Inflammation-Associated Disease Progression in B-ALL
Inflammation-Associated Extramedullary Disease Progression Following Tisagenlecleucel in a Pediatric Patient with B-ALL: A Case Report
April L. Rahrig1, Jessica Harsin1, Stacey Woodburn1, Magdalena Czader2, Audrey Hopper3, Jodi L. Skiles1*
- Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, USA
- Department of Pathology and Laboratory Medicine, Indiana University School of Medicine, Indianapolis, IN, USA
- Department of Pediatrics, Riley Hospital for Children at IU Health, Indianapolis, IN, USA
OPEN ACCESS
PUBLISHED: 31 December 2024
CITATION: RAHRIG, April L. et al. Inflammation-Associated Extramedullary Disease Progression Following Tisagenlecleucel in a Pediatric Patient with B-ALL: A Case Report. Medical Research Archives, [S.l.], v. 12, n. 12, dec. 2024. Available at: <https://esmed.org/MRA/mra/article/view/6124>.
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.18103/mra.v12i12.6124
ISSN 2375-1924
ABSTRACT
Background: Tisagenlecleucel is a chimeric antigen receptor (CAR)-T cell therapy approved for the treatment of pediatric and young adult patients with B-cell acute lymphoblastic leukemia (B-ALL) that is refractory or in second or later relapse (r/r). Although tisagenlecleucel has proven efficacy and a manageable safety profile, pseudoprogression and eventual disease progression at extramedullary sites following tisagenlecleucel infusion have been reported. Depending on the site of extramedullary disease, inflammation around the extramedullary sites and/or progression may not be clinically tolerated in some patients.
Case Report: Herein we describe a 10-year-old female patient with r/r B-ALL following multiple lines of therapy. The patient was treated with tisagenlecleucel and subsequently developed pseudoprogression at extramedullary disease sites. Post infusion the patient experienced progressive swelling at extramedullary disease sites including intracranial, periorbital, scalp, retroperitoneal, intra-abdominal, and gingival lesions. A variety of anti-inflammatory agents were utilized to manage swelling, including tocilizumab, siltuximab, and corticosteroids. Although tocilizumab at first resulted in a rapid decrease in the size of visible subcutaneous scalp lesions, each subsequent dose was found to be progressively less effective, causing the patient to suffer due to the location and extent of enlarged extramedullary lesions. After continued growth of the lesions, biopsy revealed areas of necrotic inflammation as well as areas of continued leukemia involvement concerning for refractory disease. Ultimately, this patient died of complications of worsening leukemic progression at extramedullary sites.
Conclusion: Although tocilizumab is effective for the treatment of adverse events associated with CAR-T cell therapy, this case highlights unmet needs related to the identification and treatment of patients who will develop pseudoprogression, and potentially disease progression, at extramedullary sites post tisagenlecleucel infusion.
INTRODUCTION
B-cell acute lymphoblastic leukemia (B-ALL), the most common type of ALL, is a heterogenous disease characterized by immature lymphoid cell proliferation. In the United States (US), the median age at ALL diagnosis is 17 years, with 53% of newly diagnosed patients being less than 20 years of age. Although ALL only accounts for 0.3% of all new cancers in the US each year, ALL is the most common pediatric cancer. Five-year survival rates for children diagnosed with ALL have increased to >90%. Much of the improvement in survival can be attributed to advances in treatment options, although treatment of relapsed and refractory (r/r) B-ALL has been challenging and can have poor survival rates, as low as 10%. In 2017 the US Food and Drug Administration approved tisagenlecleucel (an autologous cluster of differentiation [CD]19-directed chimeric antigen receptor [CAR]-T cell therapy) for the treatment of pediatric and young adult patients with B-ALL that is refractory or in second or later relapse. This now offers patients with r/r B-ALL another treatment option.
CAR-T cell therapy is an immunotherapy that modifies T cells to target and eliminate cancer cells. Tisagenlecleucel has proven efficacy and tolerable safety in pediatric patients with r/r CD19+ B-ALL. In the pivotal Phase II ELIANA trial (NCT02435849), overall remission rate within 3 months was 81%, with all responding patients negative for minimal residual disease. Twelve-month event-free survival (EFS) and overall survival (OS) were 50% and 76%, respectively. EFS was 44% and OS was 63% at 3 years. Despite the relative safety and efficacy of CAR-T cell therapy, some patients may experience immune and inflammatory cell accumulation at lesions that appear as disease progression by imaging modalities, including positron emission tomography (PET)-computed tomography (CT) scans. Such inflammation-associated pseudoprogression (TIAP) has been reported in several patients with hematologic malignancies. Although tocilizumab is the standard of care for managing cytokine release syndrome (CRS), it is unclear how tocilizumab may affect clinical manifestation of TIAP. Currently, there is limited literature on TIAP followed by disease progression. Herein we describe the clinical progression of TIAP and ultimate disease progression in a pediatric patient with r/r B-ALL following CAR-T cell therapy and repeated tocilizumab dosing.
Case Report
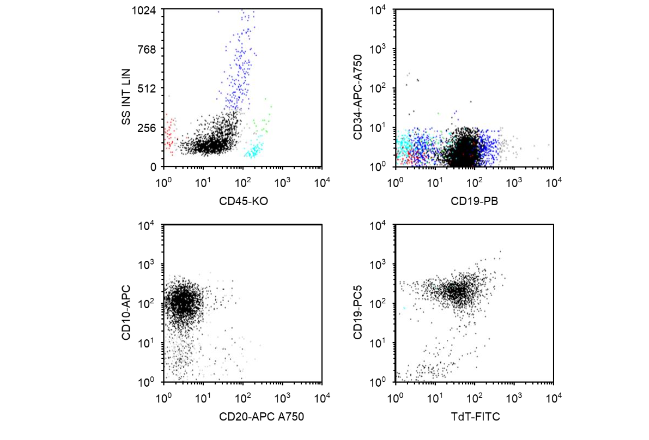
A 10-year-old Hispanic female was originally diagnosed at the age of 7 years with B-ALL with t(1;19)(q23;p13.3); TCF3-PBX cytogenetic abnormalities. Flow cytometry (FCM) analysis showed that the patient’s lymphoblasts were positive for CD19, CD10, and terminal deoxynucleotidyl transferase, which are diagnostic for B-ALL; CD34 antigen was negative (Figure 1). Next-generation sequencing at original diagnosis showed mutations of ASXL1, ATRX, PHF6, EZH2, and RAD21. The patient was treated initially with a dexamethasone-based (6 mg/m²/d, days 1–28) 3-drug induction regimen with pegaspargase (2500 IU/m², day 4), vincristine (1.5 mg/m² intravenous [IV], days 1, 8, 15, and 22), and cytarabine (70 mg intrathecal, day 1) for newly diagnosed standard-risk B-ALL per Children’s Oncology Group protocol AALL0932. She was FCM minimal residual disease (MRD)–negative at day 8 (peripheral blood) and day 29 (bone marrow). The patient’s disease relapsed approximately 2 years after diagnosis while she was receiving maintenance chemotherapy.
Figure 1. Flow cytometry analysis at the time of original diagnosis. Patient lymphoblasts (black dots) were positive for CD19, CD10, and TdT, which are diagnostic for B lymphoblastic leukemia/lymphoma. CD34 antigen was negative. APC, allophycocyanin; CD, cluster of differentiation; FITC, fluorescein isothiocyanate; KO, krome orange; PB, Pacific Blue®; PC5, phycoerythrin cyanin 5.1; SS INT LIN, side scatter intensity, linear; TdT, terminal deoxynucleotidyl transferase.
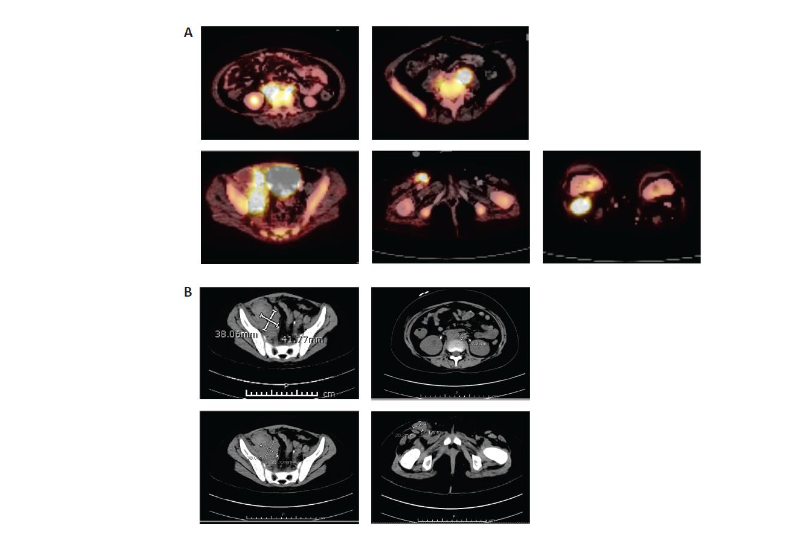
At relapse, she presented with eye pain and swelling, fever, diffuse pain, and blasts in the peripheral blood. Her bone marrow showed over 90% B-cell leukemia blasts that were CD19+ whereas her cerebrospinal fluid was negative. A CT scan to evaluate her eye pain was performed and showed preseptal soft tissue stranding over the left globe, but no signs of orbital cellulitis or overt extramedullary disease. She was treated for preseptal cellulitis, and her symptoms improved.The patient was reinduced with mitoxantrone, vincristine, asparaginase erwinia chrysanthemi, and dexamethasone per COG protocol AALL1331. Following reinduction therapy, her day 35 bone marrow evaluation revealed 47.5% blasts by FCM MRD (CD19+, CD22+ blasts). Given her r/r CD19+ B-ALL, the decision was made to pursue CAR-T cell therapy with tisagenlecleucel. Leukapheresis was performed after recovery from reinduction therapy without any difficulty. She had no peripheral blasts at the time of T-cell collection. There were no complications with the manufacturing of the product.She was then treated with inotuzumab. Disease evaluation after one cycle revealed FCM 6.2% CD19+ leukemia blasts. At this time, she had biopsy-proven new extramedullary disease noted on imaging in her inguinal, retroperitoneal, and cervical lymph nodes as well as in her calvarial bones, left zygomatic arch, and maxillary sinus. Given her suboptimal extramedullary disease control, the patient received aggressive bridging therapy with etoposide and cyclophosphamide followed by salvage escalating-dose blinatumomab.After one cycle of blinatumomab, bone marrow was FCM MRD positive (0.9% CD19+, CD22– blasts) and magnetic resonance imaging evaluation of extramedullary disease sites revealed disease progression (increase in right inguinal nodal mass, stable right iliac and retroperitoneal lymph nodes, progression of soft tissue mass in body orbits that extended into the superior and lateral extraconal orbits, and persistent calvarial marrow involvement with new soft tissue lesions within the left frontal and right temporal calvaria) that was biopsy proven (CD19+ blasts in right inguinal groin lymph node). She developed persistent fevers and clinical concerns for hemophagocytic lymphohistiocytosis and received intravenous (IV) immunoglobulin dexamethasone, and etoposide to control these symptoms during bridging therapy prior to CAR-T cell therapy.Disease staging prior to lymphodepleting chemotherapy demonstrated bone marrow FCM MRD 0.9% CD19+ blasts. A PET scan pre-tisagenlecleucel infusion (Figure 2) revealed periorbital, gingival, extra-axial, calvarial, sinus, inguinal, retroperitoneal, and diffuse bony disease involvement.
Figure 2. Pre–CAR-T cell therapy abdominal (A) PET and (B) CT scans. CAR, chimeric antigen receptor; CT, computed tomography; PET, positron emission tomography.
TREATMENT
Lymphodepletion chemotherapy (fludarabine (30 mg/m²/day IV for 4 days, cyclophosphamide 500 mg/m²/day IV for 2 days) was administered in preparation for tisagenlecleucel infusion (dose: 1.9 × 10⁶ CAR+ T cells/kg [85% CAR+ T-cell viability]). On day 2, the patient developed grade 2 CRS and concern for immune effector cell–associated neurotoxicity syndrome (ICANS) with visible swelling of scalp subcutaneous chloromas, flaccid paralysis of her right lower extremity, and generalized weakness. Cranial CT (Figure 3) showed no evidence of cerebral edema but did show progressive swelling at known sites of extramedullary disease, which was presumed to be consistent with TIAP post-tisagenlecleucel infusion.
The patient received her first tocilizumab dose (8 mg/kg) for CRS management. CRS resolved over the next 24 hours in addition to a rapid decrease in the size of visible subcutaneous scalp lesions and improving neurological symptoms. On day 5, the patient again developed progressive swelling at existing scalp chloromas as well as new subcutaneous scalp lesions. The patient received a second tocilizumab dose (8 mg/kg) and all scalp chloromas rapidly involuted, with near-complete resolution over 24 hours.
Figure 3. Cranial PET-CT and CT scans showing progressive swelling at known sites of extramedullary disease. Baseline pre-infusion (PET-CT) and post-infusion (CT) scans are shown. Arrows indicate sites of extramedullary disease; CT, computed tomography; PET, positron emission tomography.
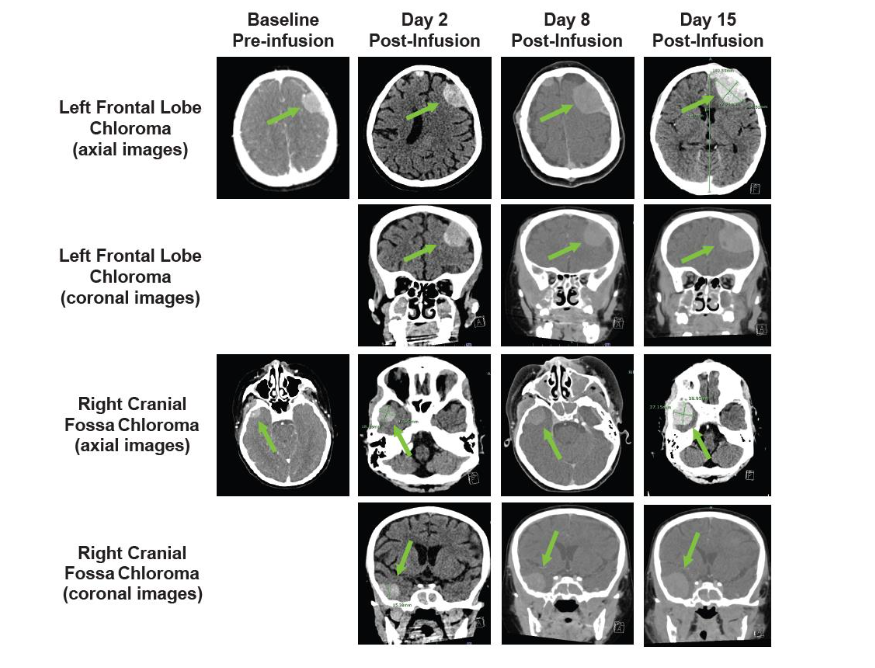
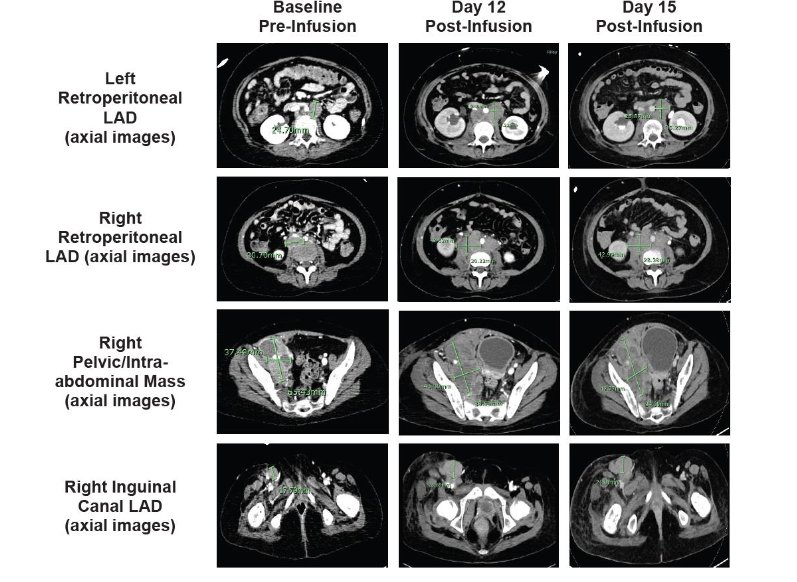
On day 8, the patient experienced progressive swelling of the mouth and gums with gingival bleeding, oral airway obstruction, transient difficulty breathing, recurrent swelling of scalp lesions, and profound nausea/vomiting. The patient simultaneously developed lower right extremity swelling and was found to have a superficial saphenous vein thrombus on ultrasound. Cranial CT (Figure 3) showed progression of existing intracranial extramedullary sites. A marked increase in known retroperitoneal disease was also observed (not shown). Given the prior reduction in extramedullary chloromas, the patient received a third tocilizumab dose (8 mg/kg) in addition to dexamethasone (10 mg IV every 6 hours). The patient’s scalp lesions decreased in size. Lower right extremity swelling and nausea/vomiting resolved completely over 36 hours.On day 12, the patient again had lower right extremity swelling. Repeat abdominal CT (Figure 4) showed a mild increase in adenopathy, with complete inferior vena cava and right external iliac vein occlusion. The patient received siltuximab (400 mg IV every 72 h) given the substantially longer half-life than tocilizumab. Unfortunately, the patient experienced no notable response and underwent emergency radiation therapy (2 Gy over 2 consecutive days). No substantial improvement in lower right extremity swelling or size of retroperitoneal adenopathy was observed at day 15 (Figure 4).
Figure 4. A. Baseline and post-infusion CT scans show marked progression of known retroperitoneal disease. Baseline pre-infusion (CT) and post-infusion (CT) scans are shown. Arrows indicate areas of known retroperitoneal disease; CT, computed tomography.
On day 15, the patient developed periorbital swelling, progressive proptosis, and worsening Cornell Assessment of Pediatric Delirium (CAPD) scores. A fourth tocilizumab dose (8 mg/kg) was administered, and dexamethasone was switched to methylprednisolone (1 mg/kg/d), which improved her tachycardia and eye swelling but provided no substantial change to lower right extremity edema.
On day 16, a fifth tocilizumab dose (8 mg/kg) was administered, with only slight improvement in gingival and orbital swelling over 48 hours. Rapid methylprednisolone taper was initiated due to the family’s concern about potential detrimental effect on CAR-T cell efficacy. On day 18, the patient had worsening bilateral proptosis and double vision, progressive swelling of scalp lesions, and worsening gum and mouth swelling. A sixth tocilizumab dose (8 mg/kg) was given, providing modest improvement over 48 hours.
At day 20, the patient again had worsening proptosis, which was associated with increased intraocular pressure (IOP), fatigue, and markedly worsening CAPD scores. A seventh tocilizumab dose (8 mg/kg) provided minimal improvement. The patient underwent bilateral canthotomy on day 22 with immediate IOP improvement and was restarted on dexamethasone (10 mg IV every 6 h).
OUTCOMES AND FOLLOW-UP
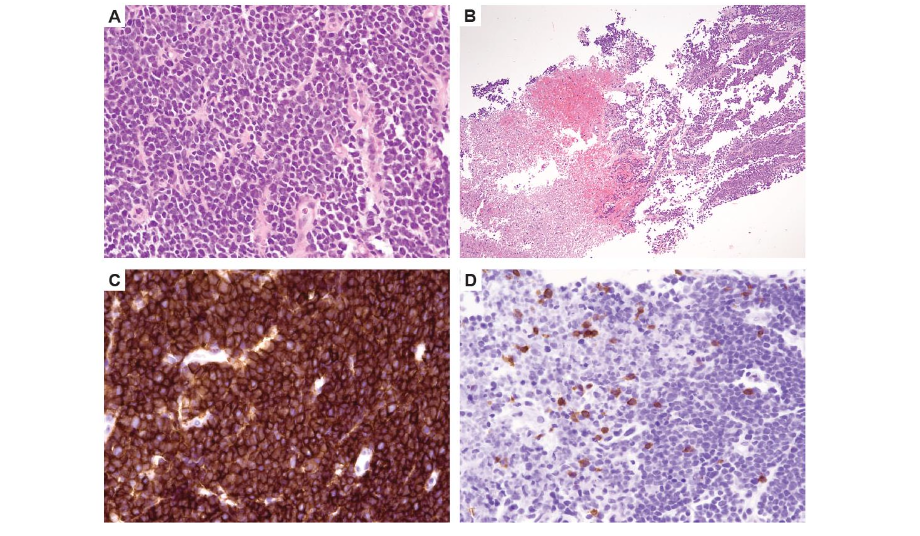
Because of diminishing response to tocilizumab and concern for disease progression, a repeat disease evaluation to help guide goals-of-care conversations was undertaken on day 22 while the patient was sedated for bilateral canthotomy. Bone marrow was FCM MRD positive (0.07% CD19+ blasts) and peripheral blood FCM showed B-cell aplasia. The scalp biopsy showed sheets of blasts and focal necrosis with T-cell aggregates (Figure 5). Due to lack of available commercial testing for quantitative tracking of tisagenlecleucel, it was unclear if the T-cell aggregates represented CAR-T cell infiltration into the extramedullary sites.
Figure 5. B-ALL in a core needle biopsy of scalp lesion. (A) Sheets of medium-sized blasts with scant cytoplasm, round to irregular nuclei, and immature chromatin (hematoxylin eosin stain, 400×). (B) Necrotic areas (hematoxylin eosin stain, 200×). (C) Strong expression of CD19 antigen seen by immunohistochemistry (400×). (D) CD3 immunohistochemical stain highlights higher T-cell density in necrotic foci (left) compared with areas with viable blasts (right) (400×). CD, cluster of differentiation.
Figure 5. Left and right inguinal CT scans (axial images) showing progressive swelling of known sites of extramedullary disease.
On day 23, due to declining urinary output with intermittent hypoxic episodes, furosemide was administered followed by an eighth tocilizumab dose (8 mg/kg) with no appreciable improvement. On day 24, CAPD scores continued to worsen, and the patient began continuous furosemide infusion due to concerns of fluid overload. On day 25, the patient was given pembrolizumab out of concern for T-cell exhaustion but showed no significant clinical improvement. The patient experienced a massive hemoptysis and subsequently died on day 27.
DISCUSSION
We describe a case of TIAP, and ultimately disease progression, at extramedullary disease sites following CAR-T cell infusion. Pseudoprogression has been well documented in patients receiving immunotherapy for solid tumors and in several patients with B-cell lymphoma and leukemia with extramedullary disease; however, the mechanisms underlying TIAP are not completely elucidated.
Following immunotherapy, inflammatory cell infiltrates, necrosis, and edema at the tumor site may lead to rapid but transient tumor enlargement, then tumor regression. Similarly, infiltrating CAR-T cells can cause local and systemic inflammation. Previous studies suggest that pseudoprogression may have an incidence <10% but can be as high as 20%.
Although pseudoprogression/delayed extramedullary disease response can extend the time interval to complete response and is often associated with a high likelihood of >1-year survival, some patients may not tolerate TIAP based on the anatomic location of the extramedullary disease. This highlights the potential utility of access to assays that differentiate CAR-T cell expansion from disease progression, which were not available at the time that this patient was receiving care.
This case also highlights the ambiguous definition of TIAP and actual extramedullary disease progression. Although the patient’s marrow disease remained low, the extramedullary biopsy was concerning for disease progression. The focal necrosis with T-cell aggregates seen on the scalp biopsy highlights the possible local inflammatory action of the CAR-T cells while residual disease was present. Imaging alone will not help determine the cellular nature of the enlarging extramedullary sites; therefore, biopsy is imperative to help distinguish between the two entities (TIAP vs actual extramedullary disease progression). Given the early onset of the extramedullary enlargement soon after CAR-T cell infusion, it is more likely that the enlargement of the extramedullary sites was due to TIAP. After the biopsy on day 22 revealed the continued presence of leukemia cells, it is possible there was also a component of disease persistence in the extramedullary sites. This persistent disease over time may lead to progressive disease, which can make the distinction between TIAP and disease progression more difficult. Although tocilizumab initially reduced extramedullary disease, subsequent tocilizumab doses (8 total) became progressively less effective, and no additional anti-inflammatory benefits occurred following siltuximab, supporting the hypothesis of initial TIAP followed by disease persistence and progression.
This raises the question whether the tocilizumab impacted CAR-T cell expansion or efficacy. Based on literature available, tocilizumab does not appear to affect the expansion or efficacy of CAR-T cells. Therefore, it is unlikely that the tocilizumab contributed to the progression of disease; however, it is unknown whether the multiple doses this patient received (8 total) could have influenced the efficacy of the CAR-T cell therapy.
Disease control with aggressive bridging therapy and salvage blinatumomab during the period of CAR-T cell manufacturing was necessary in this patient. The lack of response to blinatumomab during bridging therapy may have been a potential indicator of T-cell dysfunction or resistance to CD19-targeted therapy as recent studies suggest that nonresponse to prior blinatumomab can be associated with worse clinical outcomes and a high risk of relapse. Although this understanding and information were not available at the time, it would not have changed the patient’s course of treatment as even patients with disease that is refractory to blinatumomab therapy may still respond to CAR-T cell therapy.
Conclusions
Although CAR-T cell therapy has established benefit and safety in pediatric patients with r/r B-ALL, this case highlights the critical need for physicians to consider location and extent of extramedullary disease due to the risk of adverse events related to TIAP in the post-infusion period. Additionally, this case highlights the challenges physicians face in distinguishing between TIAP and actual disease progression, and the possibility of both occurring in the same patient over time.
Conflict of Interest:
All authors report no competing interests.
Funding Statement:
This work was supported by Novartis Pharmaceuticals Corporation.
Acknowledgements:
Medical writing support was provided by Healthcare Consultancy Group, and funded by Novartis Pharmaceuticals Corporation.
ORCID ID:
Jodi L. Skiles
https://orcid.org/0000-0002-7653-5920
REFERENCES
1. National Cancer Institute. B-cell acute lymphocytic leukemia. Accessed Accessed May 10, 2024, https://www.cancer.gov/publications/dictionaries/cancer-terms/def/b-cell-acute-lymphocytic-leukemia
2. National Cancer Institute. Cancer Stat Facts: Leukemia — acute lymphocytic leukemia (ALL). Accessed Accessed May 8, 2024, https://seer.cancer.gov/statfacts/html/alyl.html
3. National Cancer Institute. Cancer in children and adolescents. Accessed May 8, 2024, https://www.cancer.gov/types/childhood-cancers/child-adolescent-cancers-fact-sheet
4. Saleh K, Pasquier F, Bigenwald C, De Botton S, Ribrag V, Castilla-Llorente C. CAR T-cells for the treatment of B-cell acute lymphoblastic leukemia. J Clin Med. 2023;12(21):6883. doi:10.3390/jcm1 2216883
5. Kymriah [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corp; May 2022.
6. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 20 18;378(5):439-448. doi:10.1056/NEJMoa1709866
7. Laetsch TW, Maude SL, Rives S, et al. Three-year update of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory acute lymphoblastic leukemia in the ELIANA trial. J Clin Oncol. 2023;41(9):1664-1669. doi:10.1200/JCO. 22.00642
8. Ma Y, Wang Q, Dong Q, Zhan L, Zhang J. How to differentiate pseudoprogression from true progression in cancer patients treated with immunotherapy. Am J Cancer Res. 2019;9(8):1546-1553.
9. Danylesko I, Shouval R, Shem-Tov N, et al. Immune imitation of tumor progression after anti-CD19 chimeric antigen receptor T cells treatment in aggressive B-cell lymphoma. Bone Marrow Transplant. 2021;56(5):1134-1143. doi:10.1038/s4 1409-020-01156-y
10. Huang J, Rong L, Wang E, Fang Y. Pseudoprogression of extramedullary disease in relapsed acute lymphoblastic leukemia after CAR T-cell therapy. Immunotherapy. 2021;13(1):5-10. doi:10.2217/imt-2020-0188
11. Santomasso BD, Nastoupil LJ, Adkins S, et al. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO guideline. J Clin Oncol. 2021;3 9(35):3978-3992. doi:10.1200/JCO.21.01992
12. US National Library of Medicine. Treatment of patients with newly diagnosed standard risk B-lymphoblastic leukemia (B-ALL) or localized B-lineage lymphoblastic lymphoma (B-LLy). ClinicalTrials.gov identifier: NCT01190930. Posted August 30, 2010. Updated July 12, 2022. Accessed September 26, 2022. https://clinicaltrials.gov/ct2/show/NCT01190930.
13. de Boer JW, Pennings ERA, Kleinjan A, et al. Inflammatory reactions mimic residual or recurrent lymphoma on [18F]FDG-PET/CT after CD19-directed CAR T-cell therapy. Blood Adv. 2023;7(21) :6710-6716. doi:10.1182/bloodadvances.2023010665
14. Hodi FS, Hwu WJ, Kefford R, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34(13): 1510-7. doi:10.1200/jco.2015.64.0391
15. McKendry K, Desmond F, Ward C, Duffy AG. Pseudoprogression and cancer immunotherapy: a seven year retrospective study of rate, temporal course, and predictive markers in an Irish tertiary referral center. J Clin Oncol. 2021;39(15_suppl): Abstract 2651. doi:10.1200/JCO.2021.39.15_suppl.2651
16. Wang X, Zhang B, Zhang Q, et al. Impact of tocilizumab on anti-CD19 chimeric antigen receptor T-cell therapy in B-cell acute lymphoblastic leukemia. Cancer. 2024 Apr 5. doi: 10.1002/cncr.35316. Online ahead of print;doi:10. 1002/cncr.35316
17. Myers RM, Shah NN, Pulsipher MA. How I use risk factors for success or failure of CD19 CAR T cells to guide management of children and AYA with B-cell ALL. Blood. 2023;141(11):1251-1264. doi:10.1182/blood.2022016937
18. Myers RM, Taraseviciute A, Steinberg SM, et al. Blinatumomab nonresponse and high-disease burden are associated with inferior outcomes after CD19-CAR for B-ALL. J Clin Oncol. 2022;40(9):932-944. doi:10.1200/JCO.21.01405

