells, which exacerbates airway inflammation and hyperresponsiveness. Furthermore, blocking IL-18 signaling NLRP3/caspase-1 seems to alleviate airway hyperresponsiveness in mouse asthma model, and the airway hyperresponsiveness is inhibited in IL-18-deficient mice, indicating the critical role of IL-18 in asthma pathology.4.4 IL-18 AND ASTHMA SEVERITY
Studies based on human demonstrate that the gene polymorphism of IL-18 is not only relevant to asthma, but also to the severity of asthmatic adults. While IL18R1 gene is negatively associated with lung function and is highly expressed in severe asthma patients. Elevated serum level of IL-18 in adults with moderate and severe asthma but not in children with asthma is correlated with asthma attack. Upregulated IL-18 and IL-18R expressions are observed in the lungs of fatal asthma patients but not of well-controlled mild asthma patients. However, these characteristics are not applicable to asthmatic children. Particularly, serum IL-18 levels in patients with mild and moderate asthma exacerbation decrease after therapy and low serum IL-18 level may predict the effectiveness of Dupilumab in severe asthma. Furthermore, IL-18 level in sputum of patients with asthma and with severe eosinophilic asthma is elevated, which is lower in patients with severe refractory asthma than that with mild asthma. On the contrary, IL-18 level in bronchoalveolar lavage fluid is lower than that in healthy control subjects, and high serum free IL-18 is associated with decreased omalizumab efficacy. These suggest that serum IL-18 level and IL-18 gene polymorphism are more likely to be used as a potential biomarker to reflect asthma severity. Studies based on mice reveal that IL-18 increases allergic sensitization, serum IgE, Th2 cytokines and airway eosinophilia in asthmatic mice. The IL-18 gene knockout and NLRP3 inflammasome inhibition alleviate airway inflammation, remodeling and hyperresponsiveness. Till now, there is only one report that IL-18 deficiency selectively enhances allergen-induced eosinophilia and lung damage in mice. These further support the potential of IL-18 as a therapeutic target in asthma.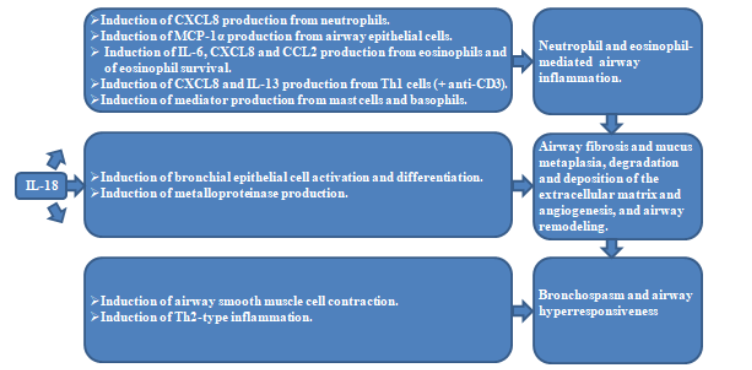
5. Future research directions and challenges
5.1 POTENTIAL RESEARCH DIRECTIONS
Future studies can further explore the specific role of IL-18 in different asthma phenotypes and endotypes, in particular the differences in the performance of refractory asthma and atypical asthma, e.g., patients with refractory asthma often do less respond to conventional therapeutics and may have unique inflammatory mechanisms in which the specific role of IL-18 has not been fully defined. These studies may help identify more effective therapeutic targets and provide novel ideas for the development of more precise therapy. In addition, the synergistic mechanism of IL-18 with other proinflammatory cytokines is also a direction worthy of further exploration. IL-18 interacts with IL-4, IL-33 and other cytokines to form a multi-factor network and regulate airway inflammation and hyperresponsiveness. Understanding the interaction between these factors, particularly the specific signaling pathways in different immune cells will reveal the multi-level effects of IL-18 in asthma, and provide a more comprehensive understanding of the complex mechanism of IL-18 and a richer scientific basis for the multi-target therapeutics of asthma.5.2 RESEARCH CHALLENGES
Indeed, IL-18-related preparations have been applied in clinical trials for the therapy of inflammatory dermatological disease. Although the development of IL-18 as a therapeutic target for asthma is promising, its practical application still faces many technical and clinical challenges. Firstly, how to mimic the complex mechanism of IL-18 in vivo accurately is an urgent problem to be figured out. Given that IL-18 has multiple functions in inflammatory response and immune regulation, how to reproduce its multi-level roles effectively in animal models is crucial to understand its specific mechanism in the pathogenesis of asthma. The development of experimental techniques such as more precise gene editing techniques and three-dimensional tissue culture models, may help to better understand the complex biological roles of IL-18. On the other hand, how to reduce the potential impact of IL-18 inhibitor or IL-18R blocking on immune system is an important issue that is worthy of deep consideration. The complete inhibition of IL-18 activity or IL-18R blocking is likely to impair the individual ability to defense pathogen. Therefore, future research is warranted to find precise methods that can effectively suppress airway inflammation without impairing immune function. In addition, the safety and efficacy of IL-18 in clinical application still need to be further verified in human trials. Although current preclinical studies have shown its potential efficacy, there are not enough data to support its full clinical application. Future clinical trials with more rigorous design are warranted to evaluate the long-term effects of different doses and different durations of medication on patients to ensure that the treatment is both safe and effective. The resolution of these challenges will be the key to the successful application of IL-18 targeted therapy in asthma.6. Conclusions
IL-18 plays a key role in the pathogenesis and development of asthma by inducing airway inflammation, remodeling and hyperresponsiveness. These effects make IL-18 a potential therapeutic target and provide a new possibility for the treatment of asthma in the future. However, although the potential of IL-18-targeted therapy is gradually being recognized, its clinical application still needs to be supported by more experimental data and clinical trials. Particularly in terms of efficacy and safety, further verification and refinement are warranted.Conflicts of interest
The authors have no conflicts of interest to declare.Acknowledgement
This project was sponsored by the Key Research and Development and Promotion Special Foundation of Henan Province (232102311022); Foundation for Doctoral Scientific Research of Henan Province (HN2022097); National Natural Science Foundation of China (81471592).References
- Schleich F, Bougard N, Moermans C, Sabbe M, Louis R. Cytokine-targeted therapies for asthma and COPD. Eur Respir Rev. 2023;32(168)doi:10.1183/16000617.0193-2022
- Asthma GIf. 2024 GINA Main Report. Oct 25, 2024, Accessed Jul 12, 2024, https://ginasthma.org/2024-report/
- Sharma BS, Shekhawat DS, Sharma P, Meena C, Mohan H. Acute Respiratory Distress in Children: Croup and Acute Asthma. Indian J Pediatr. 2015;82(7):629-36. doi:10.1007/s12098-014-1559-4
- Tang HHF, Teo SM, Sly PD, Holt PG, Inouye M. The intersect of genetics, environment, and microbiota in asthma-perspectives and challenges. J Allergy Clin Immunol. 2021;147(3):781-793. doi:10.1016/j.jaci.2020.08.026
- Luo M, Zhao F, Cheng H, Su M, Wang Y. Macrophage polarization: an important role in inflammatory diseases. Front Immunol. 2024;15:1352946. doi:10.3389/fimmu.2024.1352946
- Wang X, Kong Y, Zheng B, et al. Tissue-resident innate lymphoid cells in asthma. J Physiol. 2023;601(18):3995-4012. doi:10.1113/jp284686
- Ji T, Li H. T-helper cells and their cytokines in pathogenesis and treatment of asthma. Front Immunol. 2023;14:1149203. doi:10.3389/fimmu.2023.1149203
- Boonpiyathad T, Sözener ZC, Akdis M, Akdis CA. The role of Treg cell subsets in allergic disease. Asian Pac J Allergy Immunol. 2020;38(3):139-149. doi:10.12932/ap-030220-0754
- Gutiérrez-Vera C, García-Betancourt R, Palacios PA, et al. Natural killer T cells in allergic asthma: Implications for the development of novel immunotherapeutical strategies. Front Immunol. 2024;15:1364774. doi:10.3389/fimmu.2024.1364774
- Dinarello CA. Interleukin-18. Methods. 1999;19(1):121-32. doi:10.1006/meth.1999.0837
- Kuruvilla ME, Lee FE, Lee GB. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin Rev Allergy Immunol. 2019;56(2):219-233. doi:10.1007/s12016-018-8712-1
- Hammad H, Lambrecht BN. The basic immunology of asthma. Cell. 2021;184(6):1469-1485. doi:10.1016/j.cell.2021.02.016
- Wang J, Zhan M, Zhai Y, et al. Allergens induce upregulated IL-18 and IL-18Rα expression in blood Th2 and Th17 cells of patients with allergic asthma. Clin Exp Immunol. 2024;217(1):31-44. doi:10.1093/cei/uxae022
- Wang Z, Liu Z, Wang L, et al. Altered expression of IL-18 binding protein and IL-18 receptor in basophils and mast cells of asthma patients. Scand J Immunol. 2018;87(5):e12658. doi:10.1111/sji.12658
- Zhang H, Wang J, Wang L, Xie H, Chen L, He S. Role of IL-18 in atopic asthma is determined by balance of IL-18/IL-18BP/IL-18R. J Cell Mol Med. 2018;22(1):354-373. doi:10.1111/jcmm.13323
- Ghayur T, Banerjee S, Hugunin M, et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature. 1997;386(6625):619-23. doi:10.1038/386619a0
- Netea MG, van de Veerdonk FL, Kullberg BJ, Van der Meer JW, Joosten LA. The role of NLRs and TLRs in the activation of the inflammasome. Expert Opin Biol Ther. 2008;8(12):1867-72. doi:10.1517/14712590802494212
- Triantafilou K, Ward CJK, Czubala M, et al. Differential recognition of HIV-stimulated IL-1β and IL-18 secretion through NLR and NAIP signalling in monocyte-derived macrophages. PLoS Pathog. 2021;17(4):e1009417. doi:10.1371/journal.ppat.1009417
- Babamale AO, Chen ST. Nod-like Receptors: Critical Intracellular Sensors for Host Protection and Cell Death in Microbial and Parasitic Infections. Int J Mol Sci. 2021;22(21)doi:10.3390/ijms222111398
- Borges PV, Moret KH, Raghavendra NM, et al. Protective effect of gedunin on TLR-mediated inflammation by modulation of inflammasome activation and cytokine production: Evidence of a multitarget compound. Pharmacol Res. 2017;115:65-77. doi:10.1016/j.phrs.2016.09.015
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805-20. doi:10.1016/j.cell.2010.01.022
- Fukata M, Vamadevan AS, Abreu MT. Toll-like receptors (TLRs) and Nod-like receptors (NLRs) in inflammatory disorders. Semin Immunol. 2009;21(4):242-53. doi:10.1016/j.smim.2009.06.005
- Yang J, Zhang M, Luo Y, et al. Protopine ameliorates OVA-induced asthma through modulating TLR4/MyD88/NF-κB pathway and NLRP3 inflammasome-mediated pyroptosis. Phytomedicine. 2024;126:155410. doi:10.1016/j.phymed.2024.155410
- Yang Z, Li X, Wei L, et al. Involucrasin B suppresses airway inflammation in obese asthma by inhibiting the TLR4-NF-κB-NLRP3 pathway. Phytomedicine. 2024;132:155850. doi:10.1016/j.phymed.2024.155850
- Li M, Wang C, Xu WT, Zhong X. Sodium houttuyfonate plays a protective role in the asthmatic airway by alleviating the NLRP3-related pyroptosis and Th1/Th2 immune imbalance. Mol Immunol. 2023;160:103-111. doi:10.1016/j.molimm.2023.06.013
- Lu HF, Zhou YC, Hu TY, et al. Unraveling the role of NLRP3 inflammasome in allergic inflammation: implications for novel therapies. Front Immunol. 2024;15:1435892. doi:10.3389/fimmu.2024.1435892
- Yasuda K, Nakanishi K, Tsutsui H. Interleukin-18 in Health and Disease. Int J Mol Sci. 2019;20(3)doi:10.3390/ijms20030649
- Sugimoto T, Ishikawa Y, Yoshimoto T, Hayashi N, Fujimoto J, Nakanishi K. Interleukin 18 acts on memory T helper cells type 1 to induce airway inflammation and hyperresponsiveness in a naive host mouse. J Exp Med. 2004;199(4):535-45. doi:10.1084/jem.20031368
- Akdis CA, Arkwright PD, Brüggen MC, et al. Type 2 immunity in the skin and lungs. Allergy. 2020;75(7):1582-1605. doi:10.1111/all.14318
- Chen L, Hou W, Liu F, et al. Blockade of NLRP3/Caspase-1/IL-1β Regulated Th17/Treg Immune Imbalance and Attenuated the Neutrophilic Airway Inflammation in an Ovalbumin-Induced Murine Model of Asthma. J Immunol Res. 2022;2022:9444227. doi:10.1155/2022/9444227
- Mills KH, Dungan LS, Jones SA, Harris J. The role of inflammasome-derived IL-1 in driving IL-17 responses. J Leukoc Biol. 2013;93(4):489-97. doi:10.1189/jlb.1012543
- Revu S, Wu J, Henkel M, et al. IL-23 and IL-1β Drive Human Th17 Cell Differentiation and Metabolic Reprogramming in Absence of CD28 Costimulation. Cell Rep. 2018;22(10):2642-2653. doi:10.1016/j.celrep.2018.02.044
- Ito T, Hirose K, Nakajima H. Bidirectional roles of IL-22 in the pathogenesis of allergic airway inflammation. Allergol Int. 2019;68(1):4-8. doi:10.1016/j.alit.2018.10.002
- French AR, Holroyd EB, Yang L, Kim S, Yokoyama WM. IL-18 acts synergistically with IL-15 in stimulating natural killer cell proliferation. Cytokine. 2006;35(5-6):229-34. doi:10.1016/j.cyto.2006.08.006
- Lepretre F, Gras D, Chanez P, Duez C. Natural killer cells in the lung: potential role in asthma and virus-induced exacerbation? Eur Respir Rev. 2023;32(169)doi:10.1183/16000617.0036-2023
- Terrén I, Sandá V, Amarilla-Irusta A, et al. IL-12/15/18-induced cell death and mitochondrial dynamics of human NK cells. Front Immunol. 2023;14:1211839. doi:10.3389/fimmu.2023.1211839
- Leung BP, Culshaw S, Gracie JA, et al. A role for IL-18 in neutrophil activation. J Immunol. 2001;167(5):2879-86. doi:10.4049/jimmunol.167.5.2879
- Brabcová E, Kolesár L, Thorburn E, Stříž I. Chemokines induced in human respiratory epithelial cells by IL-1 family of cytokines. Folia Biol (Praha). 2014;60(4):180-6.
- Chow JY, Wong CK, Cheung PF, Lam CW. Intracellular signaling mechanisms regulating the activation of human eosinophils by the novel Th2 cytokine IL-33: implications for allergic inflammation. Cell Mol Immunol. 2010;7(1):26-34. doi:10.1038/cmi.2009.106
- Wang W, Tanaka T, Okamura H, et al. Interleukin-18 enhances the production of interleukin-8 by eosinophils. Eur J Immunol. 2001;31(4):1010-6. doi:10.1002/1521-4141(200104)31:4<1010::aid-immu1010>3.0.co;2-8
- Hata H, Yoshimoto T, Hayashi N, Hada T, Nakanishi K. IL-18 together with anti-CD3 antibody induces human Th1 cells to produce Th1- and Th2-cytokines and IL-8. Int Immunol. 2004;16(12):1733-9. doi:10.1093/intimm/dxh174
- Yoshimoto T, Nakanishi K. Roles of IL-18 in basophils and mast cells. Allergol Int. 2006;55(2):105-13. doi:10.2332/allergolint.55.105
- Yamagata S, Tomita K, Sato R, Niwa A, Higashino H, Tohda Y. Interleukin-18-deficient mice exhibit diminished chronic inflammation and airway remodelling in ovalbumin-induced asthma model. Clin Exp Immunol. 2008;154(3):295-304. doi:10.1111/j.1365-2249.2008.03772.x
- Thomas LH, Wickremasinghe MI, Friedland JS. IL-1 beta stimulates divergent upper and lower airway epithelial cell CCL5 secretion. Clin Immunol. 2007;122(2):229-38. doi:10.1016/j.clim.2006.10.004
- Joseph C, Tatler AL. Pathobiology of Airway Remodeling in Asthma: The Emerging Role of Integrins. J Asthma Allergy. 2022;15:595-610. doi:10.2147/jaa.S267222
- Kaur D, Chachi L, Gomez E, Sylvius N, Brightling CE. Interleukin-18, IL-18 binding protein and IL-18 receptor expression in asthma: a hypothesis showing IL-18 promotes epithelial cell differentiation. Clin Transl Immunology. 2021;10(6):e1301. doi:10.1002/cti2.1301
- Kang MJ, Choi JM, Kim BH, et al. IL-18 induces emphysema and airway and vascular remodeling via IFN-γ, IL-17A, and IL-13. Am J Respir Crit Care Med. 2012;185(11):1205-17. doi:10.1164/rccm.201108-1545OC
- Wang F, Guan M, Wei L, Yan H. IL‑18 promotes the secretion of matrix metalloproteinases in human periodontal ligament fibroblasts by activating NF‑κB signaling. Mol Med Rep. 2019;19(1):703-710. doi:10.3892/mmr.2018.9697
- Bajbouj K, Ramakrishnan RK, Hamid Q. Role of Matrix Metalloproteinases in Angiogenesis and Its Implications in Asthma. J Immunol Res. 2021;2021:6645072. doi:10.1155/2021/6645072
- Bradding P, Porsbjerg C, Côté A, Dahlén SE, Hallstrand TS, Brightling CE. Airway hyperresponsiveness in asthma: The role of the epithelium. J Allergy Clin Immunol. 2024;153(5):1181-1193. doi:10.1016/j.jaci.2024.02.011
- Vignola AM, Gagliardo R, Siena A, et al. Airway remodeling in the pathogenesis of asthma. Curr Allergy Asthma Rep. 2001;1(2):108-15. doi:10.1007/s11882-001-0077-4
- Ke Q, Yang L, Cui Q, et al. Ciprofibrate attenuates airway remodeling in cigarette smoke-exposed rats. Respir Physiol Neurobiol. 2020;271:103290. doi:10.1016/j.resp.2019.103290
- Ishikawa Y, Yoshimoto T, Nakanishi K. Contribution of IL-18-induced innate T cell activation to airway inflammation with mucus hypersecretion and airway hyperresponsiveness. Int Immunol. 2006;18(6):847-55. doi:10.1093/intimm/dxl021
- Yoshimoto T, Tsutsui H, Tominaga K, et al. IL-18, although antiallergic when administered with IL-12, stimulates IL-4 and histamine release by basophils. Proc Natl Acad Sci U S A. 1999;96(24):13962-6. doi:10.1073/pnas.96.24.13962
- Chen LX, Xu CM, Gao F, et al. Associations of IL-18 and IL-9 expressions and gene polymorphisms with asthma. Eur Rev Med Pharmacol Sci. 2020;24(12):6931-6938. doi:10.26355/eurrev_202006_21684
- El-Husseini ZW, Vonk JM, van den Berge M, Gosens R, Koppelman GH. Association of asthma genetic variants with asthma-associated traits reveals molecular pathways of eosinophilic asthma. Clin Transl Allergy. 2023;13(4):e12239. doi:10.1002/clt2.12239
- Harada M, Obara K, Hirota T, et al. A functional polymorphism in IL-18 is associated with severity of bronchial asthma. Am J Respir Crit Care Med. 2009;180(11):1048-55. doi:10.1164/rccm.200905-0652OC
- Camiolo MJ, Zhou X, Wei Q, et al. Machine learning implicates the IL-18 signaling axis in severe asthma. JCI Insight. 2021;6(21)doi:10.1172/jci.insight.149945
- Wang J, Zhang H, Zheng W, et al. Correlation of IL-18 with Tryptase in Atopic Asthma and Induction of Mast Cell Accumulation by IL-18. Mediators Inflamm. 2016;2016:4743176. doi:10.1155/2016/4743176
- Hossny EM, El-Sayed SS, El-Hadidi ES, Moussa SR. Serum interleukin-18 expression in children with bronchial asthma. World Allergy Organ J. 2009;2(5):63-8. doi:10.1097/WOX.0b013e3181a33649
- Oda H, Kawayama T, Imaoka H, et al. Interleukin-18 expression, CD8(+) T cells, and eosinophils in lungs of nonsmokers with fatal asthma. Ann Allergy Asthma Immunol. 2014;112(1):23-28.e1. doi:10.1016/j.anai.2013.09.004
- Ezzat DA, Morgan DS, Mohamed RA, Mohamed AF. Genetic association of interleukin 18 (-607C/A, rs1946518) single nucleotide polymorphism with asthmatic children, disease severity and total IgE serum level. Cent Eur J Immunol. 2019;44(3):285-291. doi:10.5114/ceji.2019.89603
- Lachheb J, Chelbi H, Ammar J, Hamzaoui K, Hamzaoui A. Promoter polymorphism of the IL-18 gene is associated with atopic asthma in Tunisian children. Int J Immunogenet. 2008;35(1):63-8. doi:10.1111/j.1744-313X.2007.00738.x
- Shaaban HH, Mohy AM, Abdel-Razek AR, Wahab AA. Interleukin-18 -607C/A gene polymorphism in Egyptian asthmatic children. Mol Diagn Ther. 2014;18(4):427-34. doi:10.1007/s40291-014-0097-0
- Tanaka H, Miyazaki N, Oashi K, et al. IL-18 might reflect disease activity in mild and moderate asthma exacerbation. J Allergy Clin Immunol. 2001;107(2):331-6. doi:10.1067/mai.2001.112275
- Watanabe S, Suzukawa M, Tashimo H, et al. Low Serum IL-18 Levels May Predict the Effectiveness of Dupilumab in Severe Asthma. Intern Med. 2024;63(2):179-187. doi:10.2169/internalmedicine.1808-23
- Mukherjee M, Bulir DC, Radford K, et al. Sputum autoantibodies in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2018;141(4):1269-1279. doi:10.1016/j.jaci.2017.06.033
- Rovina N, Dima E, Bakakos P, et al. Low interleukin (IL)-18 levels in sputum supernatants of patients with severe refractory asthma. Respir Med. 2015;109(5):580-7. doi:10.1016/j.rmed.2015.03.002
- Morimoto C, Matsumoto H, Tajiri T, et al. High serum free IL-18 is associated with decreased omalizumab efficacy: findings from a 2-year omalizumab treatment study. J Asthma. 2021;58(9):1133-1142. doi:10.1080/02770903.2020.1766061
- Wild JS, Sigounas A, Sur N, et al. IFN-gamma-inducing factor (IL-18) increases allergic sensitization, serum IgE, Th2 cytokines, and airway eosinophilia in a mouse model of allergic asthma. J Immunol. 2000;164(5):2701-10. doi:10.4049/jimmunol.164.5.2701
- Yu CX, Shi ZA, Ou GC, et al. Maresin-2 alleviates allergic airway inflammation in mice by inhibiting the activation of NLRP3 inflammasome, Th2 type immune response and oxidative stress. Mol Immunol. 2022;146:78-86. doi:10.1016/j.molimm.2022.03.118
- Fu Y, Huang FY, Dai SZ, et al. Penicilazaphilone C alleviates allergic airway inflammation and improves the immune microenvironment by hindering the NLRP3 inflammasome. Biomed Pharmacother. 2024;175:116788. doi:10.1016/j.biopha.2024.116788
- Kodama T, Matsuyama T, Kuribayashi K, et al. IL-18 deficiency selectively enhances allergen-induced eosinophilia in mice. J Allergy Clin Immunol. 2000;105(1 Pt 1):45-53. doi:10.1016/s0091-6749(00)90176-3
- Wang X, Wang L, Wen X, Zhang L, Jiang X, He G. Interleukin-18 and IL-18BP in inflammatory dermatological diseases. Front Immunol. 2023;14:955369. doi:10.3389/fimmu.2023.955369
- Kim S, Yu H, Azam T, Dinarello CA. Interleukin-18 Binding Protein (IL-18BP): A Long Journey From Discovery to Clinical Application. Immune Netw. 2024;24(1):e1. doi:10.4110/in.2024.24.e1
Interested in publishing your own research?
ESMED members can publish their research for free in our peer-reviewed journal.
Learn About Membership

