Natural vs. Induced Pluripotent Stem Cell Therapies
Comparison of Natural Multipotent Stem Cell and Induced Pluripotent Stem Cell Therapeutic Applications: Benefits and Challenges
Fu Nan Wang 1; Sebo Gene Wang 1; Ming Chu Hsu 1; Sebo Ling Wang 1; Sebo Dih Wang 1; James J. Huang 1
- TOP IVF USA, American Stem Cell Base, Los Angeles, CA, USA
OPEN ACCESS
PUBLISHED:31 October 2025
CITATION: Wang, FN., Wang, SG., et al., 2025. Comparison of Natural Multipotent Stem Cell and Induced Pluripotent Stem Cell Therapeutic Applications: Benefits and Challenges. Medical Research Archives, [online] 13(10). https://doi.org/10.18103/mra.v13i10.6936
COPYRIGHT © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI https://doi.org/10.18103/mra.v13i10.6936
ISSN 2375-1924
ABSTRACT
Natural Multipotent Stem Cell preparation is unique and safe when compared with other methods. Using this technology, American Stem Cell Base Dr. Wang has treated more than 100 kinds of diseases, made thousands of transplantations, with excellent cure rate (> 90% cured or great improvement per clinical end point measurement) and most importantly, there are no significant side effects. In 2012, the Nobel Prize-winning medical technology, Induced Pluripotent Stem Cell, invigorated stem cell research as it showed that Induced Pluripotent Stem Cell has the potential to produce every type of cell and tissue in the body. However, the inherited properties of Induced Pluripotent Stem Cell were known as tumorigenicity, immunogenicity, and heterogeneity. In his 2024 review article, Dr. Yamanaka indicated that he had dedicated two decades of research aimed at overcoming these three difficulties. Given the potential for these cells to become cancerous stem cells, the widespread application of the technology in treating various clinical diseases might require collaborative research by scholars, scientists, and clinicians and potentially could take decades to resolve. Natural Multipotent Stem Cell, on the other hand, is a mature biotechnology with specialized technologies and capabilities for mass production. It has successfully treated over 100 long-standing and intractable diseases, potentially providing widespread relief to millions afflicted with these diseases. This study provided a side-by-side comparison of two leading and promising stem cell therapy candidates, natural Multipotent Stem Cell and Induced Pluripotent Stem Cell, in clinical applications and present the challenges and promises in stem cell therapy.
Keywords
Natural Multipotent Stem Cell, Induced Pluripotent Stem Cell, stem cell therapy, tumorigenicity, immunogenicity, clinical applications
INTRODUCTION
Stem cells have self-renewing abilities and the capabilities to differentiate into cells with specialized functions. They serve as a reserved repair system for the body. They are used to regenerate tissues and cells in the body that have been damaged or destroyed by disease. Stem cells come in three different varieties: adult stem cells, embryonic stem cells and induced pluripotent stem cells (iPSCs).
The process of isolating embryonic stem cells has been controversial and raises ethical concerns as it results in the destruction of the embryo. On the other hand, adult stem cells (ASCs) represent an ethically acceptable source of extremely rare stem cells, which might be lower than 1% of a given cell population. With technology, stem cells can now be obtained and isolated from the umbilical cord, the placenta, amniotic fluid, etc.
In 2006, a Japanese team led by Shinya Yamanaka discovered that mature cells can be reprogrammed to become pluripotent stem cells. In 2012, this Nobel Prize-winning medical technology was termed induced pluripotent stem cells. This discovery has invigorated stem cell research as it showed that iPSCs have the potential to produce every type of cell and tissue in the body. Thus, iPSCs are promising candidates for tissue engineering and regenerative medicine. The treatment of human disease with iPSCs could revolutionize the health care industry with its ability to generate any cell, tissue, or even organ, “on-demand” in the laboratory.
However, iPSC research has its share of challenges. One of the major concerns with the potential clinical application of iPSCs is their propensity to form tumors, as a result of either the inducing agents or incomplete reprogramming.
At TOP IVF USA, American Stem Cell Base, Dr. Fu-Nan Wang, MD. PhD., recognized the potential benefits that stem cells have and embarked on the challenges of using allogeneic cell transplantation in stem cell therapy. When the field was still in its infancy, restrictions were placed on stem cell search making it difficult for scientists to pursue this promising medical research. However, Dr. Wang was undeterred. He envisioned that patients from around the world could safely receive allogeneic stem cell treatment at effective concentrations.
The Natural Multipotent MSC (nMS) preparation technology is a great endeavor that can be used for clinical treatment. It has not exhibited any noticeable adverse side effects as the safety ratio was found to be close to 100%. The efficacy ratio of measurable clinical endpoint was greater than 90% because of natural culture “adaptation” rather than that of induced stem cells. The lab has found and cataloged different types of stem cells preparation and has applied them to clinical treatment with great success. Regarding the successful clinical treatment of stem cells, examples of relevant case reports have been published in peer-reviewed medical journals.
The scope of this communication is to introduce a novel stem cell isolation technique and details a science-based approach and evidence-driven clinical data, a sustainable, scalable production process over three decades, where thousands of cellular treatments were delivered against more than 100 different kinds of intractable diseases. The purpose is to provide a side-by-side comparison of two leading stem cell therapy candidates, nMS and iPSC, in clinical applications and present the challenges and promises in stem cell therapy.
All iPSC data presented were obtained through PubMed literature search, published through the NIH National Library of Medicine.
METHODS
INDUCED PLURIPOTENT STEM CELL (iPSC)
Induced pluripotent stem cell is a type of pluripotent stem cell that can be generated directly from a somatic cell. Nobel prize awarded medical advancement demonstrated that overexpression of four transcription factors can reprogram the somatic cells to a pluripotent state by rearranging their epigenetic landscape, proving cellular identity is epigenetically regulated. These four transcriptional factors collectively were called Yamanaka factors, include Oct4, Sox2, Klf4 and c-Myc (OSKM).
These cocktails of transcription factors regulate signaling pathways, epigenetic modifications, and microRNAs to establish pluripotency. Human iPSCs are autologous, are established by ectopic expression or direct delivery of certain mRNAs or proteins and free from major ethical concerns, but are prone to transcriptional and epigenetic aberrations.
We searched for papers published through the NIH National Library of Medicine, the PubMed database, using the search strings [iPSC] AND [cell therapy] AND [efficacy] AND [side effect], and the search strings [iPSC] AND [clinical trial]. Relevant information in publications were cited and discussed in this manuscript.
NATURAL MULTIPOTENT STEM CELL (nMS) PREPARATION
The study design of natural multipotent mesenchymal stem cell isolation has been researched with a comprehensive and systematic approach to elucidate the most thorough and effective stem cell isolation technique. For example, about 10 -15 cm of fresh umbilical cords is received from consenting donors. The general health examination status of stem cell donors was conducted under a comprehensive evaluation. Mesenchymal stem cells (MSC) from umbilical cords were prepared by the tissue explant method as described with minor modification. Fresh umbilical cord tissue from a healthy donor was minced into small pieces and were cultured in α-MEM (Thermo Fisher Scientific, Grand Island, NY, USA) supplemented with human platelet lysate (HPL, Fisher Scientific International, Pittsburg, Pennsylvania, USA). The concentration of HPL was gradually increased to accommodate the cell growth. The medium was replaced three times a week, and adherent cells were allowed to reach 80 % – 85 % confluency before they were sub-cultured. There were no decomposing agents or digestive enzymes used during the stem cell isolation and expansion only biophysical methods such as brief cold temperature shock (4°C – 8°C) or a small brush to detach the adhesion cells from plastic surface. During the expansion process, the cell culture was incubated under a standard hypoxia condition (carbon dioxide, oxygen and balanced with dry nitrogen), and the culture was observed under an inverted microscope for cell attachment, morphology and any microbial contamination, etc. Freshly isolated stem cells were designated as P0, routine cell count, and viability assay was performed accordingly, cells passage after 7 (P7) were harvested and stored in shipping buffer. With technology, stem cells can now be obtained and isolated from many sources such as umbilical cords, the placenta, amniotic fluid, menstrual blood, gingiva, deciduous tooth, adipose tissue and skin tissue etc., except for nail, hair and keratin.
CASE REPORT DATA
The clinical results were captured in a real-time medical examination record, backed by laboratory diagnostic results, and the attending physicians’ assessment based on established measurable clinical end points were noted, and the results were recorded in each patient’s medical case summary.
RESULTS
At American Stem Cell Base, our team has isolated and cataloged stem cells from bone marrow, umbilical cord tissue, adipose tissue, menses blood, and gingival tissue. These cells exhibited standard fibroblast-like morphology without significant difference in appearances under inverted microscope. We have strong evidence that different types of stem cells have specific functionalities associated with their sources. We are in preparation of a manuscript entitled “The Miraculous nMS Lineages Successfully Treating 100 Plus Intractable Diseases – Systemic Summary”.
Natural Pluri-Potent Stem Cells (nPS stem cells), also known as embryonic stem cells (ESCs) are derived from the inner cell mass of the embryo at around the fifth day of embryonic development, also known as the Blastocyst Stage. Because they are extracted from embryonic cells, this raises ethical or sociological concerns. Pluripotent stem cells can develop into almost any cell type found in the human body, except for those that make up the placenta. This ability makes them an outstanding focus in modern biological research, offering new avenues for understanding human development and disease progression, and paving the way for advanced medical applications. The “nPS Stem Cells” differ from “iPS Stem Cells”. The “i” in iPS stands for “induced,” meaning they are artificially induced. The iPS cells are generated using various chemical drugs, viruses, and radiation to induce normal somatic cells into pluripotent stem cells, which are then converted into the desired types of stem cells for clinical application.
However, the major drawback and risk with iPS stem cells is the significant potential of becoming a cancerous threat. Clinically, a considerable ratio of patients using iPS cells can experience various types and degrees of side effects, including the rapid induction of cancer in severe cases. iPSCs can accumulate chromosomal abnormalities, genetic instability, copy number variants and loss of heterozygosity over a period in vitro culture and expansion since these cells are maintained in culture for prolonged periods of time. As such, tumorigenicity, immunogenicity, and heterogeneity may hamper attempts to deploy this technology therapeutically.
On the other hand, MSC genetic stability is well documented. Cells that are cultured at physiological oxygen levels delay senescence and inhibit senescence-related genes, preventing cell cycle arrest. Even at the molecular level, microenvironmental control is multi-component and involves different players. The freshly isolated cells were considered P0 and they should have saved the “most primitive, original stem cells” or the “progenitor” of umbilical cord mesenchymal stem cells. It is believed that these “ancestors” were the most effective MSC of clinical treatment. Cells can be propagated for more than 20 passages and this natural preparation MSC is called “nMS”. Given the fact that stem cells are so sensitive to environmental influence, the isolation process we have developed might be the most profound and effective preparation.
These extensively expanded and cultured cells may lead to a gradual loss of the pluripotency of the cells and aging of the MSCs. Therefore, precise laboratory preparation environments and quality techniques are extremely important, including reducing the use of enzymes, minimizing manual laboratory procedures, etc. Many potential drawbacks have raised concerns among scientists, as the expansion of cultured cells may alter the expected biological functions of MSCs, potentially hindering their clinical therapeutic potential.
Currently, the preparation process of MSC aims to obtain “Natural Multipotent Stem Cell, nMS,” which is considered the safest and most reliable technique. Although MSCs have been extensively studied, promoted, and applied in many laboratories worldwide, their isolation, expansion, and phenotypic identification standard protocols have not been fully established yet. There is not an individual biomarker that distinguishes MSCs from other types of cells. American Stem Cell Base has isolated MSC from various source and fully characterized and subjected them for medical application. For example, gingiva-derived MSC contains a population of neurocrest-derived stem cells, possess multipotent differentiation capacities and potent immunomodulatory effects, and exhibit a superior capacity for neurogeneration and are more valuable in potential applications for neurodegenerative diseases and nerve regeneration. Bone marrow isolated stem cells are used in cancers, and blood disorder diseases. We have validated morphology, spindle shaped and fibroblast-like cells, and the expression of classical surface markers on these nMS cells, isolated from each umbilical cord donor; surface clusters were conformed to the International Society for Cellular Therapy (ISCT) cluster marker display characterization.
| Item Parameters | nMS | iPSC |
|---|---|---|
| 1 Source of cell | Primary explant | Adult somatic cells |
| 2 Ethical or legal issue | None | None |
| 3 Immunogenic limitation | None | HLA matching required |
| 4 Isolation-culture expansion, Growth condition | Biological programming of safe culture expansion. No feeder cell, Hypoxia growth condition | Reprogramming of genome. Need feeder cell, Standard CO2 Incubator |
| 5 Addition of transcription factors | None | Oct4, Sox2, c-Myc, Klf4 |
| 6 Chemicals and enzymes used in isolation-creation | None | Yes |
| 7 Life span | Well-controlled 60-80 generations with normal chromosome karyotype | Renew indefinitely |
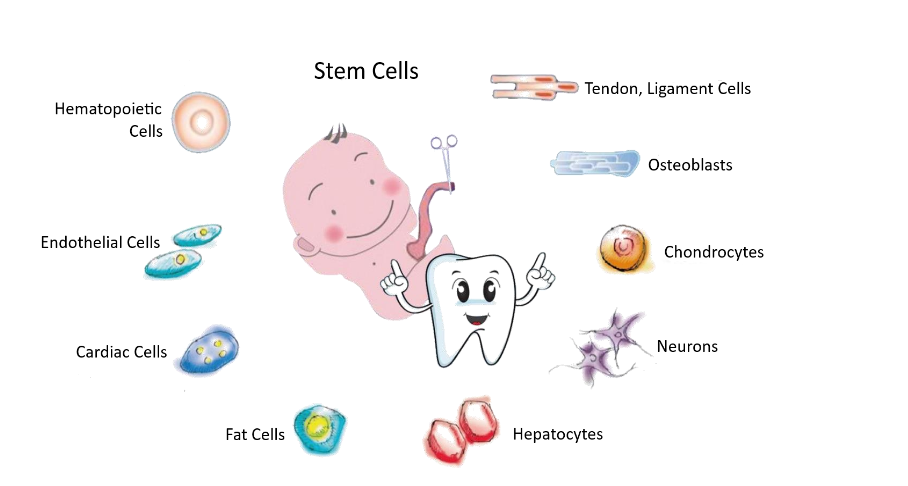
| 8 Differentiated cell types | nMS isolation from various cell types: Hematopoietic cells, osteoblasts, chondrocyte, neuron, cardiac cells, etc. | Differentiate into many different cell types; pancreatic-β cells, liver hepatocytes, cardiomyocytes, hematopoietic cells, and dopaminergic neurons |
| 9 Targeted disease treatment | Almost every intractable disease, even at the end stage of colon cancer, prostate cancer, multiple myeloma | Safety concern abound: Diabetes, leukemia, and Parkinson’s disease, cancer therapy |
| 10 Successful cell transplantation | Successfully treated thousands of patients with excellent efficacy, without noticeable side effect | Successfully in animal models of Parkinson’s disease and liver cirrhosis respectively |
| 11 New drug screening | Yes, with reservations | Unknown. |
| 12 Roadblock to clinical: Safety | No safety concern, reliable, reproducible | Safety concern: Immune rejection and compatibility |
| 13 Validated differentiating SOP | GMP-compliant cultural SOP | Followed GMP Guidance: 21 CFR 210 & 21 CFR 211 |
| 14 Epigenetic memory | No adverse impact | Evidence of adverse impact |
| 15 Cost and Time for each treatment | Reasonable and affordable (1×108 cells / Transplantation depending on disease status); cost is affordable even at high cumulative dose | It is too expensive and time-consuming |
| 16 Targeted patient | Allogeneic, autogenetic | Autogenetic |
| 17 Tumorigenicity | Genetic stability | Teratoma and tumors |
| 18 Immunogenicity | Minor or non-significant | Immune rejection |
| 19 Heterogeneity | Minor or insignificant | Genetic background, integrity, impact /defect in differentiation |
| 20 Is there a psychological burden on patients and medical staff during cell transplantation? | No, safe, effective and reliable | Side effect, potential tumor formation |
| 21 What is the rate of disease control after treatment? | ~90% | Unknown |
| 22 Safety, sterility, efficacy | Excellent based on results of treating thousands of patients | Unknown, severe adverse side effects |
| 23 Finished product handling | Store at ambient temperature for up to one week | Unknown or ultra-low temperature storage |
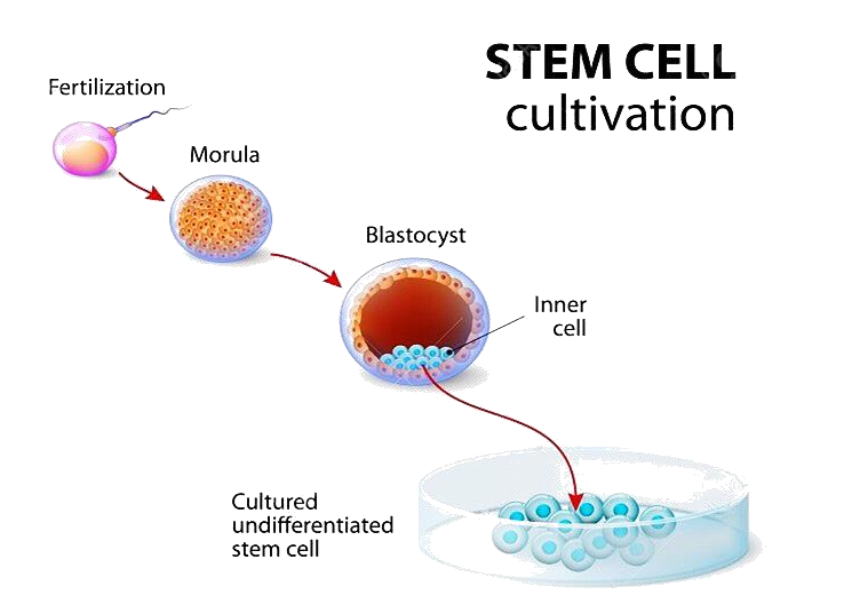
Figure 3: Cell morphological features of the nMS lineage culture.
Figure 4. Micrograph from inverted microscopy showing Natural Multipotent Stem Cell (nMS) lineage after subculture from umbilical cord. Cells were maintained under standard hypoxic conditions (5% CO₂, 37 °C, and 96% humidity). Approximately 50 million viable stem cells were present on the culture dish surface, appearing alive, active, sterile, and free of debris. Original magnification: 100×.
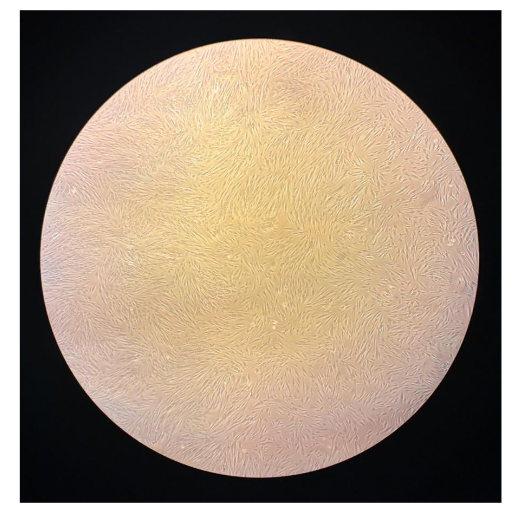 Figure 5. Comparison of nMS (left) and iPSC (right) stem cells, highlighting their differences, advantages, and disadvantages. Left: nMS stem cells were sub-cultured from a boy’s deciduous tooth tissue and maintained under hypoxic conditions (5% CO₂, 37 °C, 96% humidity). Cells on the culture plate appeared viable, active, sterile, and free of debris, without the addition of growth factors or chemical inducers. Right: iPSC stem cells were derived from adult skin tissue and maintained in suspension culture under the same incubation conditions described above.
Figure 5. Comparison of nMS (left) and iPSC (right) stem cells, highlighting their differences, advantages, and disadvantages. Left: nMS stem cells were sub-cultured from a boy’s deciduous tooth tissue and maintained under hypoxic conditions (5% CO₂, 37 °C, 96% humidity). Cells on the culture plate appeared viable, active, sterile, and free of debris, without the addition of growth factors or chemical inducers. Right: iPSC stem cells were derived from adult skin tissue and maintained in suspension culture under the same incubation conditions described above.
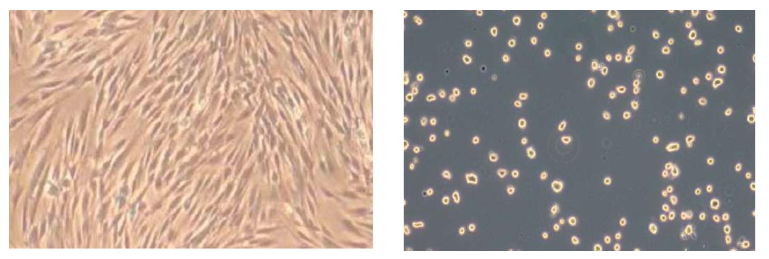
Figure 6. Top: Micrograph from inverted microscopy showing Natural Multipotent Stem Cell (nMS) lineage after subculture from gingival biopsy tissue under the same culture conditions described in Figure 5. The cells display active spreading and attachment, appearing viable, sterile, and free of debris or contamination (original magnification ×300). Bottom: nMS in shipping buffer, showing stable morphology under ambient conditions for up to seven days without controlled incubation (37 °C, CO₂, O₂, and humidity) (original magnification ×600).
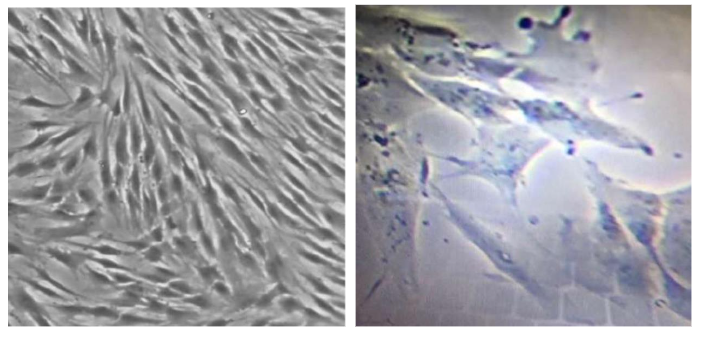
Figure 7. Micrograph from inverted microscopy showing Natural Multipotent Stem Cell (nMS) lineage after subculture from umbilical cord tissue. The attached cells exhibit fibroblast-like morphology characteristic of mesenchymal stem cells (MSC). Original magnification ×200.
DISCUSSION
iPSC medical technology has invigorated stem cell research as it shows that it has the great potential to produce every type of cell and tissue in the body. Thus, iPSCs are promising candidates for tissue engineering and regenerative medicine. The treatment of human disease with iPSCs might revolutionize the health care industry with its ability to generate any cell, tissue, or even organ, “on-demand” in the laboratory, albeit with limited success.
However, iPSC research has its share of challenges. One of the major concerns with the potential clinical application of iPSCs is their propensity to form tumors, contributed by either the inducing agents, oncogenic gene set used for pluripotency induction, or incomplete reprogramming. Per review articles, iPSC’s tumorigenicity could be due to residual PSC, reprogramming factors or by genetical abnormality. These inherited properties of iPSC were better known as tumorigenicity, immunogenicity, and heterogeneity. In his review article in 2024, Dr. Yamanaka indicated that he had dedicated two decades of research aimed at overcoming these three difficulties.
The main issue is the use of retroviruses to generate iPSCs as they are associated with cancer. More specifically, retroviruses can insert their DNA anywhere in the genome and subsequently trigger cancer-causing gene expression. Kamarda et al. showed that 84% of iPSCs transplanted in mice became weaker or died because of tumor development. Cancer-iPSCs have more potential risks. Their use in therapy should be used more cautiously. Stimulation or inhibition of specific factors increases the tumorigenicity risk of iPSCs.
The technology of Natural Multipotent Stem Cell (nMS) preparation is unique and safe when compared with other methods. For example, tissue of gingiva or umbilical cord is processed by using biophysical technology bypassing chemicals and medicine, such as digestive enzymes (hyaluronidase or trypsin) for dissociation of the tissue, and antibiotics (Penicillin G Sodium, Streptomycin Sulfate and Amphotericin B, etc.) These nMS mesenchymal stem cell (MSC) preparations are unique and are used currently in this laboratory.
After more than thirty years of research and experiment, “Dr. Wang’s nMS Stem Cell Lineage” has been developed into a highly effective and efficient technique to culture stem cells with remarkable survivability and resilience. The MSC is able to survive without a fixed incubator at 37 degrees Celsius, without fixed 5% carbon dioxide, without fixed 20% oxygen, and without fixed humidity for more than seven (7) days, while maintaining physiological function for clinical application. This characteristic enables scaled nMS production, nMS bulk cells transportation and large-scale clinical administration a reality.
Dr. Wang’s Team has successfully treated more than 100 diseases, made thousands of transplantations, treated just about every disease condition, with excellent treatment responses (> 90% cured or great improvement per clinical end point measurement) and most importantly, there are no significant side effects. Dr. Wang has documented all case studies, published books and many peer-reviewed journal articles.
Over the past 30 years, Dr. Wang and his team have made great strides to master unique skills. The team (1) is proficient in stem cell identification and preparation; (2) has proven efficacy in the clinical application of these stem cells; (3) has made breakthroughs in medicine and science by demonstrating nMS is a multi-target stem cell and capable of repairing damaged cells or tissues. His facility has successfully treated thousands of patients and has improved the largest variety of major and difficult human diseases via IV parenteral administration of MSCs. The records show Wang’s team has broken through, greatly improved, and/or cured major intractable human diseases.
“Endogenous stem cells”, the resident tissue-specific cells are unique, vital, and require special attention. They have not been fully understood or appreciated for their contributions in our daily lives, cell reproduction, cell alternation, cell repair, cell reproduction, and the aging process, but these cells continue to fulfill their role. In short, the unique and novel “Stem Cell Lineage” treatment regimen was developed, after performing thousands of stem cell transplantations. Our Stem Cell Lineage has successfully cured or significantly improved more than 100 kinds of human intractable diseases and their complications, including but not limited to, regenerative illnesses, cancer, congenital and hereditary diseases.
CONCLUSIONS
The new medical technology of iPSCs has energized stem cell research as it shows that it has the potential to produce every type of cell and tissue in the body. Thus, iPSCs are promising candidates for tissue engineering and regenerative medicine, and could revolutionize the health care industry. However, tumorigenicity, immunogenicity, and heterogeneity may hamper attempts to deploy this technology therapeutically. It is recommended that iPSC should be used for research purposes only and not for clinical treatment at present.
Over the last three decades, the team at American Stem Cell Base has made great efforts to develop stem cell treatment and research. The team (1) is proficient in stem cell identification and preparation; (2) has met good manufacturing practice (GMP) guidance with finished product rigorously tested to ensure sterility, potency and strength; (3) has proven efficacy in the clinical application of these stem cells; (4) has made breakthroughs in medicine and science by demonstrating nMS is potentially a multi-target or broad-spectrum stem cell lineage and capable of repairing damaged cells or tissues.
In the pursuit of frontier medicine, providing the safe, effective, affordable intervention and reproducible clinical product would be the goal and purpose of every medical researcher. It is both our duty and responsibility to report and communicate our novel nMS isolation technology, sustainable process, high quality finished products, affordable treatment regimen, and outstanding treatment results, as one of the most advantageous options available to the stem cell therapy community. Further, the harvested finished product nMS stored in shipping buffer remains active and effective for at least one week at ambient temperature and general environment, without a fixed 37 °C incubator, fixed CO2 and O2 and humidity control. There is solid evidence that depicts the effectiveness of nMS cells for regenerative therapies and increasing evidence supports their effectiveness in regeneration and repair, as such that makes nMS the gold standard of stem cell therapy for patients.
REFERENCES
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006; 126 (4): 663–76
- Rippon HJ, and Bishop AE. Embryonic stem cells. Cell Prolif. 2004; 37(1):22-23
- Knoepfler PS. Deconstructing stem cell tumorigenicity: a roadmap to safe regenerative medicine. Stem Cells. 2009; 27 (5): 1050–6
- Yamanaka S. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell. 2020 Oct 1; 27(4):523-531. doi: 10.1016/j.stem.2020.09.014
- Wang SG, Hsu, NC, Wang, SM, and Wang, FN. Successful Treatment of Plaque Psoriasis with Allogeneic Gingival Mesenchymal Stem Cells: A Case Study. Dermatol Medicine 2020: 1-4
- Wang SG, Hsu, NC, Wang, SM, Hsu, MC and Wang, FN. First-in-human High Cumulative Dose Mesenchymal Stem Cell Therapy in Multiple Myelomas: A Case Report. J. Hematol. and Transfu. 2021 8(1):1090
- Wang FN, Hsu MC, Wang SL, Wang SG, Chen WC, Lee TD, Wang SM, and Doong H. Stem cell therapy: A Possibility for Coronavirus. J. Hematol and Transfus. 2021 8 (2):1094
- Wang SG, Wang SM, Hsu MC, Wang FN. Mesenchymal stem cell treatment improves renal failure and multiple episodes of nephrolithiasis. Journal of Urology and Research. 2022 91(1):1130
- Zhong C, Liu M, Pan X, Zhu A. Tumorigenicity risk of iPSCs in vivo: nip it in the bud. Precision Clinical Medicine, Volume 5, Issue 1, March 2022, pbac004
- Drishty B. Sarker DB, Xu Y, Mahmud F, Jocelyn JA, and Sang QA. Interconversion of Cancer Cells and Induced Pluripotent Stem Cells. Cells 2024, 13(2), 125; doi.org/10.3390/cells13020125
- Doss MX, Agapios Sachinidis A. Current Challenges of iPSC-Based Disease Modeling and Therapeutic Implications. Cells. 2019 Apr 30; 8(5):403. doi: 10.3390/cells8050403
- Shamsian A, Sahebnasagh R, Norouzy A, Hussein SH, Ghahremani MH, Azizi Z. Cancer cells as a new source of induced pluripotent stem cells. Stem Cell Res Ther. 2022 Sep 5; 13:459. doi: 10.1186/s13287-022-03145-y
- Peterson SE, Loring JF. Genomic instability in pluripotent stem cells: implications for clinical applications. J Biol Chem. 2014 Feb 21; 289(8):4578-84. doi: 10.1074/jbc.R113.516419. Epub 2013 Dec 20
- Gore, A. Li, Z. Fung, HL, Young JE, Agarwal S, Antosiewicz-Bourget J, Canto I. Giorgetti A, Isreal MA, Kiskinis E, Lee J et al Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011; 471:63-67
- Orgueira, AM, Antelo, BR, Arias, JAD, Varela ND, Vence NA, Pereze MSG, Lopez JLB. Novel Mutation Hotspots within Non-Coding Regulatory Regions of the Chronic Lymphocytic Leukemia Genome. Sci. Rep. 2020; 10:2407
- Zhang J, Jia Z, Pan H, Ma W, Liu Y, Tian X, Han Y, Wang Q, Zhou C, Zhang C. From induced pluripotent stem cell (iPSC) to universal immune cells: literature review of advances in a new generation of tumor therapies. Transl Cancer Res. 2025 Apr 30; 14(4):2495-2507. doi: 10.21037/tcr-24-1087. Epub 2025 Apr 15
- Jiang Y, Zhang L, Zhang F, Bi W, Zhang P, Yu X, Rao S, Wang S, Li Q, Ding C, Jin Y, Yang H. Dual human iPSC-derived cardiac lineage cell-seeding extracellular matrix patches promote regeneration and long-term repair of infarcted hearts. Bioact Mater. 2023 May 27:28:206-226. doi: 10.1016/j.bioactmat.2023.05.015. eCollection 2023 Oct
- Soma T, Oie Y, Takayanagi H, Matsubara S, Yamada T, Nomura M, Yoshinaga Y, Maruyama K, Watanabe A, Takashima K, Mao Z, Quantock AJ, Hayashi R. Induced pluripotent stem-cell-derived corneal epithelium for transplant surgery: a single-arm, open-label, first-in-human interventional study in Japan. Lancet. 2024 Nov 16; 404(10466):1929-1939. doi: 10.1016/S0140-6736(24)01764-1. Epub 2024 Nov 7
- Raniga K, Nasir A, Vo NTN, Vaidyanathan R, Dickerson S, Hilcove S. Mosqueira D, Mirams GR, Clements P, Hicks R, Pointon A, Stebbeds W, Francis J, Denning C. Strengthening cardiac therapy pipelines using human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell. 2024 Mar 7; 31(3):292-311. doi: 10.1016/j.stem.2024.01.007. Epub 2024 Feb 15
- Wyles SP, Brandt EB, Nelson TJ. Stem Cells: The Pursuit of Genomic Stability. Int J Mol Sci. 2014; 15(11):20948–20967. doi: 10.3390/ijms151120948
- Panchalingam KM, Jung S, Rosenberg L, Leo A. Behie LA. Bioprocessing strategies for the large-scale production of human mesenchymal stem cells: a review. Stem Cell Research & Therapy (2015) 6:225. doi: 10.1186/s13287-015-0228-5
- Mas-Bargues C, Sanz-Ros J, Román-Domínguez A, Inglés M, Gimeno-Mallench L, Alami ME, Viña-Almunia J, Gambini J, Viña J, Borrás C. Relevance of Oxygen Concentration in Stem Cell Culture for Regenerative Medicine. Int J Mol Sci. 2019; 20(5):1195. doi: 10.3390/ijms20051195
- Kim D, Lee AE, Xu Q, Zhang Q, Le AD. Gingiva-Derived Mesenchymal Stem Cells: Potential Application in Tissue Engineering and Regenerative Medicine – A Comprehensive Review. Front Immunol. 2021 Apr 16:12:667221. doi: 10.3389/fimmu.2021.667221. eCollection 2021
- Kumar D, Tanwar R. World’s first: stem cell therapy reverses diabetes. Stem Cell Res Ther. 2024 Dec 20; 15(1):487. PMC11662597. doi: 10.1186/s1328-024-04036-0
- Doi D, Magotani H, Kikuchi T, Ikeda M, Hiramatsu S, Yoshida K, Amano N, Nomura M, Umekage M, Morizane A, Takahashi J. Pre-clinical study of induced pluripotent stem cell-derived dopaminergic progenitor cells for Parkinson’s disease. Nat Commun. 2020 Jul 6; 11(1):3369. doi: 10.1038/s41467-020-17165-w
- Wenker SD, Leal MC, Farías MI, Zeng X, Pitossi FJ. Cell therapy for Parkinson’s disease: Functional role of the host immune response on survival and differentiation of dopaminergic neuroblasts. Brain Res. 2016 May 1; 1638(Pt A):15-29. doi: 10.1016/j.brainres.2015.06.054. Epub 2015 Aug 1
- Mandai M, Watanabe A, Kurimoto Y, Mandai M, Watanabe A, Hirami Y, Daimon T, Fujihara M, Akimaru H, Sakai N, et al. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017; 376:1038-1046
- Sugita S, Mandai M, Hirami Y, Takagi S, Maeda T, Fujihara M, Matsuzaki M, Yamamoto M, Iseki K, Hayashi N Hono A, et al. HLA-Matched Allogeneic iPS Cells-Derived RPE Transplantation for Macular Degeneration. J. Clin. Med. 2020; 9:2217
- Deuse T, Hu X, Agbor-Enoh S, Koch M, Spitzer MH, Gravina A, Alawi M, Marishta A, Peters B, Kosaloglu-Yalcin Z, et al. De novo mutations in mitochondrial DNA of iPSCs produce immunogenic neoepitopes in mice and humans. Nat. Biotechnol. 2019; 37:1137-1144
- Gornalusse GG, Hirata RK, Funk SE, Riolobos L, Lopes VS, Manske G, Prunkard D, Colunga A, Hanafi L, Clegg DO, et al. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat. Biotechnol. 2017; 35:765-772
- Koyanagi-Aoi M, Ohnuki M, Takahashi K, Okita K, Noma H, Sawamura Y, Teramoto I, Narita M, Sato Y, Ichisaka T, et al. Differentiation-defective phenotypes revealed by large-scale analyses of human pluripotent stem cells. Proc. Natl. Acad. Sci. USA. 2013; 110:20569-20574
- Theunissen TW, Friedli M, He Y, Planet E, O’Neil RC, Markoulaki S, Pontis J, Wang H, Iouranova A, Imbeault M, et al. Molecular Criteria for Defining the Naive Human Pluripotent State. Cell Stem Cell. 2016; 19:502-515
- Avior Y, Eggan K, Benvenisty N. Cancer-Related Mutations Identified in Primed and Naive Human Pluripotent Stem Cells. Cell Stem Cell. 2019; 25:456-461
- Avior Y, Eggan K, Benvenisty N. Cancer-Related Mutations Identified in Primed and Naive Human Pluripotent Stem Cells. Cell Stem Cell. 2019; 25:456-461
- Kamada M, Mitsui Y, Kumazaki T, Kawahara Y, Matsuo T, Takahashi T. Tumorigenic risk of human induced pluripotent stem cell explants cultured on mouse SNL76/7 feeder cells. Biochem Biophys Res Commun. 2014 Oct 24; 453(3):668-73. doi: 10.1016/j.bbrc.2014.10.009. Epub 2014 Oct 8
- Zou Y, Hui R, Song L, The era of clinical application of gene diagnosis in cardiovascular diseases is coming. Chronic Dis Transl Med. 2020; 5(4):214–220. doi: 10.1016/j.cdtm.2019.12.005
- Mitrano TI, Grob MS, Carri F, Nova-Lamperti E, Luz PA, Fierro FS, Quintero A, Chaparro A, Sanz 48. Cirino AL, Harris S, Lakdawala NK, Michels M, Olivotto I, Day SM, Abrams DJ, Charron P, Caleshu C, Semsarian C, Ingles J, Rakowski H, Judge DP, Ho CY. Role of Genetic Testing in Inherited Cardiovascular Disease: A Review JAMA Cardiol. 2017; 2(10):1153-1160. doi: 10.1001/jamacardio.2017.2352
- Simonson OE, Domogatskaya A, Volchkov P, Rodin S. The safety of human pluripotent stem cells in clinical treatment. Ann Med. 2015; 47(5):370-80. doi: 10.3109/07853890.2015.1051579. Epub 2015 Jul 6.
- Patel S, Athirasala A, Menezes P, Ashwanikuma A, Zou T, Sahay G, and Bertassoni L. Messenger RNA Delivery for Tissue Engineering and Regenerative Medicine Applications. Tissue Eng Part A; 2019; 25(1-2): 1-112
- Maziarz A, Kocan B, Bester M, Budzik S, Cholewa M, Ochiya T and Banas A. How electromagnetic fields can influence adult stem cells: positive and negative impact. Stem cell Research and Therapy 2016 7, Article 54
- Wang FN, Ed, American Stem Cell Medicine Ace Review. Chapter 1, Section 7 pp91-101, Clinical Application, pp. 369-436, Book Series of The World Medical Health Organization of The United Nations. American Stem Cell Base, Publisher, United States, 2024; ISBN 979-8-218-97499-2

