Physiological CTG Interpretation: Enhancing Fetal Monitoring
Physiological interpretation of cardiotocograph (CTG): Inter-observer and “Observer-AI” agreements in recognising types of fetal hypoxic stress
Chandraharan E1, Griffiths K2, Edmondson M3, Ingram C4, Mohamed T5, Zivkovic N6, Kovacev N7, Velemir L8
- Global Academy of Medical Education and Training Ltd, London, UK.
- Royal College of Midwives, UK
- Fetal Monitoring Midwife, Medical and Health Care Educator. MSc. Advanced Midwifery Practice. PgCert: Medical and Health Care Education.UK
- Barking, Havering and Redbridge University Hospitals NHS Trust, UK
- Forth Valley Royal Hospital, UK
- Rubik’s Code, Berlin, Germany
- Polyclinic OrtoMD, Futoska 117, 21000 Novi Sad, Serbia
- Gynecology Institute of Nice, 5 rue Cronstadt 06000 Nice, France
OPEN ACCESS
PUBLISHED 30 November 2024
CITATION Chandraharan, E., Griffiths, K., et al., 2024. Physiological interpretation of cardiotocograph (CTG): Inter-observer and “Observer-AI” agreements in recognising types of fetal hypoxic stress. Medical Research Archives, [online] 12(11). https://doi.org/10.18103/mra.v12i11.5980
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.18103/mra.v12i11.5980
ISSN 2375-1924
ABSTRACT
Introduction: Interobserver reliability in interpreting cardiotocographs (CTGs) using traditional categorization into “normal,” “suspicious,” and “pathological” is typically very low ranging from Kappa 0.3 to 0.6. Physiological CTG interpretation focuses on identifying specific features of different types of fetal hypoxic stress and a combination of features which are associated with adverse perinatal outcomes.
Objective: To evaluate the agreement among members of the Editorial Board (EBM) of the international expert consensus statement on physiological CTG interpretation, members of the international expert consensus panel (ICP), and the Tweris Mini App (TMA), which is an AI-based CTG interpretation tool developed based on international expert consensus statement.
Materials & Methods: Thirty 10–15-minute CTG trace segments, representing different types of fetal hypoxic stress (chronic, gradually evolving compensated, gradually evolving decompensated, subacute, and acute) and abnormal CTG patterns (atypical sinusoidal or the “Poole Shark Teeth”, typical sinusoidal and the ZigZag patterns), were independently reviewed by 3 editorial board members and 3 international expert consensus panel members. An orthopedic surgeon independently analyzed the same traces using the TMA. Fleiss’ Kappa and Z-scores were used for statistical analysis.
Results: Inter-observer agreement was 0.8 (95% CI: 0.72-0.87, p < .001) among EBM and 0.68 (95% CI: 0.60-0.76, p < .001) among ICP, with a statistically significant difference between these groups (p < .05). Agreement between EBM and the Tweris Mini App was higher than between ICP and the Tweris Mini App (0.81 vs 0.73, p = .06).
Conclusion: The inter-observer agreement when using physiological CTG interpretation surpasses that of the inter-observer agreement reported with traditional systems of CTG classification, with higher interobserver agreement among editorial board members compared to international expert consensus panel members. There was a substantial agreement between editorial board members and the Tweris Mini app which was higher than between ICP and the Tweris Mini App. These findings highlight the potential of AI-assisted tools, such as the Tweris Mini App, based on physiological CTG interpretation, to provide expert-level diagnostic accuracy in clinical practice. The Tweris Mini App was found to be superior in consistently recognising rare fetal heart rate patterns.
Keywords
cardiotocograph (CTG); physiological CTG; artificial intelligence (AI); hypoxia; false positive; Tweris Mini App (TMA)
Introduction
Cardiotocograph (CTG) trace was introduced into clinical practice to improve perinatal outcomes without increasing unnecessary intrapartum interventions such as emergency caesarean sections and operative vaginal births to the mother. The aim was to timely identify the features of the fetal heart rate which were suggestive of the onset of decompensation so that immediate actions could be instituted either to immediately improve fetal oxygenation and reverse the observed changes or to expedite birth to avoid hypoxic-ischaemic encephalopathy (HIE) and its long term sequalae such as cerebral palsy, learning difficulties and / or intrapartum-related perinatal deaths. However, due to the introduction of the technology directly into clinical practice without any prior robust scientific research or randomised controlled trials resulted in confusion and anxiety regarding the changes which were observed on the CTG trace. The features which were normal physiological cardioprotective reflex responses to reduce fetal myocardial workload (fetal heart rate decelerations) were illogically considered as “reassuring or non-reassuring” based on the apparent morphology or arbitrarily pre-determined time limits by several CTG guidelines, leading to an overall classification of CTG traces into “Normal, Suspicious, Pathological” or “Category I, II or III”. Repeated Cochrane Systematic Reviews on electronic fetal heart rate monitoring have highlighted that this unscientific approach without incorporation and the application of the knowledge of fetal pathophysiology has been associated with a significant increase in the rate of caesarean sections and operative vaginal births (vacuum and forceps) without any improvement in perinatal outcomes. A more recent systematic review and metanalysis of such a “pattern-recognition” methodology of randomly grouping different features of the fetal heart rate into pre-defined categories, has concluded that 98% of fetuses with “category II” fetal heart rate tracings, had normal perinatal outcomes.
Lack of scientific evidence supporting improvement in outcomes using the 3-tier CTG classification
It has been shown that despite the exponential increase in the rate of caesarean sections since the introduction of CTG into routine clinical practice in 1968, there has been no significant changes in cerebral palsy or perinatal deaths. This implies that the vast majority of emergency caesarean sections and operative vaginal births (vacuum or forceps) performed by obstetricians globally for presumed fetal compromise (twenty years ago, illogically called “fetal distress” a term often used to mask the “obstetrician’s distress due to observation of “concerning” CTG patterns) were totally unnecessary. This has been clearly shown by Karin Nelson et al, in 1996 who analysed > 150,000 CTG traces and their resultant perinatal outcomes, and concluded that, even in the presence of repetitive late decelerations and reduced baseline fetal heart rate variability, 99.8% of neonates were born with normal umbilical cord pH. Translating this information to clinical practice, over 99% of “emergency caesarean sections and operative vaginal births” performed for presumed fetal compromise after illogically classifying CTG traces as “abnormal, pathological or Category III” are potentially unnecessary. The inability of abnormal CTG features and classifications systems which are based on “pattern-recognition”, without any consideration of fetal pathophysiological responses to ongoing stress, to predict neonatal acidosis had been highlighted again, more than 20 years after the publication by Karin Nelson et al.
Very high false positive rates of cardiotocographs using the 3-tier classification system
Honest reflection by intrapartum care providers would confirm that no test that is currently used in clinical medicine has such an extremely high false positive rate of 99.8%. The adverse impact on women due to increased rate of emergency caesarean sections and operative vaginal births are significant: postpartum haemorrhage, wound infection, prolonged hospitalisation, sepsis, venous thromboembolism, uterine rupture, placental accreta spectrum and post-traumatic stress disorder- to mention a few. If these are due to the extremely high false positive rate (and therefore, 99.8% of these serious complications are potentially avoidable), then this raises serious ethical questions. Therefore, it is not surprising that several authors have recently questioned the continuing use of CTG in clinical practice.
Very high false positive rate of CTG and misinterpretation: What is the problem?
One may argue that the interpretation of a CTG trace is similar interpreting an ECG trace, and therefore, unlike physicians and nurses who interpret ECG traces with relatively much lower false positive and negative rates, obstetricians and midwives have a knowledge gap. This argument is valid because it is the deficiency of knowledge of fetal pathophysiology that has led to poor interpretation of CTG traces resulting in poor maternal and perinatal outcomes, however, it is important to appreciate that the frontline obstetricians and midwives are not responsible for the current predicament facing the specialty. The fundamental flaw was the introduction of CTG into clinical practice without any prior robust scientific studies which resulted in grouping random features of fetal heart rate into different categories. More importantly, fetal heart rate decelerations which are physiological cardioprotective reflexes to compensate for hypoxic stress, similar to adults increasing the rate and depth of respiration whilst running, were considered as “pathological” based on their apparent morphology resulting in an exponential increase in unnecessary intrapartum caesarean sections. These historical unscientific practices, and illogical classification systems used have been recently highlighted in several publications.
Emphasizing and coercing intelligent obstetricians and midwives to embark on a hazardous, and scientifically impotent journey of “pattern recognition” by classifying the observed fetal heart rate decelerations into “early, variable, late, typical, atypical, complicated, uncomplicated, reassuring, non-reassuring’ by those who produced CTG guidelines, resulted in the anticipated outcome of any attempts at human pattern recognition: inconsistent recognition of these patterns. Several scientific publications have highlighted both inter and intra-observer disagreements in recognition of fetal heart rate changes, especially morphology of decelerations, with the range of agreements between very poor to modest (Kappa 0.3-.0.6). Even the presumed or those who assumed themselves as “experts” in CTG interpretation, and those who went to courts due to such presumed expertise to provide medico-legal opinions on the standard of CTG interpretation by their fellow obstetricians were also subjected to the same pitfalls of pattern recognition resulting in poor agreement. More worryingly, once the neonatal outcomes were revealed to the experts, based on the knowledge of outcomes, they changed their own earlier classification. This highlights the perils of depending on such experts without the knowledge of fetal pathophysiology, who base their retrospective opinions on patterns with the benefit of their hindsight of knowing the perinatal outcomes, and this calls into question their ability to provide honest medico-legal opinions against their peers.
Human costs of misinterpretation of cardiotocographs
The impact of the failure to apply the knowledge of fetal pathophysiology whilst interpreting CTG traces and solely relying on grouping random features of FHR into different “categories” (normal, suspicious, pathological or category I , II or III) with resultant significant inter-and intra-observer disagreements in pattern recognition, not only resulted in an increase in the intrapartum operative interventions to the mother and resultant complications, but it also resulted in significant harm to babies. Four successive “Each Baby Counts” Reports produced by the Royal College of Obstetricians and Gynaecologists in the UK annually concluded that over 1000 babies sustained severe intra-partum related hypoxic-ischaemic brain injuries and/or died during the first 7 days of life in the UK every year, and unfortunately, 70% of these sad outcomes a different care would have given rise to different outcomes. More importantly, > 60% of these adverse perinatal outcomes were due to errors in CTG interpretation and fetal scalp blood sampling (FBS) and unfortunately, without rectifying these errors immediately after the publication of the first report in 2016 by introducing a pathophysiological approach to CTG interpretation, subsequent reports were produced. As would be expected by any clinician who understands logic, the outcomes had worsened during the subsequent years of counting with an additional 200 babies sustaining severe hypoxic ischaemic brain injuries in the 2020 Report as compared to 2016. The medico-legal costs to the National Health Service (NHS) and the UK taxpayer due to these avoidable clinical negligence cases have been substantial. The NHS Resolution, which is a body set up to indemnify hospitals against medico-legal claims in the UK concluded that very little has changed with regard to errors in CTG misinterpretation, and its 2019 Report, highlighted 70% of cases of severe hypoxic-ischaemic encephalopathy (HIE) were due to CTG misinterpretation. Incorrect CTG classification had contributed to 42.8 % of these cases.
Financial costs of misinterpretation of cardiotocographs
The latest NHS Resolution Annual Report & Accounts (2023/24) has highlighted that the maternity service had contributed to 62% of the total clinical negligence provision (£ 58.5 billion) of the NHS and in one year (2023/24) 49% of total (£ 5.1 billion) clinical negligence cost of harm was contributed by the UK maternity service. This translates to approximately £13.7 million paid out every day due to clinical negligence in the UK maternity service, and CTG misinterpretation resulting in cerebral palsy, learning difficulties and perinatal deaths being the major contributor.
What was the response to poor maternal and perinatal outcomes due to CTG misinterpretation?
“Knee-jerk reactions” are a known common response to lack of knowledge in clinical medicine. Fetal scalp blood sampling (FBS) was hastily introduced as an “adjunct” to CTG to reduce the false positive rate of the CTG and to reduce unnecessary intrapartum operative interventions, in lieu of improving the knowledge of fetal pathophysiology. Those who advocated FBS and those who propagated it through national guidelines failed to realise that the fetal scalp is a peripheral non-essential tissue which undergoes catecholamine-mediated vasocontraction to centralise blood flow. Therefore, similar to marathon runners, the lactic acidosis in the skin of the fetal scalp due this effective re-distribution reflects fetal compensation. It was obvious to those who understood basic human physiology that this illogical, unscientific approach of considering the lactic acidosis due to compensatory fetal response as “abnormal indicating of fetal distress” would lead to unnecessary intrapartum emergency caesarean sections, and operative vaginal births increasing the risks of maternal morbidity and mortality. Repetitive Cochrane Systematic Reviews highlighting lack of reduction in emergency caesarean sections, and improvement in perinatal outcomes, and FBS was stopped in the USA and many countries around the world approximately 30 years ago. Unfortunately, due to historical beliefs and lack of knowledge, FBS was continued to be performed in some European and Scandinavian countries and a handful of “common-wealth” countries which simply followed UK guidelines despite the Cochrane Reviews and many publications highlighting the dangers of FBS. Even after the scientific publications concluding that the use of FBS increased the rate of caesarean sections and operative vaginal births, very unfortunately, FBS was continued to be performed due to the lack of knowledge on fetal physiology, despite the full knowledge of serious maternal complications that may occur due to unnecessary intrapartum emergency caesarean sections. It FBS was stopped by the NICE CTG guideline only in 2022, despite the Confidential Enquiries into Maternal Deaths in the UK highlighting in 2018 a near doubling of maternal deaths due top postpartum haemorrhage, predominantly contributed by placenta accreta spectrum (PAS) which was mainly due to previous caesarean sections. Similar attempts at assessing oxygenation saturation around the fetal face using pulse oximetry also resulted in dismal failure, due to the assessment of the wrong fetal tissue.
The use of illogical fetal scalp stimulation test
Some obstetricians, especially from Republic of Ireland had embarked on “fetal scalp stimulation” as an adjunctive test due to the lack of appreciation of the fact that if the skin of the adult with severe sepsis is stimulated, they may move their limbs in response to that stimulus. However, such movement induced by the stimulation of the skin does not indicate that they are well. Similarly, fetuses with chorioamnionitis and fetal inflammatory response syndrome may show a response on the CTG trace and by considering this response to suggest a as “normal, uncompromised fetus” would lead to disastrous consequences. It is indeed very regrettable that despite the recent Cochrane systematic Review questioning the safety of fetal scalp stimulation, a randomised trial is currently being conducted despite the knowledge that babies may be harmed due to the false negative result during this randomised controlled trial.
Use of fetal electrocardiograph using illogical CTG guidelines
Fetal ECG or ST-Analyser (STAN) was introduced into clinical practice as an adjunct test, and it showed a great promise because pathophysiology on which the technology was based, was scientifically very sound. Unfortunately, due to the use of a CTG guideline which was solely based on pattern recognition (normal, intermediary, abnormal), the potential benefits of the STAN technology have been blunted. This is illustrated by a recent systematic review and a meta-analysis of nine randomised controlled trials questioning the clinical utility of STAN. Recently, it has been suggested the use of STAN should no longer recommended using the current CTG guideline tool introduced by the manufacturer and a physiological approach to CTG interpretation to harness the true benefits of STAN to improve outcomes for women and babies is essential.
Use of computer software wrongly programmed on “normal, suspicious, pathological”
Some clinicians embarked on the use of computerised intrapartum CTG analysis to eliminate the impact of human error and to reduce inter and intra-observer variation in interpretation. Unfortunately, they had programmed the unsuspecting computer software on the unphysiological “normal, suspicious, pathological” classification system. Therefore, it was not surprising that the two large randomised controlled trials: the multicentre INFANT Trial from the UK, and the multicentre, European “FM-Alert” Trial have both concluded that computerised Analysis of intrapartum CTG did not improve maternal or perinatal outcomes. A more recent systematic review on intrapartum computerised CTG interpretation has also confirmed its lack of benefit.
The birth of Physiological Interpretation of CTG and the Tweris Mini App (AI)
The concepts of physiological interpretation of CTG were pioneered and implemented at a leading teaching hospital in London, UK in response to an increased number of babies who had sustained hypoxic-ischaemic encephalopathy (CTG) due to the use of “normal, suspicious, pathological” classification system and resultant CTG misinterpretation. After the implementation of physiological interpretation of the CTG, interpretation was based on the application of knowledge of fetal physiology and the wider clinical context, a classification system based on the types of fetal hypoxia was developed. The main aim was to reduce unnecessary intrapartum operative interventions, and to totally eliminate the illogical FBS by the application of fetal physiology to understand the features of compensation to differentiate from features of decompensation, as well as to reduce avoidable intrapartum hypoxia related hypoxic-ischaemic brain injuries and perinatal deaths. There was approximately a 50% reduction in the rate of HIE and emergency caesarean sections after the introduction of physiological interpretation of CTG, which was the driver for the wider dissemination of the knowledge of physiological interpretation of CTG, by conducting over 150 Physiological CTG Masterclasses in over 20 countries from 2006 to 2024. This resulted in several other maternity units showing similar improvement in perinatal outcomes, and the publication of the first international consensus guidelines on physiological CTG interpretation in 2018. The concepts of physiological interpretation of CTG have been implemented in more than 20 countries and a publication from Spain suggested that this had a better predictive value for neonatal acidosis as compared to NICE, FIGO and ACOG CTG guidelines. In view of several publications supporting the concepts of physiological CTG interpretation, the physiological CTG guideline was revised in 2024.
Tweris CTG AI Mini App (named after Taouris, a protective goddess of childbirth and fertility in Ancient Egyptian Religion), was a collaborative effort between teams in France, UK, Serbia and Germany to ensure the democratisation of the knowledge of physiological interpretation of CTG to protect inherited intelligent potential of human fetuses and to protect their mothers from avoidable harm due to unnecessary intrapartum operative interventions due to CTG misinterpretation (https://tweris.com/our-solution/). It is based on the classification tool based on types of fetal hypoxia recommended by the latest international expert consensus statement on physiological interpretation of CTG, and the Tweris CTG AI Mini App (TMA) is being continuously upgraded based on submission of data from clinicians and several hospitals from in Europe, Asia, China, and the Middle East to continuously improve its accuracy.
Objective
The objective of this study was to evaluate the agreement among members of the Editorial Board (EBM) of the international expert consensus statement on physiological CTG interpretation, members of the international expert consensus panel (ICP), and the Tweris Mini App (TMA), which is an AI-based tool CTG interpretation tool developed based on international expert consensus statement.
Materials & Methods
Thirty 10–15-minute, anonymised segments of CTG traces, representing different types of fetal hypoxic stress (chronic, gradually evolving compensated, gradually evolving decompensated, subacute, and acute) and abnormal CTG patterns (atypical sinusoidal or the “Poole Shark Teeth”, typical sinusoidal and the ZigZag patterns) were randomly selected. An independent online link was created and this link was sent to 3 EBM and 3 ICP members, who independently reviewed the CTG traces. Their responses were independently collated and analysed by the online software. An orthopedic surgeon independently analyzed the same traces using the Tweris Mini App. Statistical analysis of the database was performed using the Fleiss’ Kappa and Z-scores to determine statistical significance. Ethics approval was not required because this was a retrospective study with no patient identifiable data, and with no interventions.
Results
The overall inter-observer agreement amongst all six expert assessors was 0.78 (Table 1), and this agreement was higher with a Fleiss’ Kappa score of 0.8 (95% CI: 0.72-0.87, p < .001) among the editorial board members as compared to 0.68 (95% CI: 0.60-0.76, p < .001) among international expert consensus panel members. This observed difference in the degree of inter-observer agreement between EBM and ICP was statistically significant (p < .05). The level of agreement between EBM and the Tweris Mini App was 0.81 (95% CI: 0.76-0.87, p < .001), and between ICP and The Tweris Mini App was 0.73 (95% CI: 0.67-0.79, p < .001). The agreement between editorial board members and the Tweris Mini App was higher than between the international expert consensus panel and the Tweris Mini App (0.81 vs 0.73, p = .06). However, this difference in the observer-AI agreement between the two groups of experts (EBM & ICP) and the Tweris Mini App did not reach statistical significance.
| DIAGNOSTIC RELIABILITY | Fleiss Kappa | Standard Error | 95% CI | P value |
|---|---|---|---|---|
| Among EBM | 0.80 | 0.04 | 0.72-0.87 | <.001 |
| Among ICP | 0.68 | 0.04 | 0.60-0.76 | <.001 |
| Between EBM and ICP | 0.77 | 0.02 | 0.73-0.80 | <.001 |
| Between EBM and TMA | 0.81 | 0.03 | 0.76-0.87 | <.001 |
| Between ICP and TMA | 0.73 | 0.03 | 0.67-0.79 | <.001 |
| Among the 6 observers | 0.78 | 0.01 | 0.75-0.81 | <.001 |
Discussion
To our best knowledge, this is the first study which analysed the interobserver agreement between clinicians using the principles of pathophysiological interpretation in their daily clinical practice, as well as the CTG AI App (TMA), which has been exclusively trained on the principles of physiological interpretation of CTG. All the experts had undergone training by attending physiological CTG masterclasses, and the members of the expert group (EBM) have demonstrated changes in the maternal and perinatal outcomes after the implementation of physiological interpretation of CTG in scientific publications, and the members of the international consensus panel (ICP) are in different stages in their journey into physiological interpretation of CTG.
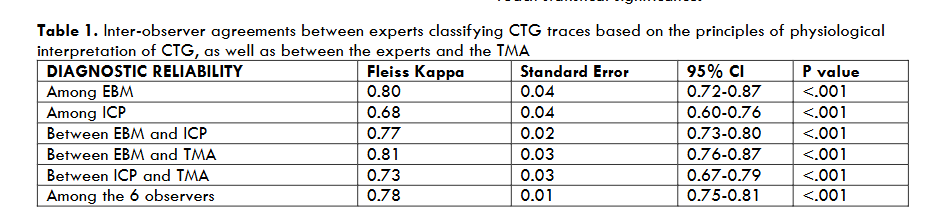
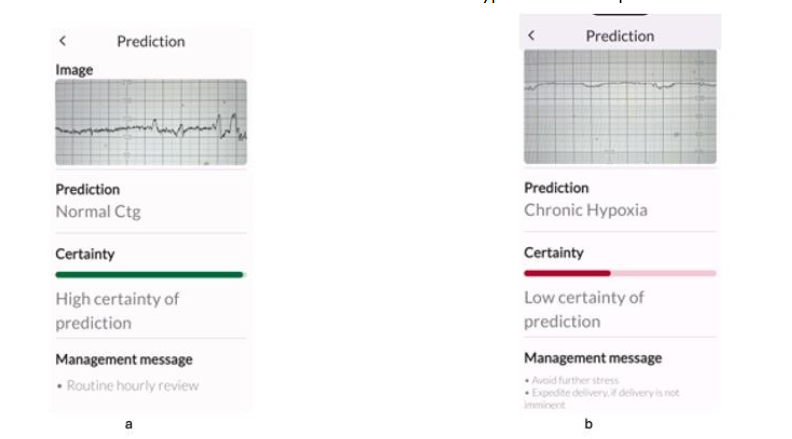
All experts correctly identified the features of normal CTG as well as chronic hypoxia (Figure 1), and there was 100% agreement with the TMA. This agreement is important not only to avoid unnecessary interventions, but also to ensure timely birth for fetuses with pre-existing hypoxia with decompensation without misclassifying them as “suspicious”. Similarly, all the six experts and the TMA correctly identified acute hypoxia due to a sudden and profound interruption of fetal oxygenation (Figure 2a), and the ZigZag pattern due to a rapidly evolving hypoxia (Figure 2b). This has important clinical implications because timely recognition is important to rapidly restore oxygenation to fetal central organs to avoid hypoxic ischaemic brain injuries and/or perinatal deaths, and to accomplish immediate birth if reversal of the hypoxic insult is not possible.
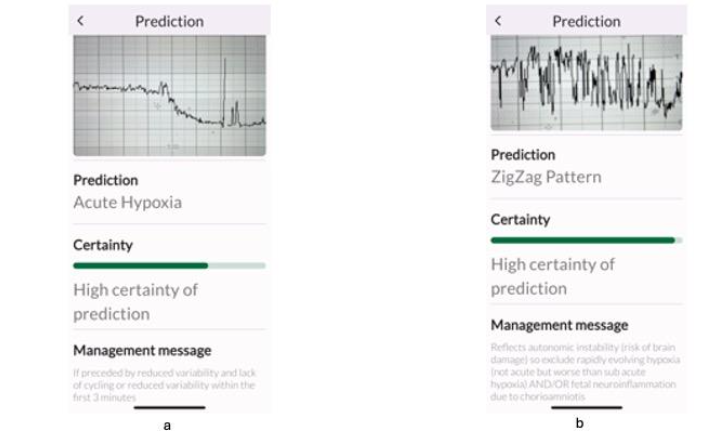
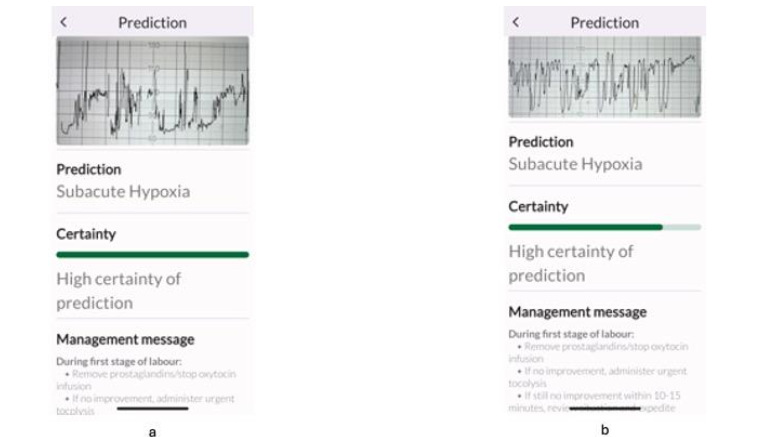
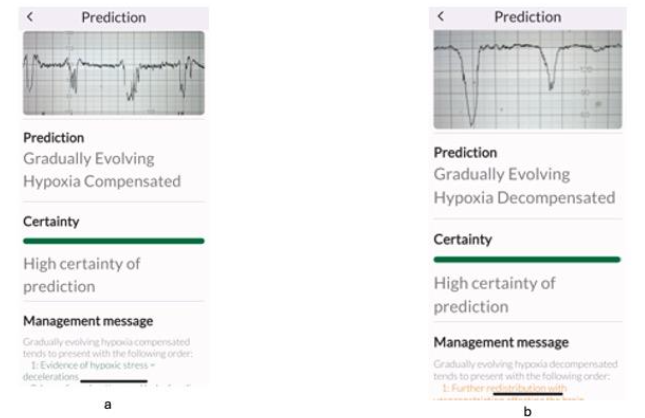
All experts correctly identified the subacute hypoxic pattern (Figure 3a), but, when subacute hypoxic pattern was interspersed with the ZigZag pattern (Figure 3b), some experts classified this only as “ZigZag” pattern which was predominant. However, this disagreement is very unlikely to affect clinical management because the immediate recommended management by both international expert consensus statement, and the TMA is the same for both subacute hypoxic pattern and the ZigZag pattern: immediately stop ongoing stress and ensure rapid intrauterine resuscitation to restore oxygenation to the fetal central organs.
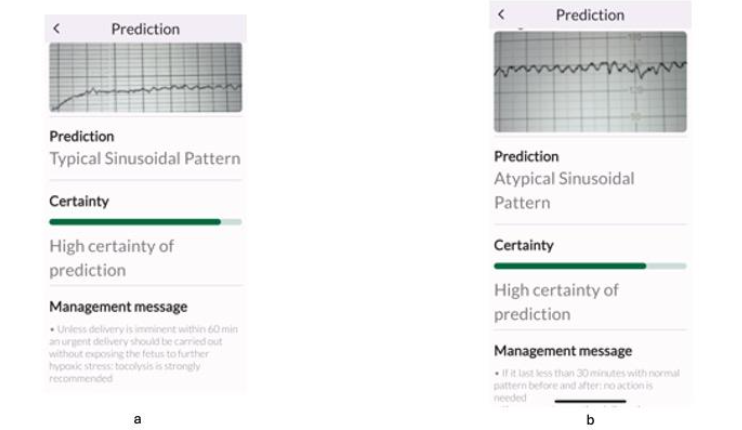
The greatest variation in agreements between the experts as well as between the experts and the TMA was observed whilst recognising the features of typical (Figure 4 a) and atypical (the Poole Shark Teeth Pattern) sinusoidal patterns (Figure 4b). The management of chronic fetal anaemia and acidosis (typical sinusoidal pattern), and acute fetal hypovolemia and hypotension (atypical sinusoidal or the “Poole Shark Teeth” pattern) is similar. Both require expediting birth, but the degree of urgency is greater in the latter. However, the TMA consistently identified these correctly, and this illustrates the role of AI in recognising and assisting clinicians to make the correct diagnosis when encountering rare FHR patterns.
Our study highlights that the importance of fetal pathophysiological knowledge based interpretation of CTG traces enhances the level of agreement between experts, and the degree of agreement observed is much higher than the reported rates by studies using the 3-tier CTG classification systems. Out of desperation, some even proceeded to a 5-tier classification system, but simply increasing the number of tiers without incorporating the application of fetal pathophysiology is likely to increase confusion without improving outcomes. It is important to appreciate that the level of agreement reported in the study is for the correct recognition of seven parameters (four types of fetal hypoxic stress: chronic, gradually evolving, subacute and acute, as well as for 3 different patterns suggestive of abnormal CNS function (the ZigZag, Typical sinusoidal and Atypical sinusoidal or the Poole Shark Teeth patterns), as opposed to several studies reporting a lower level of agreement for recognition of only 3 parameters (normal, suspicious, pathological). This illustrates that the application of the knowledge of fetal pathophysiology to recognise a combination of features of FHR (chronic and subacute hypoxic stress) or a sequence of features (gradually evolving hypoxia: compensated or decompensated) enables a higher degree of interobserver agreement. This is important because this pathophysiological approach to the interpretation of observed FHR changes will help reduce variation in clinical management potentially leading to improved, and consistent maternal and perinatal outcomes by eliminating inter-observer variability. Our study highlights the importance of the Tweris AI App, which has been exclusively trained on the recognition of FHR changes based on fetal pathophysiology in reducing interobserver variability, and thereby, potentially improving maternal and perinatal outcomes by reducing human error.
The main strength of the study is the application of fetal pathophysiology whilst interpreting CTG traces based on the principles of stipulated by the international expert consensus statement of physiological interpretation of CTG. This approach differs from the traditional random grouping of features into different categories (“normal, suspicious, pathological” or “category I, II or III”) because the clinicians have to consider a combination of CTG features to recognise chronic hypoxia and subacute hypoxia, and a sequence of evolving features to recognise a gradually evolving hypoxia by the application of the knowledge of fetal pathophysiology. Moreover, it involves differentiating features suggestive of fetal compensation to ongoing hypoxic stress from features of decompensation.
In addition, we compared the level of agreements of experts with the novel TMA, which is exclusively trained to recognise different types of hypoxic stress. It is user friendly and easy to use and also has a ChatBot for frontline clinicians to get additional information relating to physiological interpretation of CTG.
It may be argued that the limitation of our study is that we only analysed 30 CTG traces. However, several other studies which determined the interobserver variability using intrapartum CTG traces have used a similar or fewer CTG traces to determine inter-observer agreement. We had 7 experts comprising of 3 obstetricians, 3 midwives and an independent orthopaedic surgeon who had expertise in AI, which was unique. Moreover, we have 3 experts from EBM and 3 experts from ICP to reflect the real-life situation that expertise in pathophysiological interpretation evolves with time, to make our study clinically applicable.
Implications for Clinical Practice
To our best knowledge, this is the first scientific study that determined the level of interobserver agreement amongst experts who use physiological interpretation of CTG in their daily clinical practice. In comparison to earlier studies determining the interobserver agreement reporting only poor or modest agreements (Kappa 0.3 to 0.6) for classification systems using “normal, suspicious, pathological’ (i.e., 3-tier classification systems), the use of physiological interpretation of CTG is associated with overall good agreement (Kappa=0.78), and excellent agreement amongst the editorial board members (Kappa = 0.81). However, the levels of agreement between the two groups of experts (EBM & ICP) and Tweris Mini App did not reach statistical significance which suggests that the Tweris Mini App may have a vital role in eliminating interobserver variation by eliminating human errors relating to pattern recognition. This may not only improve the consistency in the interpretation of intrapartum fetal heart rate changes, but also may help avoid human and financial costs of CTG misinterpretation.
Conclusion
The inter-observer agreement when using physiological CTG interpretation surpasses that of the inter-observer agreement reported with traditional systems of CTG classification, with higher interobserver agreement among EBM compared to ICP. There was a substantial agreement between EBM and TMA, which was higher than between ICP and TMA. These findings highlight the potential of AI-assisted tools, such as TMA, based on physiological CTG interpretation, to provide expert-level diagnostic accuracy in clinical practice. TMA was found to be superior in consistently recognising rare fetal heart rate patterns such as typical and atypical (Poole Shark Teeth patterns).
Contribution to Authorship
EC conceptualised the study, and LV and EC designed the study. LV, EC, ME, KG, CI and TM classified the CTG traces. NK independently analysed the CTG traces using the TMA. LV analysed the data and NZ was involved in the technical development of the TMA. All authors contributed to the writing of the manuscript and reviewed and approved the manuscript.
Conflict of Interest
EC, CI and KG are members of the Editorial Board, and TM, LV and ME are members of the International Expert Consensus Statement on Physiological Interpretation of CTG, published by > 50 CTG experts from > 20 countries in 2024. LV, EC and NK are the founding members of Tweris S.A.S which is developing the TMA and is hosted and supported by Start-up Incubator Paca Est, France. The TMA has been currently made available free of charge in Apple and Android Stores for midwives and obstetricians worldwide with its aim to improve perinatal outcomes and to reduce unnecessary intrapartum operative interventions due to CTG misinterpretation.
References
1. Fetal monitoring in labour. NICE Guideline [NG 229]. 2022. (https://www.nice.org.uk/guidance/ng229).
2. Ayres-De-Campos D, Spong CY, Chandraharan E. FIGO Intrapartum Fetal Monitoring Expert Consensus Panel. FIGO consensus guidelines on intrapartum fetal monitoring: cardiotocography. Int J Gynaecol Obstet 2015;131:13–24.
3. American College of Obstetricians and Gynecologists ACOG practice bulletin No 106: intrapartum fetal heart rate monitoring: nomenclature, interpretation, and general management principles. Obstet Gynecol 2009;114:192-202.
4. Alfirevic Z, Devane D, Gyte GM, Cuthbert A. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev 2017;2:CD006066.
5. Zullo F, Di Mascio D, Raghuraman N, Wagner S, Brunelli R, Giancotti A, Mendez-Figueroa H, Cahill AG, Gupta M, Berghella V, Blackwell SC, Chauhan SP. Three-tiered fetal heart rate interpretation system and adverse neonatal and maternal outcomes: a systematic review and meta-analysis. Am J Obstet Gynecol. 2023 Oct;229(4):377-387.
6. Clark SL, Hankins GD. Temporal and demographic trends in cerebral palsy—fact and fiction. Am J Obstet Gynecol 2003;188:628-33.
7. Nelson KB, Dambrosia JM, Ting TY, Grether JK. Uncertain value of electronic fetal monitoring in predicting cerebral palsy. N Engl J Med 1996;334:613-8.
8. Clark SL, Hamilton EF, Garite TJ, et al. The limits of electronic fetal heart rate monitoring in the prevention of neonatal metabolic acidemia. Am J Obstet Gynocol 2017; 216: 163.e1–163.e6.
9. Sartwelle TP, Johnston JC, Arda B, Zebenigus M. Cerebral palsy, cesarean sections, and electronic fetal monitoring: All the light we cannot see. Clinical Ethics. 2019;14(3):107-114.
10. Nelson KB, Sartwelle TP, Rouse DJ. Electronic fetal monitoring, cerebral palsy, and caesarean section: assumptions versus evidence. BMJ. 2016 Dec 1;355:i6405.
11. Chandraharan E Intrapartum care: An urgent need to question historical practices and ‘non-evidence’-based, illogical foetal monitoring guidelines to avoid patient harm. Journal of Patient Safety and Risk Management. 2019; 24(5): 210-217.
12. Chandraharan E, Tahan ME, Pereira S. Each fetus matters: an urgent paradigm shift is needed to move away from the rigid “CTG Guideline Stickers” so as to individualize intrapartum fetal heart rate monitoring and to improve perinatal outcomes. Obstet Gynecol Int J 2016;5:00168.
13. Chandraharan E. Updated NICE Cardiotocograph (CTG) guideline: Is it suspicious or pathological?. J Clin Med Surgery. 2023; 3(2): 1129.
14. Bernardes J, Costa-Pereira A, Ayres-de-Campos D, van Geijn HP, Pereira-Leite L. Evaluation of interobserver agreement of cardiotocograms. Int J Gynaecol Obstet 1997;57:33–7.
15. Blackwell SC, Grobman WA, Antoniewicz L, Hutchinson M, Gyamfi Bannerman C. Interobserver and intraobserver reliability of the NICHD 3-tier fetal heart rate interpretation system. Am J Obstet Gynecol 2011;205:378.e1–5.
16. Rei M, Tavares S, Pinto P, et al. Interobserver agreement in CTG interpretation using the 2015 FIGO guidelines for intrapartum fetal monitoring. Eur J Obstet Gynecol Reprod Biol 2016;205:27–31.
17. Beaulieu MD, Fabia J, Leduc B, et al. The reproducibility of intrapartum cardiotocogram assessments. Can Med Assoc J 1982;127: 214–6.
18. Santo S, Ayres-de-Campos D, Costa-Santos C, et al. Agreement and accuracy using the FIGO, ACOG and NICE cardiotocography interpretation guidelines. Acta Obstet Gynecol Scand 2017;96:166–75.
19. Santo S, Ayres-de-Campos D. Human factors affecting the interpretation of fetal heart rate tracings: an update. Curr Opin Obstet Gynecol 2012;24:84–8.
20. Hruban L, Spilka J, Chudacek V, et al. Agreement on intrapartum cardiotocogram recordings between expert obstetricians. J Eval Clin Pract 2015; 21: 694–702.
21. Sabiani L, Le Dû R, Loundou A, et al. Intra- and interobserver agreement among obstetric experts in court regarding the review of abnormal fetal heart rate tracings and obstetrical management. Am J Obstet Gynecol 2015;213:856.e1-8.
22. Ayres-de-Campos D, Arteiro D, Costa-Santos C, Bernardes J. Knowledge of adverse neonatal outcome alters clinicians’ interpretation of the intrapartum cardiotocograph. BJOG 2011;118:978-84.
23. Figueras F, Albela S, Bonino S, et al. Visual analysis of antepartum fetal heart rate tracings: inter- and intra-observer agreement and impact of knowledge of neonatal outcome. J Perinat Med 2005;33:241–5.
24. Reif P, Schott S, Boyon C, et al. Does knowledge of fetal outcome influence the interpretation of intrapartum cardiotocography and subsequent clinical management? A multicentre European study. BJOG 2016.
25. Zain HA, Wright JW, Parrish GE, Diehl SJ. Interpreting the fetal heart rate tracing. Effect of knowledge of neonatal outcome. J Reprod Med 1998;43:367–70.
26. Royal College of Obstetricans and Gynaecologists. Each baby Counts: key messages from 2015. London: RCOG. 2016. (https://www.rcog.org.uk/media/3fopwy41/each-baby-counts-2015-full-report.pdf).
27. Royal College of Obstetricians and Gynaecologists. Each Baby Counts: 2018 Progress Report. London: RCOG. 2018. (https://www.rcog.org.uk/media/dswjqyin/each-baby-counts-re¬port-2018-11-12.pdf).
28. Royal College of Obstetricians and Gynaecologists. Each Baby Counts: 2019 Progress Report. London: RCOG. 2020. (https://www.rcog.org.uk/media/qhzlelnc/each-baby-counts-2019-progress-report.pdf).
29. Royal College of Obstetricians and Gynaecologists. Each Baby Counts: 2020 Final Progress Report. London: RCOG. 2021. (https://www.rcog.org.uk/media/a4eg2xnm/ebc-2020-final-progress-report.pdf).
30. Anderson A. Ten years of maternity claims: an analysis of the NHS Litigation Authority data—key findings. Clin Risk 2013;19:24-31 10.1177/1356262213486434.
31. Wise J. Litigation in maternity care is rising, says National Audit Office. BMJ 2013;347:f6737.
32. Donn SM, Chiswick ML, Fanaroff JM. Medico-legal implications of hypoxic-ischemic birth injury. Semin Fetal Neonatal Med 2014;19:317-21.
33. The Early Notification scheme progress report: collaboration and improved experience for families. NHS Resolution, September 2019. https://resolution.nhs.uk/wp-content/uploads/2019/09/NHS-Resolution-Early-Notification-report.pdf
34. NHS Resolution. Annual Report & Accounts 2023/24. Published on 23 July 2024. https://resolution.nhs.uk/wp-content/uploads/2024/07/NHS-Resolution-Annual-report-and-accounts_23-24_Access.pdf
35. Chandraharan E. Fetal scalp blood sampling during labour: is it a useful diagnostic test or a historical test that no longer has a place in modern clinical obstetrics? BJOG 2014; 121: 1056–1062.
36. Chandraharan E. Should national guidelines continue to recommend fetal scalp blood sampling during labor? J Matern Fetal Neonatal Med. 2016 Nov;29(22):3682-5.
37. Chandraharan E. Fetal scalp blood sampling should be abandoned: FOR: FBS does not fulfil the principle of first do no harm. BJOG. 2016 Oct;123(11):1770.
38. Holzmann M, Wretler S, Cnattingius S, et al. Neonatal outcome and delivery mode in labors with repetitive fetal scalp blood sampling. Eur J Obstet Gynecol Reprod Biol 2015; 184: 97–102.
39. Stål I, Wennerholm UB, Nordstrom L, Ladfors L, Wiberg-Itzel E. Fetal scalp blood sampling during second stage of labor – analyzing lactate or pH? A secondary analysis of a randomized controlled trial. J Matern Fetal Neonatal Med. 2022 Mar;35(6):1100-1107.
40. Al Wattar BH, Lakhiani A, Sacco A, et al; AB-FAB Study Group. Evaluating the value of intrapartum fetal scalp blood sampling to predict adverse neonatal outcomes: a UK multicentre observational study. Eur J Obstet Gynecol Reprod Biol 2019; 240: 62–67.
41. O’Heney J, McAllister S, Maresh M, Blott M. Fetal monitoring in labour: summary and update of NICE guidance. BMJ. 2022; 379: 2854.
42. Knight M, Bunch K, Tuffnell D, Jayakody H, Shakespeare J, Kotnis R, Kenyon S, Kurinczuk JJ (Eds.) on behalf of MBRRACE-UK. Saving Lives, Improving Mothers’ Care – Lessons learned to inform maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2014-16. Oxford: National Perinatal Epidemiology Unit, University of Oxford 2018. https://www.npeu.ox.ac.uk/assets/downloads/mbrrace-uk/reports/MBRRACE-UK%20Maternal%20Report%202018%20-%20Web%20Version.pdf
43. East CE, Begg L, Colditz PB, Lau R. Fetal pulse oximetry for fetal assessment in labour. Cochrane Database of Systematic Reviews 2014, Issue 10. Art. No.: CD004075. DOI: 10.1002/14651858.CD004075.pub4
44. Tahir Mahmood U, O’Gorman C, Marchocki Z, O’Brien Y, Murphy DJ. Fetal scalp stimulation (FSS) versus fetal blood sampling (FBS) for women with abnormal fetal heart rate monitoring in labor: a prospective cohort study. J Matern Fetal Neonatal Med. 2018 Jul;31(13):1742-1747.
45. Shakouri F, Iorizzo L, Edwards HMK, Vinter CA, Kristensen K, Isberg PE, Wiberg N. Effectiveness of fetal scalp stimulation test in assessing fetal wellbeing during labor, a retrospective cohort study. BMC Pregnancy Childbirth. 2020 Jun 5;20(1):347.
46. Murphy DJ, Devane D, Molloy E, Shahabuddin Y. Fetal scalp stimulation for assessing fetal well‐being during labour. Cochrane Database of Systematic Reviews 2023, Issue 1. Art. No.: CD013808. DOI: 10.1002/14651858.CD013808.pub2. Accessed 13 October 2024.
47. Murphy DJ, Shahabuddin Y, Yambasu S, O’Donoghue K, Devane D, Cotter A, Gaffney G, Burke LA, Molloy EJ, Boland F. Digital fetal scalp stimulation (dFSS) versus fetal blood sampling (FBS) to assess fetal wellbeing in labour-a multi-centre randomised controlled trial: Fetal Intrapartum Randomised Scalp Stimulation Trial (FIRSST NCT05306756). Trials. 2022 Oct 4;23(1):848.
48. Chandraharan E. Foetal electrocardiograph (ST-analyser or STAN) for intrapartum foetal heart rate monitoring: a friend or a foe? J Matern Fetal Neonatal Med 2018;31:123–7.
49. Blix E, Brurberg KG, Reierth E, Reinar LM, Øian P. ST waveform analysis vs cardiotocography alone for intrapartum fetal monitoring: An updated systematic review and meta-analysis of randomized trials. Acta Obstet Gynecol Scand. 2024 Mar;103(3):437-448.
50. Chandraharan E. Fetal Electrocardiograph (ST-Analyser or STAN): Is it time for the Requiem?. J Clin Med Surgery. 2023; 3(2): 1111.
51. Brocklehurst P, Field D, Greene K, Juszczak E, Kenyon S, Linsell L, Mabey C, Newburn M, Plachcinski R, Quigley M, Steer P, Schroeder L, Rivero-Arias O. Computerised interpretation of the fetal heart rate during labour: a randomised controlled trial (INFANT). Health Technol Assess. 2018 Feb;22(9):1-186.
52. Nunes I, Ayres-de-Campos D, Ugwumadu A, Amin P, Banfield P, Nicoll A, Cunningham S, Sousa P, Costa-Santos C, Bernardes J; Fetal Monitoring and Alert (FM-ALERT) Study Group. Central Fetal Monitoring With and Without Computer Analysis: A Randomized Controlled Trial. Obstet Gynecol. 2017 Jan;129(1):83-90.
53. Campanile M, D’Alessandro P, Della Corte L, Saccone G, Tagliaferri S, Arduino B, Esposito G, Esposito FG, Raffone A, Signorini MG, Magenes G, Di Tommaso M, Xodo S, Zullo F, Berghella V. Intrapartum cardiotocography with and without computer analysis: a systematic review and meta-analysis of randomized controlled trials. J Matern Fetal Neonatal Med. 2020 Jul;33(13):2284-2290.
54. Chandraharan E, Arulkumaran S. Prevention of birth asphyxia: responding appropriately to cardiotocograph (CTG) traces. Best Pract Res Clin Obstet Gynaecol 2007;21:609–24.
55. Chandraharan E. Rational Approach to electronic fetal monitoring in all resource settings. SL J Obst & Gynaecol 2010; 32: 77-84
56. McDonnell S, Chandraharan E, The Pathophysiology of CTGs and Types of Intrapartum Hypoxia, Current Women`s Health Reviews 2013; 9(3).
57. Pinas A, Chandraharan E. Continuous cardiotocography during labour: analysis, classification and management. Best Pract Res Clin Obstet Gynaecol 2016;30:33–47.
58. Chandraharan E, Preti M, Lowe V, Archer A, Ugwumadu A, Arulkumaran S. Effectiveness of ‘George’s Intrapartum Monitoring Strategy’ on Operative
Delivery and Perinatal Outcomes at a Teaching Hospital in London:
a 5 Year Experience. Book of Abstracts. COGI Conference, Vienna, 2013.
59. Chandraharan E. Handbook of CTG interpretation: from patterns to physiology. Cambridge, United Kingdom: Cambridge University Press; 2017.
60. Pereira S, Chandraharan E. Recognition of chronic hypoxia and pre-existing foetal injury on the cardiotocograph (CTG): urgent need to think beyond the guidelines. Porto Biomed J 2017;2: 124–9.
61. Oikonomou M, Chandraharan E. Fetal heart rate monitoring in labor: from pattern recognition to fetal physiology. Minerva Obstet Gynecol 2021;73:19–33.
62. Griffiths K, Gupta N, Chandraharan E. Intrapartum fetal surveillance: a physiological approach,Obstetrics, Gynaecology & Reproductive Medicine, Volume 32, Issue 8, 2022,Pages 179-187.
63. Bolten M, Chandraharan E. The Significance of ‘Non-Significant’ Meconium Stained Amniotic Fluid (MSAF): Colour versus Contents. J Adv Med Med Res. 2019. doi:10.9734/jammr/2019/v30i530192
64. Physiological interpretation of CTG: From Knowledge to Practice. Volumes 1-3. Glob Acad Med Edu Train, London, UK. KDP. 2022 (https://www.amazon.co.uk/s?k=chandraharan&crid=1VKO4VCPZJ5IV&sprefix=chandraharan%2Caps%2C310&ref=nb_sb_noss_1)
65. Yanamandra N, Chandraharan E. Saltatory and sinusoidal fetal heart rate (FHR) patterns and significance of FHR ‘overshoots’. Curr Wom Health Rev. 2013;9:1e8
66. Al Fahdi B & Chandraharan E. True vs Spurious Intrapartum Fetal Heart Rate Accelerations on the Cardiotocograph (CTG): An Urgent Need for Caution. Glob J Reprod Med. 2020; 7 (5): 5556722. DOI: 10.19080/GJORM.2019.07.555722.
67. Afors K, Chandraharan E. Use of continuous electronic fetal monitoring in a preterm fetus: clinical dilemmas and recommendations for practice. J Pregnancy. 2011;2011:848794.
68. Saeed F, Abeysuriya S, Chandraharan E. Erroneous Recording of Maternal Heart Rate as Fetal Heart Rate During Second Stage of Labour: Isn’t it Time to Stop this? J Biomed Res Environ Sci. 2021 May 11; 2(5): 315-319. doi: 10.37871/jbres1233.
69. Nurani R, Chandraharan E, Lowe V, Ugwumadu A, Arulkumaran S. Misidentification of maternal heart rate as fetal on cardiotocography during the second stage of labor: the role of the fetal electrocardiograph. Acta Obstet Gynecol Scand. 2012 Dec;91(12):1428-32. doi: 10.1111/j.1600-0412.2012.01511.x. Epub 2012 Sep 18.
70. Ingram C, Gupta N, Mustafa S, Singh M, Chandraharan E. Impact of Physiological CTG Guidelines on Intrapartum Hypoxic injuries and brain cooling. World Congress of the Royal College of Obstetricians and Gynaecologists (RCOG) 2021.
71. Jia YJ, Ghi T, Pereira S, Gracia Perez-Bonfils A, Chandraharan E. Pathophysiological interpretation of fetal heart rate tracings in clinical practice. Am J Obstet Gynecol. 2023 Jun;228(6):622-644.
72. Reeves K, Scully R, Dutta A, Bullen-Bull R, Singh M, Chandraharan E. Training and support on Physiological CTG Interpretation: Does it reduce the hypoxic encephalopathy (HIE) rate? European Congress on Intrapartum Care. 2021.
73. Chandraharan E, Evans SA, Krueger D, Pereira S, Skivens S, et al. (2018) Physiological CTG interpretation. Intrapartum Fetal Monitoring Guideline. https://physiological-ctg.com/guideline.html.
74. Zamora Del Pozo C, Chóliz Ezquerro M, Mejía I, Díaz de Terán Martínez-Berganza E, Esteban LM, Rivero Alonso A, Castán Larraz B, Andeyro García M, Savirón Cornudella R. Diagnostic capacity and interobserver variability in FIGO, ACOG, NICE and Chandraharan cardiotocographic guidelines to predict neonatal acidemia. J Matern Fetal Neonatal Med. 2022 Dec;35(25):8498-8506.
75. Ghi T, Di Pasquo E, Dall’Asta A, et al. Intrapartum fetal heart rate between 150 and 160 bpm at or after 40 weeks and labor outcome. Acta Obstet Gynecol Scand 2021;100:548–54.
76. Preti M, Chandraharan E. Importance of fetal heart rate cycling during the interpretation of the cardiotocograph (CTG). Int J Gynecol Reprod Sci 2018;1:10–2.
77. Pereira S, Lau K, Modestini C, Wertheim D, Chandraharan E. Absence of fetal heart rate cycling on the intrapartum cardiotocograph (CTG) is associated with intrapartum pyrexia and lower Apgar scores. J Matern Fetal Neonatal Med. 2021 Jun 22:1-6.
78. Gracia-Perez-Bonfils A, Martinez-Perez O, Llurba E, Chandraharan E. Fetal heart rate changes on the cardiotocograph trace secondary to maternal COVID-19 infection. Eur J Obstet Gynecol Reprod Biol. 2020 Sep;252:286-293.
79. di Pasquo E, Commare A, Masturzo B, Paolucci S, Cromi A, Montersino B, Germano CM, Attini R, Perrone S, Pisani F, Dall’Asta A, Fieni S, Frusca T, Ghi T. Short-term morbidity and types of intrapartum hypoxia in the newborn with metabolic acidaemia: a retrospective cohort study. BJOG. 2022 Oct;129(11):1916-1925.
80. Descourvieres L, Ghesquiere L, Drumez E, Martin C, Sauvage A, Subtil D, Houfflin-Debarge V, Garabedian C. Types of intrapartum hypoxia in the newborn at term with metabolic acidemia: A retrospective study. Acta Obstet Gynecol Scand. 2022 Nov;101(11):1276-1281.
81. Yatham SS, Whelehan V, Archer A, Chandraharan E. Types of intrapartum hypoxia on the cardiotocograph (CTG): do they have any relationship with the type of brain injury in the MRI scan in term babies? J Obstet Gynaecol. 2020 Jul;40(5):688-693.
82. Pereira S, Patel R, Zaima A, Tvarozkova K, Chisholm P, Kappelou O, Evanson J, Chandraharan E, Wertheim D, Shah DK. Physiological CTG categorization in types of hypoxia compared with MRI and neurodevelopmental outcome in infants with HIE. J Matern Fetal Neonatal Med. 2022 Dec;35(25):9675-9683.
83. Chandraharan E. Physiological Interpretation of Cardiotocograph: Does the Emerging Scientific Evidence Suggest a Reversal in the “Thunder and Lightning” Phenomenon?. J Clin Med Surgery. 2023; 3(1): 1098.
84. Jia YJ, Chen X, Cui HY, Whelehan V, Archer A, Chandraharan E. Physiological CTG interpretation: the significance of baseline fetal heart rate changes after the onset of decelerations and associated perinatal outcomes. J Matern Fetal Neonatal Med. 2021 Jul;34(14):2349-2354.
85. Gracia-Perez-Bonfils A, Vigneswaran K, Cuadras D, Chandraharan E. Does the saltatory pattern on cardiotocograph (CTG) trace really exist? The ZigZag pattern as an alternative definition and its correlation with perinatal outcomes. J Matern Fetal Neonatal Med. 2019 Nov 13:1-9.
86. Galli L, Dall’Asta A, Whelehan V, Archer A, Chandraharan E. Intrapartum cardiotocography patterns observed in suspected clinical and subclinical chorioamnionitis in term fetuses. J Obstet Gynaecol Res. 2019 Dec;45(12):2343-2350. doi: 10.1111/jog.14133. Epub 2019 Oct 16.
87. Sukumaran S, Pereira V, Mallur S, Chandraharan E. Cardiotocograph (CTG) changes and maternal and neonatal outcomes in chorioamnionitis and/or funisitis confirmed on histopathology. Eur J Obstet Gynecol Reprod Biol. 2021 May;260:183-188. doi: 10.1016/j.ejogrb.2021.03.029. Epub 2021 Mar 30.
88. di Pasquo E, Fieni S, Chandraharan E, Dall’Asta A, Morganelli G, Spinelli M, Bettinelli ML, Aloe R, Russo A, Galli L, Perrone S, Ghi T. Correlation between intrapartum CTG findings and interleukin-6 levels in the umbilical cord arterial blood: A prospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2024 Mar;294:128-134.
89. Chandraharan E, Pereira S, Ghi T, Gracia Perez-Bonfils A, Fieni S, Jia YJ, Griffiths K, Sukumaran S, Ingram C, Reeves K, Bolten M, Loser K, Carreras E, Suy A, Garcia-Ruiz I, Galli L, Zaima A. International expert consensus statement on physiological interpretation of cardiotocograph (CTG): First revision (2024). Eur J Obstet Gynecol Reprod Biol. 2024 Oct 2;302:346-355. doi: 10.1016/j.ejogrb.2024.09.034. Epub ahead of print.
90. Kikuchi H, Noda S, Katsuragi S, Ikeda T, Horio H. Evaluation of 3-tier and 5-tier FHR pattern classifications using umbilical blood pH and base excess at delivery. PLoS One 2020;15:e0228630.
91. Cappe M, Deruelle P, Depret S, Houfflin-Debarge V, Ghesquière L, Garabedian C. Fetal heart rate classification in routine use: Do your prefer a 3-tier or a 5-tier classification? J Gynecol Obstet Hum Reprod. 2018 Nov;47(9):477-480.
92. Gyamfi Bannerman C, Grobman WA, Antoniewicz L, Hutchinson M, Blackwell S. Assessment of the concordance among 2-tier, 3-tier, and 5-tier fetal heart rate classification systems. Am J Obstet Gynecol. 2011 Sep;205(3):288.e1-4.
93. Magawa S, Tanaka H, Furuhashi F, Maki S, Nii M, Toriyabe K, Kondo E, Ikeda T. Intrapartum cardiotocogram monitoring between obstetricians and computer analysis. J Matern Fetal Neonatal Med. 2021 Mar;34(5):787-793.

