Acute Anterior Uveitis Post COVID-19 mRNA Vaccination
Acute Anterior Uveitis following Pfizer-BioNTech mRNA COVID-19 (BNT162b2) Vaccination – Case Report
Yigit C. Akduman, MD, 1 Pamela Martin, MD, 2 William J. Anderson, MD, 1 Sabrina M. Shultz, OD, 3 Jinghua Chen, MD, 2 Henry J. Kaplan, MD, 1,4 Niloofar Piri, MD 1*
- SSMHealth Medical Group, Department of Ophthalmology, Saint Louis University, School of Medicine
- Department of Ophthalmology, University of Florida, Gainesville
- Quantum Vision, 12 Professional Park Dr, Maryville, IL, USA
- SSMHealth Medical Group, Department of Ophthalmology, Saint Louis University, School of Medicine; Department of Biochemistry & Molecular Biology, Saint Louis University, School of Medicin
OPEN ACCESS
PUBLISHED: 31 March 2025
CITATION: Akduman, Y., C., Martin, P., et al., 2025. Acute Anterior Uveitis following Pfizer-BioNTech mRNA COVID-19 (BNT162b2) Vaccination – Case Report. Medical Research Archives, [online] 13(3). https://doi.org/10.18103/mra.v13i3.6345
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI https://doi.org/10.18103/mra.v13i3.6345
ISSN 2375-1924
ABSTRACT
Background: Vaccine-associated intraocular inflammation has been reported with several vaccines, including the Pfizer-BioNTech mRNA COVID-19 (BNT162b2) vaccine. While rare, these ocular adverse events warrant further investigation to better understand their clinical course, ideal management, and long-term outcomes. Anterior uveitis has been the most commonly reported manifestation following mRNA COVID-19 vaccination, but optimal treatment strategies and risk of recurrence remain unclear.
Aims: To report three cases of acute anterior uveitis occurring within weeks of COVID-19 vaccination, assess their response to treatment and follow up course, and review existing literature on vaccine-associated uveitis.
Methods: This case report analyzes three patients who developed acute anterior uveitis following COVID-19 vaccination. All patients underwent a comprehensive ophthalmic evaluation at presentation, including best-corrected visual acuity, slit-lamp examination, intraocular pressure measurement, and dilated fundus exam. These patients were managed with topical corticosteroids and were followed over the course of 30 months to assess response to treatment as well as any recurrence or complications.
Results: Each case of uveitis was successfully treated with topical corticosteroids, leading to complete resolution of inflammation without requiring systemic immunosuppressants. One patient experienced a mild recurrence eight weeks after the second vaccine dose, which was controlled with a second course of topical corticosteroids. No further recurrences were observed with long-term follow-up extending up to 30 months. Laboratory and imaging evaluations were negative for systemic inflammatory or infectious causes.
Conclusion: All three cases of acute anterior uveitis after COVID-19 vaccination responded well to topical corticosteroids, with no evidence of persistent or recurrent inflammation or long-term complications. These findings suggest that, in the absence of previously documented systemic autoimmune disease or other concerning features, COVID-19 vaccine associated acute anterior uveitis can be effectively managed with topical therapy alone, without the need for extensive systemic workup. Larger studies are needed to further investigate these findings and management strategies.
Keywords
acute anterior uveitis, COVID-19 vaccination, Pfizer-BioNTech, mRNA vaccine, ocular inflammation
INTRODUCTION
Vaccination has been a cornerstone of public health, reducing the burden of infectious diseases and preventing millions of deaths worldwide. While vaccines are generally safe and well-tolerated, they can be associated with immune-mediated adverse effects, including intraocular inflammation, and particularly, uveitis. A comprehensive review of vaccine-associated uveitis cases identified several vaccines linked to this adverse event; notably, the hepatitis B vaccine accounted for the highest number of cases. Other vaccines implicated include the human papillomavirus (HPV) vaccine, the influenza vaccine, the Bacille Calmette-Guerin (BCG) vaccine, the measles-mumps-rubella (MMR) vaccine, and the varicella vaccine. The pathogenesis of vaccine-associated uveitis is not fully understood; however, proposed mechanisms involve molecular mimicry, where vaccine-derived peptides resemble ocular antigens, leading to an autoimmune response, and antibody-mediated hypersensitivity reactions. While these occurrences are relatively rare, they underscore the importance of monitoring and reporting ocular inflammatory responses following vaccination to better understand and manage this potential adverse event. New vaccine technology surfaced with the COVID-19 pandemic, namely mRNA, and was found to have strong effectiveness in preventing hospitalization and mortality rate caused by this pathogen. Just like its predecessors, this new mRNA vaccine technology has been found in several studies to have some association with intraocular inflammation. Reported inflammatory manifestations include anterior uveitis, anterior scleritis, acute macular neuroretinopathy, panuveitis, paracentral acute middle maculopathy (PAMM), and subretinal fluid. The most common manifestation was acute anterior uveitis in studies investigating ocular effects of vaccination. Further studies focusing on demographic trends of COVID-19 vaccine associated uveitis have found that most cases occur in middle aged females who do not have previous medical history of uveitis. Similar to other vaccines, the ocular complications observed following COVID-19 vaccination are believed to result from vaccine-induced immunologic responses, including molecular mimicry and antibody-mediated hypersensitivity reactions. While these adverse events remain rare, they highlight the need for clinicians to remain vigilant for potential ocular side effects to ensure timely diagnosis and appropriate management. In the following manuscript, we report three cases of anterior uveitis that developed within three weeks of Pfizer-BioNTech mRNA COVID-19 (BNT162b2) vaccination and describe their response to treatment and follow up course over a 30-month follow-up period.
METHODS
This case report was conducted by retrospective chart review of three patients who developed acute anterior uveitis following vaccination with the BNT162b2 mRNA COVID-19 vaccine. Data, including patient demographics, ocular and systemic medical histories, symptom onset in relation to vaccination, clinical exam findings, laboratory investigations, imaging, treatments regimens, and follow up outcomes were all collected from clinical records. All patients underwent comprehensive ophthalmic examinations at presentation, including best corrected visual acuity, slit-lamp exam, intraocular pressure measurement, and dilated fundus examination. Additional testing and imaging were performed when clinically indicated. Patients were treated with topical corticosteroids and cycloplegics according to the degree of anterior chamber inflammation. Long-term follow-up was performed to monitor for recurrence or complications.
RESULTS
CASE 1
A 64-year-old male presented with redness, decreased vision and light sensitivity in the right eye (OD) for 3 weeks. There was no significant past medical history for systemic disease, and he was not on any medications. His past ocular history was unremarkable except for bilateral mild nuclear sclerotic cataracts; there was no previous history of intraocular inflammation. His visual acuity was 20/20 in both eyes one year before this visit. His symptoms started three days after receiving the second dose of the BNT162b2 mRNA COVID-19 vaccine. His best corrected visual acuity was 20/50 OD and 20/20 in the left eye (OS). Intraocular pressure (IOP) in both eyes (OU) was normal. Anterior segment examination OD was significant for 1+ conjunctival and episcleral injection. The anterior chamber OD had small fine keratic precipitates (KPs), 2+ cells and 1+ flare. Posterior synechiae was present from 6 to 9 o’clock, with mild nuclear sclerosis and trace inflammatory cells in the anterior vitreous. Anterior segment examination OS was normal. Dilated fundus examination OU showed an attached retina without retinal hemorrhage, vasculitis or choroidal detachment. Macular Spectral-Domain Optical Coherence Tomography (SD-OCT) OD (Figure 1 Top) revealed significant cystoid macular edema (CME).
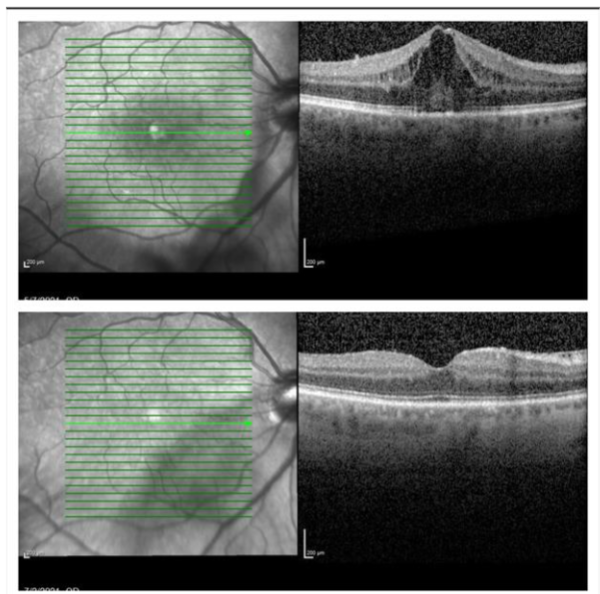
He was diagnosed with acute anterior uveitis and CME OD. Topical Prednisolone Acetate 1% was started four times a day (QID) as well as Cyclopentolate 1% twice a day (BID). Laboratory evaluation was negative, which included Human Leukocyte Antigen B-27 (HLA-B27), complete blood count with differential, C-reactive protein, erythrocyte sedimentation rate, Syphilis serology, Lyme Antibody by Enzyme-Linked Immunosorbent Assay (ELISA), Lysozyme, Angiotensin Converting Enzyme, Rheumatoid Factor, Anti-Nuclear Antibody, Human Immunodeficiency Virus (HIV), Quantiferon Gold, and a chest X-ray. Since the patient did not present with any nasopharyngeal or systemic symptoms and had no history of a previous infection, polymerase chain reaction (PCR) testing for the Covid-19 virus was not performed. At the time of his presentation PCR testing was restricted to symptomatic patients. On his next visit (day 8) visual acuity improved to 20/40 OD. Anterior segment examination OD revealed a few fine KPs, and 1+ cells with no flare in the anterior chamber. Topical medications were continued as initially prescribed. One month after initial presentation visual acuity OD improved to 20/25. Anterior segment examination showed no residual inflammation with resolution of CME on SD-OCT. Cyclopentolate was discontinued and prednisolone acetate tapered weekly. No evidence of acute inflammation or CME in either eye after 26 months follow up.
CASE 2
A 43-year-old female nurse presented with the complaint of filmy vision and redness OD for 6 days. She reported no similar symptoms in the past. Her past medical and ocular history were unremarkable, and she was on no systemic medications. The patient was vaccinated with the first dose of the BNT162b2 mRNA Covid-19 vaccine three weeks prior to the start of her symptoms. She denied a history of SARS-CoV-2 infection and had no previous COVID antigen or antibody testing. Her visual acuity was 20/40 OD and 20/30 OS. Intraocular pressures were normal OU. Slit lamp examination revealed 3+ cell and 1+ flare in the anterior chamber and a few non-granulomatous fine KPs OU. The right pupil was irregular with posterior synechiae. The left pupil and lens were unremarkable. Posterior segment examination including the vitreous, retina, choroid and optic nerve were normal OU. The patient underwent a uveitis work-up for anterior uveitis. She had an unremarkable comprehensive regular health check-up with diagnostic tests by her primary care physician recently. Her uveitis diagnostic workup included a normal complete blood count (CBC) with differential, a negative HLA B27, and a normal chest x-ray. Since the patient did not present with Covid-19 infectious symptoms and had no history of previous infection, PCR testing for the virus was not performed. The patient was diagnosed with anterior uveitis secondary to the BNT162b2 mRNA Covid-19 vaccine. Her uveitis was treated with topical Difluprednate 0.05% QID with gradual weekly tapering based on the response to therapy. Vision returned to 20/20 in both eyes in 1 month with complete resolution of her anterior uveitis OU. She discontinued her topical corticosteroids. Eight weeks after the second vaccine dose, she returned to clinic with similar complaints as in the initial visit. She was found to have recurrent acute anterior uveitis OU with 3+ cells, 1+ flare and fine KPs. Intraocular pressure was normal OU. Topical Difluprednate 0.05% drop QID was restarted and tapered weekly. No recurrences of anterior uveitis were noted off corticosteroids up to 28 months.
CASE 3
A 38-year-old female with past medical history of gout, chronic kidney disease – stage 2, asthma, and alopecia areata presented with bilateral eye pain and photosensitivity for one day. Her only additional relevant history was a second dose of the BNT162b2 vaccine three weeks prior to presentation. A limited uveitis work-up for tuberculosis (TB) and syphilis were negative. On examination, her visual acuity was 20/20 OU. She was found to have non-granulomatous acute anterior uveitis in OU with 3+ cells, 1+ flare, and fine KPs. Posterior segment examination was unremarkable. She was started on topical Prednisolone Acetate 1% Q2h, as well as Cyclopentolate 1% BID initially with complete resolution of inflammation within 2 weeks. Cyclopentolate was discontinued at 2 weeks and prednisolone acetate was tapered over 6 weeks. No recurrence of anterior uveitis off topical corticosteroids was detected up to 31 months. She was diagnosed with COVID vaccine related uveitis. Since the patient did not present with Covid-19 infectious symptoms and had no history of previous infection, no PCR testing for the virus was performed.
DISCUSSION
Several vaccines have previously been reported to cause intraocular inflammation. Vaccine associated uveitis has been described for hepatitis B (HBV), human papillomavirus (HPV), influenza virus, Bacille Calmette-Guerin (BCG), measles-mumps-rubella (MMR), and others. The recent COVID-19 pandemic has led to the rapid development of mRNA vaccines. The extent and severity of the pandemic necessitated the fast and massive production of a vaccine. mRNA technology was very convenient and effective with minimal adverse events in short-term follow up, which led to quick approval of two mRNA vaccines for emergent use. Like their predecessors, these new COVID vaccines have been studied to reveal new adverse ocular associations. Both anterior and nonanterior uveitis have been documented secondary to vaccination. A study by Kim et al. analyzed 473,934 individuals with a history of uveitis who received COVID-19 vaccinations. The findings revealed that the 3 month and 1 year cumulative incidences of post-vaccination uveitis were 8.6% and 16.8%, respectively. Notably, in this study, a higher risk was observed between the first and second vaccine doses, and the risk decreased after subsequent vaccinations. Similarly, Tomkins-Netzer et al. conducted a population-based study involving over 2.6 million individuals who received the BNT162b2 mRNA COVID-19 vaccine. They reported 100 and 88 cases of noninfectious uveitis within 21 days after the first and second vaccine doses, respectively, noting a slight increase in risk compared to the general population (estimated attributable risk of 1.12 cases per 100,000 vaccinations after the first dose and estimated attributable risk of 0.86 cases per 100,000 vaccinations after the second dose). Furthermore, Chang et al. performed a nationwide retrospective cohort study in South Korea, comparing vaccinated and unvaccinated cohorts. The study found a modestly higher risk of uveitis within 180 days of COVID-19 vaccination, especially among females, noting that a history of noninfectious uveitis was a significant risk factor. There are multiple previous case series detailing management of both COVID vaccine-induced uveitis and other vaccine-induced uveitis with topical and systemic corticosteroids combined, all documenting resolution of symptoms at the end of the follow up period. Watad et al. discussed autoimmune/inflammatory syndromes induced by vaccine adjuvants in general, highlighting cases where exposure to vaccine adjuvants led to autoimmune conditions, including uveitis, which were managed successfully by corticosteroids. More specific to COVID vaccination, Pan et al. reported a case of bilateral uveitis following COVID-19 vaccination, which was treated with a combination of topical and systemic corticosteroids, resulting in complete resolution of symptoms. Though severity of presenting symptoms certainly plays a role in treatment decisions, our study proposes that the systemic portion of the treatment regimen may not be necessary for patients presenting with uveitis secondary to COVID vaccination, especially if degree of inflammation is not greater than those seen in our cases. Specifically, as we saw resolution of all three cases with use of only topical corticosteroids as treatment, it is not unreasonable to suggest that topical corticosteroids alone may be enough to treat the complication in the short term as well as prevent recurrence far into the future. This is a point that needs to be further researched in larger studies in the future. Several hypotheses have been proposed to explain vaccine induced uveitis, but the exact pathogenesis is often unknown. The mRNA vaccine induces the host to produce a specific immunoglobulin and cellular response against the virus, which allows host immune cells to establish immunity. However, the immune response may produce molecular mimicry with immunogenic uveal tissue peptides and result in intraocular inflammation. A similar mechanism has been offered as an explanation for the development of thyroiditis and hepatitis after vaccination. In the setting of live attenuated vaccines, uveitis may be triggered by direct infection by the virus. Another proposed mechanism involves vaccine adjuvants causing hypersensitivity reactions, which has been described and referred to as autoimmune conditions induced by adjuvants or Shoenfeld Syndrome. Aluminum salt is the adjuvant that is frequently reported to have immunogenic potential in vaccines and is incorporated in the Hepatitis A vaccine. The most widely used vaccine preservative, polyethylene glycol, has also been reported to cause anaphylactic reactions. Specific to the BNT162b2 mRNA COVID-19 vaccine, Rabinovitch et al. proposed that vaccine-induced type 1 interferon secretion may create autoimmune manifestations in patients with a previous history or susceptibility to autoimmune reactions. The uveitis associated with vaccines is usually a transient anterior uveitis responsive to topical corticosteroids.
We observed three cases of intraocular inflammation that developed within 3 weeks following vaccination with the BNT162b2 mRNA Covid-19 vaccine. Long term follow-up for these patients (average 30 months) demonstrated no recurrences after initial treatment, further corroborating the BNT162b2 mRNA Covid-19 vaccine as the presumed etiology of intraocular inflammation in these cases. Many studies have retrospectively reviewed incidence of intraocular inflammation following vaccination, but few have followed patients’ specific treatment regimens and long-term recovery. Although our three cases do not establish a causal role for the vaccine, these patients had no other obvious cause, work up for systemic inflammatory diseases were negative, and none of them showed any signs of recurrence with up to 30 months of follow up. A retrospective study using CDC database and Prevention Vaccine Adverse Event Reporting System analyzed 853 reported cases of vaccine associated uveitis following BNT162b2 vaccination and found that only 1.17% of patients had previous history of systemic autoimmune disorders. The findings of our case report align with those reported in the aforementioned article, collectively indicating that a comprehensive systemic workup may not be imperative for patients presenting with uveitis following COVID-19 vaccination, particularly in cases where resolution is achieved through topical corticosteroid treatment. This is further supported in the cases we presented as none of the patients had a recurrence after treatment was completed. At this point, it is unknown whether new mRNA vaccine technology, preservatives, and/or adjuvant effects are responsible for the emergence of uveitis in these cases. We have already reported these cases as potential vaccine-related adverse events to the Food and Drug Administration (FDA). Vaccine administrators should remain aware that intraocular inflammation may be associated with BNT162b2 mRNA Covid-19 vaccination. With widespread vaccination, there has already been reports not only of other types of intraocular inflammation, but also more systemic inflammation. Most notably, cases of myocarditis and pericarditis have been reported, particularly among younger males following mRNA vaccination. These events, while rare, have prompted ongoing surveillance and research to better understand their incidence and underlying mechanisms.
CONCLUSION
This case report describes three patients who developed acute anterior uveitis following vaccination with the BNT162b2 mRNA COVID-19 vaccine. All cases responded well to topical corticosteroid therapy without requiring systemic immunosuppressive treatment. Importantly, no recurrences were observed with follow-up periods up to 30 months. These findings suggest that anterior uveitis following COVID-19 vaccination may be effectively managed with topical corticosteroids alone, without the need for an extensive diagnostic work-up in the absence of other concerning clinical features. Long-term follow-up of affected patients demonstrated favorable outcomes without persistent or recurrent inflammation or visual complications. Further studies involving larger cohorts are necessary to confirm these findings and establish definitive management guidelines for COVID-19 vaccine associated uveitis.
Conflict of Interest:
None
Funding Statement:
None.
Acknowledgements:
None.
REFERENCES:
- Cunningham ET Jr, Moorthy RS, Fraunfelder FW, Zierhut M. Vaccine-associated uveitis. Ocul Immunol Inflamm. 2019;27(4):517-520. doi:10.1080/09273948.2019.1626188.
- Benage M, Fraunfelder FW. Vaccine-associated uveitis. Mo Med. 2016;113(1):48-52. Available from: https://www.ncbi.nlm.nih.gov/pubmed/27039491.
- Fraunfelder FW, Suhler EB, Fraunfelder FT. Hepatitis B vaccine and uveitis: an emerging hypothesis suggested by review of 32 case reports. Cutan Ocul Toxicol. 2010;29(1):26-29. doi:10.3109/15569520903427717.
- Mudie LI, Zick JD, Dacey MS, Palestine AG. Panuveitis following vaccination for COVID-19. Ocul Immunol Inflamm. 2021;1-2. doi:10.1080/09273948.2021.1949478.
- Mambretti M, Huemer J, Torregrossa G, Ullrich M, Findl O, Casalino G. Acute macular neuroretinopathy following coronavirus disease 2019 vaccination. Ocul Immunol Inflamm. 2021;1-4. doi:10.1080/09273948.2021.1946567.
- Testi I, Brandao-de-Resende C, Agrawal R, Pavesio C, C-VOIES Group. Ocular inflammatory events following COVID-19 vaccination: a multinational case series. J Ophthalmic Inflamm Infect. 2022;12(1):4. doi:10.1186/s12348-021-00275-x.
- Cherif YYS, Djeffal C, Abu Serhan H, et al. The characteristics of COVID-19 vaccine-associated uveitis: a summative systematic review. Vaccines (Basel). 2022;11(1):69. Published December 28, 2022. doi:10.3390/vaccines11010069.
- Rabinovitch T, Ben-Arie-Weintrob Y, Hareuveni-Blum T, et al. Uveitis after the BNT162b2 mRNA vaccination against SARS-CoV-2 infection: a possible association. Retina. 2021;41(12):2462-2471. doi:10.1097/IAE.0000000000003277.
- Pichi F, Aljneibi S, Neri P, Hay S, Dackiw C, Ghazi NG. Association of ocular adverse events with inactivated COVID-19 vaccination in patients in Abu Dhabi. JAMA Ophthalmol. 2021;139(10):1131. doi:10.1001/jamaophthalmol.2021.3477.
- Kim J, Kwon HY, Ahn SJ. COVID-19 vaccine-associated uveitis in patients with a history of uveitis. JAMA Ophthalmol. 2024;142(6):522-528. doi:10.1001/jamaophthalmol.2024.0973.
- Tomkins-Netzer O, Sar S, Barnett-Griness O, Friedman B, Shyriaieva H, Saliba W. Association between vaccination with the BNT162b2 mRNA coronavirus disease 2019 vaccine and noninfectious uveitis: a population-based study. Ophthalmology. 2022;129(10):1087-1095. doi:10.1016/j.ophtha.2022.05.015.
- Lee YK, Huang YH. Ocular manifestations after receiving COVID-19 vaccine: a systematic review. Vaccines (Basel). 2021;9(12). doi:10.3390/vaccines9121404.
- Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi:10.1056/NEJMoa2035389.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/NEJMoa2034577.
- Chang MS, Kim HR, Kim S, et al. Noninfectious uveitis risk after COVID-19 vaccination: a nationwide retrospective cohort study. Am J Ophthalmol. 2024;258:22-31. doi:10.1016/j.ajo.2023.09.015.
- Watad A, David P, Brown S, Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants and thyroid autoimmunity. Front Endocrinol (Lausanne). 2016;7:150. doi:10.3389/fendo.2016.00150.
- Pan L, Zhang Y, Cui Y, Wu X. Bilateral uveitis after inoculation with COVID-19 vaccine: a case report. Int J Infect Dis. 2021;113:116-118. doi:10.1016/j.ijid.2021.09.075.
- Sim HE, Hwang JH. New onset of acute uveitis following COVID-19 vaccination. Graefes Arch Clin Exp Ophthalmol. 2023;261:555-560. doi:10.1007/s00417-022-05798-0.
- Watad A, Quaresma M, Brown S, et al. Autoimmune/inflammatory syndrome induced by adjuvants (Shoenfeld’s syndrome) – an update. Lupus. 2017;26(7):675-681. doi:10.1177/0961203316686406.
- Wenande E, Garvey LH. Immediate-type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy. 2016;46(7):907-922. doi:10.1111/cea.12760.
- Singh RB, Parmar UPS, Kahale F, Agarwal A, Tsui E. Vaccine-associated uveitis after COVID-19 vaccination: Vaccine Adverse Event Reporting System database analysis. Ophthalmology. 2023;130(2):179-186. doi:10.1016/j.ophtha.2022.08.027.
- Husby A, Hansen JV, Fosbøl E, et al. SARS-CoV-2 mRNA vaccination and myocarditis in a Nordic cohort study of 23 million residents. BMJ. 2021;375:e068665. doi:10.1136/bmj-2021-068665.

