Impact of Water Drinking Test on IOP in Advanced Glaucoma
Water drinking test in patients with advanced-terminal stage glaucoma and maximal topical hypotensive therapy
Eva de los Ángeles Medina1, Marcos A. Geria1, Javier F. Casiraghi1
- Glaucoma section, Ophthalmology department, Hospital de Clínicas José de San Martín. Buenos Aires City, Argentina
OPEN ACCESS
PUBLISHED: 30 November 2024
CITATION:Medina, EA., Geria, MA., et al., 2024. Water drinking test in patients with advanced-terminal stage glaucoma and maximal topical hypotensive therapy. Medical Research Archives, [online] 12(11). https://doi.org/10.18103/mra.v12i11.5841
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.18103/mra.v12i11.5841
ISSN 2375-1924
ABSTRACT
Objective: To assess the impact of the water drinking test (WDT) on intraocular pressure (IOP) in advanced-terminal stage glaucoma patients, treated with maximal topical hypotensive therapy.
Method: This retrospective clinical study included patients with advanced or end-stage open-angle glaucoma, with or without prior glaucoma surgery and medication. Participants underwent the WDT, where they consumed 1 liter of water over 5 minutes. IOP was measured at baseline, and then at 15-, 30-, and 45-minutes post-administration.
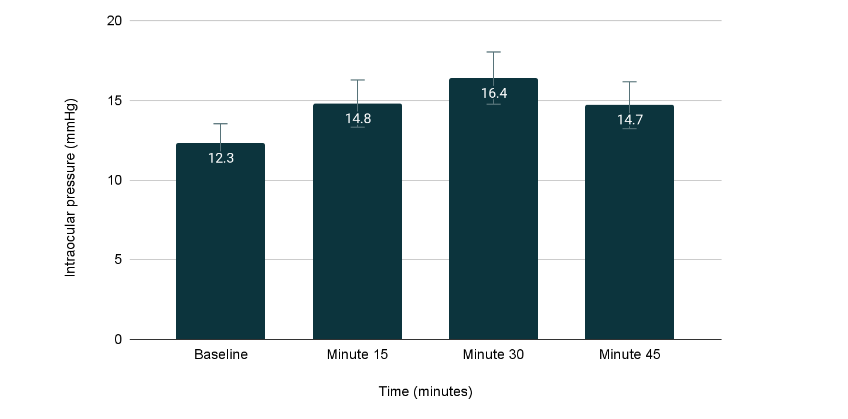
Results: The IOP measurements (mean ± standard deviation) were as follows: baseline: 12.3 ± 2.1 mmHg; at minute 15: 14.8 ± 3.1 mmHg; at minute 30: 16.4 ± 3.3 mmHg and at minute 45: 14.7 ± 2.6 mmHg. A significant increase in IOP was observed at minute 30 compared to baseline (p = 0.01).
Conclusion: Ingesting 1000 ml of water within 5 minutes raises IOP in patients with advanced or end-stage glaucoma and maximal topical hypotensive therapy. Further research is needed to explore the relevance of these findings in medical scenarios requiring significant fluid intake, such as kidney-bladder, prostate, and gynecological ultrasounds.
Keywords
water drinking test, glaucoma, intraocular pressure.
INTRODUCTION
Considering that glaucoma is an entity that expresses itself silently and whose chronic and slow progression can also go unnoticed, detection and follow-up methods are constantly being improved. These methods propose the detection of both structural changes in the optic nerve and functional changes in vision and in the hydrodynamics of the aqueous humor.1-6 Likewise, great advances are being made in the diagnosis and follow-up of glaucoma, especially in the field of diagnostic imaging, which is now beginning to be assisted by artificial intelligence.7,8 This implies the need for using sophisticated technology and costly devices that are not accessible to all physicians, especially in less economically developed regions of the world.9-12 Therefore, more accessible options are being sought to diagnose and monitor glaucoma, for example, through the use of smartphones.13-16 Also, the information provided by simpler clinical studies, such as the water drinking test, has re-emerged and is gaining clinical value.17,18
The water overload test or water drinking test (WDT) began to spread in the early 1950s with the publication of Leydhecker,19 which other authors later confirmed as one of the tests used to evaluate patients with glaucoma.20-22 The importance of fluid intake and diuresis on intraocular pressure (IOP)23 has been known for many decades. Although its usefulness was questioned,24 it is still valid today in evaluating glaucoma patients who have undergone different pharmacological and surgical treatments according to recent publications.25-34 A recent systematic review35 evaluated the value of the water drinking test WDT and observed after analyzing all the evidence published up to the beginning of the year 2024, that there is a strong positive correlation between WDT peak IOP measurements and daytime IOP monitoring in glaucoma patients. Also, the peak IOP value observed on the WDT demonstrated good reproducibility and may be associated with greater future visual field progression.
Among other things, the WDT allows the evaluation of glaucomatous progression of the visual field, is useful for the follow-up of patients with glaucoma, and at the same time provides indirect information on trabecular function.18,35 However, considering that this type of test may have a different impact depending on the type of glaucoma and also on the evolutionary stage of the disease. The present study aimed to evaluate the effect of the water overload test on IOP in patients with advanced-terminal open-angle glaucoma, treated with maximal topical hypotensive therapy.
METHODS
DESIGN
A retrospective study was conducted to evaluate cases attended between March 2022 and March 2023 at the Hospital de Clínicas José de San Martín in the city of Buenos Aires, Argentina. The study was approved by the ethics committee of the institution and was developed following the guidelines of the Declaration of Helsinki. The data of the patients included in this review were treated anonymously, protecting their identity, and all participants signed an initial informed consent form, accepting that their data could be used for scientific academic purposes.
POPULATION AND PARAMETERS
Patients over 18 years of age of both sexes, with a visual acuity of 1 to 4 tenths, who attended the glaucoma service of the hospital in the mentioned period, who had open-angle glaucoma treated with maximal hypotensive topical therapy, and who had not undergone any glaucoma surgery were included. The visual field and optic nerve evaluation showed that the glaucoma was in advanced terminal stage according to the Hodapp-Parrish-Anderson damage criteria (MD less than -12 dB, n points p-5% greater than 27 [50%], n points less than 1%: 14 and in 5 central degrees: any point 0 dB and in both hemifields points equal to or less than 15dB) and a papillary excavation of 0.8 or worse. Any patient with narrow-angle or closed-angle glaucoma with active ocular surface infections, corneal alterations, difficulty collaborating in applanation tonometry, renal failure, cardiac failure, and those who refused informed consent were excluded.
PROCEDURE FOR PERFORMING THE WDT
The WDT consisted of a 5-minute intake of 1,000 ml of drinking water after a total fast of at least four hours. IOP was recorded before drinking water (basal intake) and then at different times: 15, 30, and 45 minutes. IOP measurements were always taken by the same physician, with the same Goldmann Haag Streit flattening tonometer whose calibration was previously controlled and with the patient seated in front of the slit lamp.
STATISTICS
The data were analyzed by performing descriptive and comparative statistical tests using the XLMiner Analysis ToolPack software. After checking the normality of the data, the analysis of variance test (ANOVA) of repeated measurements was used to evaluate the existence of statistically significant differences considering p < = 0.05. To establish when these differences appeared, the pairs of averages were compared with each other (Bonferroni test).
RESULTS
A total of 40 eyes with open-angle glaucoma were included. They were patients with maximum medication (3 drugs). The IOP values found at different times were (mean and standard deviation): baseline: 12.3 ± 2.1 mmHg; at minute 15: 14.8 ± 3.1 mmHg; at minute 30: 16.4 ± 3.3 mmHg and at minute 45: 14.7 ± 2.6 mmHg. After analyzing the results, IOP at minute 30 was found to increase statistically significantly with respect to baseline values (p = 0.01). With a 95% confidence interval for the differences between baseline and 30-minute IOP averages, which ranged from 0.68 to 6.1 mmHg.
DISCUSSION
In the present study, after performing the WDT with the rapid intake of 1 liter of water, a statistically significant increase in IOP was generated after half an hour in a group of patients with advanced end-stage glaucoma. This test is still very relevant today because, in spite of technological advances, it is accessible for all the ophthalmologists, is simple to perform and inexpensive. Its main usefulness is that it allows the follow-up of patients with glaucoma, but it is not useful for its diagnosis due to its low sensitivity (15.6%) despite being highly specific (96.7%).25,36-38 There may be variations in its performance, depending on the amount of fluid administered (800 or 1000 ml) 15 or the time between IOP measurements. For example, Danesh-Meyers et al. in their work extended the time of intake (15′) and IOP measurements up to 60 minutes, showing that the pressure decreases as time passes and that the peaks are lower in patients operated on for trabeculectomy with mitomycin C, compared to those exposed to medical therapy.38
In our study, the decrease in IOP was already evident after 30 minutes. It should be noted that different diagnostic medical practices are usually performed in complementary studies, where abundant fluid intake is required in a short period of time, such as in reno-vesical, prostate and gynecological ultrasounds. As seen in this study, this could determine the increase in IOP in patients with advanced glaucoma. There are studies that have evaluated visual field compromise in patients with advanced-terminal glaucoma by WDT.25,34-38 These are data from this work that should be shared with other specialties, such as medical clinic and diagnostic imaging, and also with the patients themselves, explaining to them that they should avoid ingesting too much fluid in a short time.
As some of our study’s limitations, taking into account the mechanism of action of WDT, the two populations of cases evaluated (operated and non-operated) could have been compared, since there are publications that talk about the different test results in operated patients, although this was not the primary objective of the present study and it may be interesting to evaluate it in the future, in an experimental design specifically set up for this purpose. Another limitation is related to the small sample size analyzed, in a single-center study. The study design was simple, which was a decision, considering that it was performed without any type of sponsorship with few resources in a public health hospital setting.
Finally, we would like to point out something indirectly related to our study, but which is potentially of great clinical relevance and hopefully may motivate other groups in the development of further research. What we have described is associated with understanding the impact of water intake on ocular pressure. Although there are different studies that analyze the impact of water intake on general health, renal and urinary disorders, skin conditions, cardiovascular system and even psychic performance,39-44 there are not many studies that provide evidence of what is the critical level of water intake in relation to the intake time, so that it does not affect, potentially negatively, the intraocular pressure. In addition, it may be important to include in the clinical chart, how much liquid the patient ingests, in what time and, what type of liquids (water, sweetened sodas, alcoholic beverages, coffee, tea and other infusions).
CONCLUSION
After consuming 1 liter of water within 5 minutes, patients with advanced-terminal glaucoma experience an increase in intraocular pressure (IOP). Given the fragile visual condition of these patients and the imperative of maintaining low and stable IOP levels, it is advisable to caution against excessive fluid intake over short durations. Additionally, they should be aware of potential risks during common medical procedures like ultrasound scans that necessitate significant fluid intake. Furthermore, future research involving a larger sample size will be essential to validate these findings.
REFERENCES
- Pitha I, Du L, Nguyen TD, Quigley H. IOP and glaucoma damage: The essential role of optic nerve head and retinal mechanosensors. Prog Retin Eye Res. 2024; 99:101232. doi: 10.1016/j.preteyeres.2023.101232.
- Johnstone M, Xin C, Tan J, Martin E, Wen J, Wang RK. Aqueous outflow regulation – 21st century concepts. Prog Retin Eye Res. 2021; 83:100917. doi: 10.1016/j.preteyeres.2020.100917.
- Englmaier VA, Storp JJ, Leclaire MD, Lahme L, Brücher VC, Biermann J, Diener R, Eter N. Accuracy of Bruch’s membrane opening minimum rim width and retinal nerve fiber layer thickness in glaucoma diagnosis depending on optic disc size. Graefes Arch Clin Exp Ophthalmol. 2024; 262(6):1899-1910. doi: 10.1007/s00417-024-06375-3.
- Shen R, Chan LKY, Yip ACW, Chan PP. Applications of optical coherence tomography angiography in glaucoma: current status and future directions. Front Med (Lausanne). 2024; 11:1428850. doi: 10.3389/fmed.2024.1428850.
- Jin SW, Bouris E, Morales E, Caprioli J. Long-Term Rate of Optic Disc Rim Loss in Glaucoma Patients Measured From Optic Disc Photographs With a Deep Neural Network. Transl Vis Sci Technol. 2024; 13(9):9. doi: 10.1167/tvst.13.9.9.
- Acosta PCO, de Leon JMS. Correlation of peripapillary retinal nerve fiber layer and macular ganglion cell-inner plexiform layer in early to moderate glaucoma using the Cirrus ® widefield analysis (PanoMap ®). Indian J Ophthalmol. 2024; 72(3):412-416. doi: 10.4103/IJO.IJO_697_23.
- Huang X, Islam MR, Akter S, Ahmed F, Kazami E, Serhan HA, Abd-Alrazaq A, Yousefi S. Artificial intelligence in glaucoma: opportunities, challenges, and future directions. Biomed Eng Online. 2023; 22(1):126. doi: 10.1186/s12938-023-01187-8.
- Zhu Y, Salowe R, Chow C, Li S, Bastani O, O’Brien JM. Advancing Glaucoma Care: Integrating Artificial Intelligence in Diagnosis, Management, and Progression Detection. Bioengineering (Basel). 2024; 11(2):122. doi: 10.3390/bioengineering11020122.
- McDermott CE, Salowe RJ, Di Rosa I, O’Brien JM. Stress, Allostatic Load, and Neuroinflammation: Implications for Racial and Socioeconomic Health Disparities in Glaucoma. Int J Mol Sci. 2024; 25(3):1653. doi: 10.3390/ijms25031653.
- Davuluru SS, Jess AT, Kim JSB, Yoo K, Nguyen V, Xu BY. Identifying, Understanding, and Addressing Disparities in Glaucoma Care in the United States. Transl Vis Sci Technol. 2023; 12(10):18. doi: 10.1167/tvst.12.10.18.
- Obasuyi OC, Yeye-Agba OO, Ofuadarho OJ. Factors limiting glaucoma care among glaucoma patients in Nigeria: A scoping review. PLOS Glob Public Health. 2024; 4(1):e0002488. doi: 10.1371/journal.pgph.0002488.
- Vision Loss Expert Group of the Global Burden of Disease Study; GBD 2019 Blindness and Vision Impairment Collaborators. Global estimates on the number of people blind or visually impaired by glaucoma: A meta-analysis from 2000 to 2020. Eye (Lond). 2024; 38(11):2036-2046. doi: 10.1038/s41433-024-02995-5.
- Bragança CP, Torres JM, Soares CPA, Macedo LO. Detection of Glaucoma on Fundus Images Using Deep Learning on a New Image Set Obtained with a Smartphone and Handheld Ophthalmoscope. Healthcare (Basel). 2022; 10(12):2345. doi: 10.3390/healthcare10122345.
- Nida EK, Bekele S, Geurts L, Vanden Abeele V. Acceptance of a Smartphone-Based Visual Field Screening Platform for Glaucoma: Pre-Post Study. JMIR Form Res. 2021; 5(9):e26602. doi: 10.2196/26602.
- Li F, Song D, Chen H, Xiong J, Li X, Zhong H, Tang G, Fan S, Lam DSC, Pan W, Zheng Y, Li Y, Qu G, He J, Wang Z, Jin L, Zhou R, Song Y, Sun Y, Cheng W, Yang C, Fan Y, Li Y, Zhang H, Yuan Y, Xu Y, Xiong Y, Jin L, Lv A, Niu L, Liu Y, Li S, Zhang J, Zangwill LM, Frangi AF, Aung T, Cheng CY, Qiao Y, Zhang X, Ting DSW. Development and clinical deployment of a smartphone-based visual field deep learning system for glaucoma detection. NPJ Digit Med. 2020 Sep 22;3:123. doi: 10.1038/s41746-020-00329-9. Erratum in: NPJ Digit Med. 2022; 5(1):38. doi: 10.1038/s41746-022-00585-x.
- Rao DP, Shroff S, Savoy FM, S S, Hsu CK, Negiloni K, Pradhan ZS, P V J, Sivaraman A, Rao HL. Evaluation of an offline, artificial intelligence system for referable glaucoma screening using a smartphone-based fundus camera: a prospective study. Eye (Lond). 2024; 38(6):1104-1111. doi: 10.1038/s41433-023-02826-z.
- Johnstone M, Xin C, Tan J, Martin E, Wen J, Wang RK. Aqueous outflow regulation – 21st century concepts. Prog Retin Eye Res. 2021; 83:100917. doi: 10.1016/j.preteyeres.2020.100917.
- Yap TE, Gao Y, Ahmad H, Susanna F, Susanna R, Normando EM, Bloom PA, Cordeiro MF. Comparison of intraocular pressure profiles during the water drinking test and the modified diurnal tension curve. Eye (Lond). 2024; 38(8):1567-1574. doi: 10.1038/s41433-024-02954-0.
- Leydhecker W. The water-drinking test. Br J Ophthalmol 1950; 34: 457-479.
- Leydhecker W. Comparative provocative tests in glaucoma. Br J Ophthalmol 1950; 34: 535-544.
- Campbell DA, Gloster J, Tonks EL. Some observations on the water drinking test in glaucomatous and non-glaucomatous subjects. Br J Ophthalmol 1955; 39: 193-203.
- Winder AF, Siddiqui AA, Donovan HC. Ocular hypertension and systemic responses to the water-drinking test. Br J Ophthalmol 1978; 62: 414-419.
- Campbell DA. Diuretics and the eye. Br Med J 1961; 2: 467-474.
- Roth JA. Inadequate diagnostic value of the water-drinking test. Br J Ophthalmol 1974; 58: 55-61.
- Susanna R Jr, Clement C, Goldberg I, Hatanaka M. Applications of the water drinking test in glaucoma management. Clin Exp Ophthalmol 2017; 45: 625-631.
- Özyol P, Özyol E, Baldemir E. Intraocular pressure dynamics with prostaglandin analogs: a clinical application of the water-drinking test. Clin Ophthalmol 2016; 10: 1351-1356.
- Muñoz CR, Macias JH, Hartleben C. Reproducibilidad de la prueba de sobrecarga hídrica. Arch Soc Esp Oftalmol 2015; 90: 517-521.
- Razeghinejad MR, Tajbakhsh Z, Nowroozzadeh MH, Masoumpour M. Water drinking test: intraocular pressure changes after tube surgery and trabeculectomy. J Ophthalmic Vis Res 2017; 12: 390-396.
- Razeghinejad MR, Tajbakhsh Z, Nowroozzadeh MH et al. The water-drinking test revisited: an analysis of test results in subjects with glaucoma. Semin Ophthalmol 2018; 33: 517-524.
- Prata TS, Dias DT. The water-drinking test and glaucoma progression: considerations regarding the test usefulness as an independent risk assessment tool. J Glaucoma 2018; 27: e25-e26.
- De Moraes CG, Susanna R Jr, Sakata LM, Hatanaka M. Predictive value of the water drinking test and the risk of glaucomatous visual field progression. J Glaucoma 2017; 26: 767-773.
- Scoralick ALB, Gracitelli CPB, Dias DT et al. Lack of association between provocative test-based intraocular pressure parameters and functional loss in treated glaucoma patients. Arq Bras Oftalmol 2019; 82: 176-182.
- Mursch-Edlmayr AS, Luft N, Podkowinski D et al. Differences in optic nerve head blood flow regulation in normal tension glaucoma patients and healthy controls as assessed with laser speckle flowgraphy during the water drinking test. J Glaucoma 2019; 28: 649-654.
- The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol 2000; 130: 429-440.
- Jin E, Goh CXY, Betzler BK, Heng CP, Ang BCH. Assessing the value of the water drinking test in glaucoma-a systematic review and meta-analysis. Eye (Lond) 2024. doi:10.1038/s41433-024-03107-z
- Susanna R Jr, Vessani RM, Sakata L et al. The relation between intraocular pressure peak in the water drinking test and visual field progression in glaucoma. Br J Ophthalmol 2005; 89: 1298-1301.
- Cronemberger S, Nassim C, Vieira Filho HM et al. Provocative tests, functional exams and daily curve of intraocular pressure in glaucoma suspects Vision Pan-America 2012; 11: 80-84.
- Danesh-Meyer HV, Papchenko T, Tan YW, Gamble GD. Medically controlled glaucoma patients show greater increase in intraocular pressure than surgically controlled patients with the water drinking test. Ophthalmology 2008; 115: 1556-1570.
- Goulet EDB, Claveau P, Simoneau IL, Deshayes TA, Jolicoeur-Desroches A, Aloui F, Hoffman MD. Repeatability of Ad Libitum Water Intake during Repeated 1 h Walking/Jogging Exercise Sessions Conducted under Hot Ambient Conditions. Nutrients. 2023 Oct 24;15(21):4500. doi: 10.3390/nu15214500.
- Li S, Xiao X, Zhang X. Association between plain water intake and risk of hypertension: longitudinal analyses from the China Health and Nutrition Survey. Front Public Health. 2024 Jan 9;11:1280653. doi: 10.3389/fpubh.2023.1280653.
- Carroll HA, Ericson U, Ottosson F, Enhörning S, Melander O. The association between water intake and future cardiometabolic disease outcomes in the Malmö Diet and Cancer cardiovascular cohort. PLoS One. 2024 Jan 19;19(1):e0296778. doi: 10.1371/journal.pone.0296778.
- Armstrong LE, Bergeron MF, Muñoz CX, Kavouras SA. Low daily water intake profile-is it a contributor to disease? Nutr Health. 2024 Sep;30(3):435-446. doi: 10.1177/02601060241238826.
- Khil J, Chen QY, Lee DH, Hong KW, Keum N. Water intake and obesity: By amount, timing, and perceived temperature of drinking water. PLoS One. 2024 Apr 25;19(4):e0301373. doi: 10.1371/journal.pone.0301373.
- Lee JW, Kim Y. Association of plain water intake with self-reported depression and suicidality among Korean adolescents. Epidemiol Health. 2024;46:e2024019. doi: 10.4178/epih.e202401

