CAR-NK Therapy in Solid Tumors: Overcoming Immunosuppression
CAR-NK therapy approaches for targeting the immunosuppressive tumor microenvironment in solid tumors
Priya Hays, MS, PhD1
- Hays Documentation Specialists LLC, San Mateo, CA USA
OPEN ACCESS
PUBLISHED: 31 October 2024
CITATION: Hays, P., 2024. CAR-NK therapy approaches for targeting the immunosuppressive tumor microenvironment in solid tumors. Medical Research Archives, [online] 12(10). https://doi.org/10.18103/mra.v12i10.5750
COPYRIGHT: © 2024 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.18103/mra.v12i10.5750
ISSN 2375-1924
ABSTRACT
Background: Adoptive cellular therapies have gained considerable ground while becoming an established form of therapy for hematologic malignancies, now a fifth pillar of treatment after chemotherapy, surgery, radiation and targeted therapies. The most prominent example is chimeric antigen T cell receptor therapies. These therapies have shown superior clinical efficacy in hematologic malignancies, but are less effective against solid tumors. This paper describes the combination of CAR T cells with the innate immune cells natural killer cells in solid tumors in the immunosuppressive tumor microenvironment.
Results: CAR NK cells have been shown in a number of solid tumors in the context of the immunosuppressive tumor microenvironment to have clinical efficacy and exhibit superior safety profiles when compared with original CAR T cells. In preclinical models and ongoing clinical studies, anti-tumor activity has been shown in prostate cancer, hepatocellular carcinoma, glioblastoma and ovarian cancer that benefits from the inherent cytotoxicity of natural killer cells.
Conclusion: Future studies should focus on using the advantages of CAR NK cells in clinical and translational settings.
Keywords: CAR T cells, Natural Killer Cells, CAR-NK cells, tumor microenvironment, solid tumors
Introduction
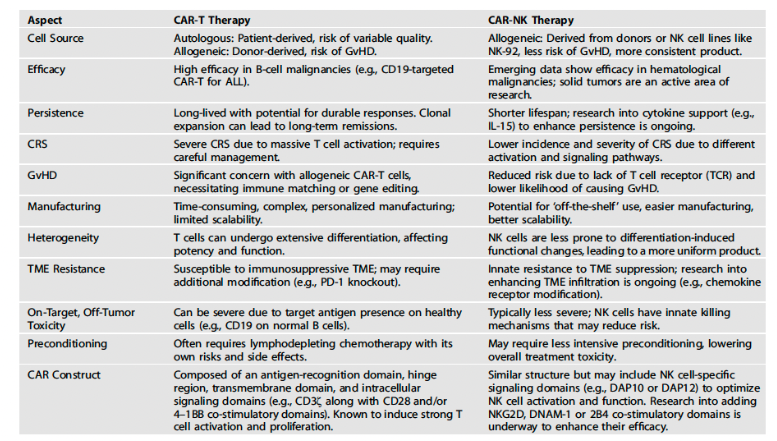
Adoptive cellular therapy has demonstrated increasing success in the treatment of hematologic malignancies such as acute lymphoblastic leukemia and diffuse lymphocytic B cell lymphoma (e.g., tisa cel, axi cel), and serves as an example of active stimulation of the immune system. Chimeric antigen T cell receptors, or CAR T receptors, are one example and when engineered in T cells they exhibit anti-tumor killing. However, when translated in the clinic they have resulted in significant toxicity, with cytokine release syndrome and neurotoxicity being observed in patients receiving CAR T cell therapies, In addition, there are issues with long-term persistence of these engineered cells. These personalized therapies also face significant production and manufacturing challenges.
The anatomy of the CAR T cell construct consists of a 2nd generation CAR construct which is the CAR T cell construct mainly approved for clinical use. They are composed of an antigen binding domain, which in turn is composed of a single chain variable derived from an immunoglobulin molecule, an intracellular domain from a CD3 chain and costimulatory domain, generally identified as the intracellular signaling domain. It is the presence of this co-stimulatory domain that allows for the CAR mediated activation of T cells, obviating the need for antigen presenting cells in an HLA-independent manner. The CAR receptor is derived from a murine mAb that can also be encoded to express a naturally occurring surface receptor that has affinity for tumor specific ligands. The second generation CAR is designed for increased intracellular effector signaling by carrying multiple additional c-o-stimulatory endodomains, being either CD28 or 4-1BB.
Combination CAR constructs have utilized immunostimulatory mechanisms to overcome the immunosuppressive tumor microenvironment in bypassing the limitations of the traditional CAR construct. As chimeric antigen T cell receptor therapies have been established as standard of care immunotherapies for hematologic malignancies, CAR T cell therapy poses a number of challenges in the tumor microenvironment. Rapid exhaustion and rejection are inherent problems. A tumor suppressive environment dampens CAR T cell activity, which also makes them less effective in solid tumors, and they have shown less clinical success against solid tumors. Solid tumors, particularly in later stages, exhibit an inhibitive tumor microenvironment, mainly due to hypoxia, low pH, presence of suppressive cytokines, among other factors. In the recent SPEARHEAD trial, the adoptive cellular therapy afami cel was recently approved by the Food and Drug Administration for the treatment of the solid tumor synovial cancer, being the first example of an engineered T cell therapy approved for clinical use against solid tumors.
A number of combination CAR T constructs have been developed, namely, CAR-Natural Killer cells (CAR NK), CAR-Macrophages (CAR-M), CAR-monoclonal antibodies, CAR-Bispecific antibodies, CAR-Immune checkpoint inhibitors and CAR small molecule inhibitor combinations, along with oncolytic viral CAR T cell strategies. CAR NK cells, CAR-M cells and oncolytic viral CAR T cell combinations are notable for having preclinical and clinical efficacy against solid tumors such as pancreatic cancer, ovarian cancer, glioblastoma and breast cancer and target the tumor microenvironment with relatively greater efficacy. This review will focus on CAR-natural killer cells, where there is significant literature on their role in solid tumors in targeting the inherently immunosuppressive tumor microenvironment. Natural killer cells are part of the innate immunity that possesses inherent cytotoxic killing of tumor cells (among other non-self-cells) that when harnessed with CAR T therapy through the design and construct of CAR-NK cell therapy, synergistically enhances the anti-tumor activities of each type of therapy individually. Natural killer cells also have the advantage of not requiring prior sensitization. Despite their advantages however, they are less effective in solid tumors in their clinical application, especially when faced with a harsh tumor microenvironment (TME), and CAR-NK therapies face significant formidable barriers in this area.
The Anti-Tumor Characteristics of Natural Killer Cells and Challenges faced in an Immunosuppressive Environment
Natural killer (NK) cells constitute 5-15% of human peripheral blood leukocytes and are characterized by lack of expression of CD3 and TCR, and express CD56. The CD56dimCD16high receptor category of NK cells are mature developmentally and have high cytotoxic function. With a high degree of natural potency against tumor cells, they are similar functionally to CD8+ T cells without the need of an antigen specific TCR, and the amount of killing is dependent on the balance between inhibitory and activating signals in the microenvironment. They are key mediators of antibody dependent cellular cytotoxicity (ADCC) in which NK cell cytotoxicity is mediated by bound antibodies. NK cells also express CD16 that leads to Fc portion of IgG bound to the target cell surface. CD16 expression is sufficient for inducing NK cell activation. NK cells also secrete cytokines and chemokines and recruit and activate T cells, dendritic cells and macrophages.
NK cells are also known as peripheral blood lymphocytes whose distribution in healthy cells varies due to unique chemokine receptors and are found in bone marrow, liver, spleen and peripheral blood. Their name derived from their unique ability to directly kill tumor cells by direct cytolytic activity without immunization. They produce large numbers of the cytokine interferon-gamma and immunosuppressive cytokines such as TNF alpha, interleukin 10 and GM-CFS and IL3. Several chemokines, such as CCL2 (MCP-1), CCL3 (MIP1-a), CCL4 (MIP1-b), CCL5 (RANTES), XCL1 (lymphotactin) and CXCL8 (IL-8), are also produced by NK cells. Their ability to produce chemokines attracts other lytic cells such as dendritic cells. NK cells also release granzymes and perforins to increase transmembrane permeability for perforation leading to osmotic lysis. Allogenic NK cells are highly toxic against tumor cells.
T cells also interact with NK cells through entering secondary lymphoid compartments. MHC molecules are downregulated in tumor cells, however, this does not pose a problem for NK cells since they can be activated and subsequently kill tumor cells by the “missing self-mechanism.” NK activation is triggered by an induced self-mechanism whereby killer Ig like receptor (KIR) recognizes induced self-ligands that are highly expressed on cancer cells that appear in response to activation of tumor associated pathways. These ‘missing-self’ and ‘induced-self’ changes are characterized by cellular ageing, DNA damage response and tumor suppressor genes that stimulate the expression of ligands for activating receptors, which leads to NK cell activation due to the influence of these activating receptors. Thus, target cells are eliminated either directly via NK cell mediated cytotoxicity or indirectly via pro-inflammatory cytokine-mediated killing.
ADCC is another way to target tumor cells. NK cells are characterized by the abundance of CD16, which has both an IgG1 and IgG3 receptor, and leads to NK mediated ADCC wherein cytotoxic granules perforin and granzymes, FAS ligands and TNF-related apoptosis-inducing ligand death receptors are all involved. A major pathway of NK cell mediated cytotoxicity is the Fas/FasL pathway that sends a death signal to the targeted cells to undergo apoptosis. IFN -gamma and IL-10 production from NK cells enhance macrophage function. NK cells spare normal cells and kill tumor cells as result of their recognition of MHC molecules and are not associated with graft versus host disease (GvHD), unlike CAR T cell therapies. They are not associated with the toxicities of CAR T cell therapy and as an adoptive cellular therapy do not result in events of cytokine release syndrome and neurotoxicity, and have unique advantages in the allogeneic setting.
The harsh TME poses challenges for effective NK antitumor therapy. It is hypoxic, devoid of glucose and amino acids, and acidic. In solid tumor, the hypoxia of the TME is notable, and is “a common driver of immune cell dysfunction.” This is exacerbated by the presence of regulatory T cells and MDSC, among other immunosuppressive molecules. Hypoxia response signaling of HIF1a signaling enhances NK cell potency and leads to NK cell dependent antitumor activity. However, tumors as a result of high levels of lactic acid, adenosine and reactive oxygen species and increase in toxic catabolites exhibit aberrant metabolic behavior, and lead to dysfunctional immune effector cells due to this uncontrolled proliferation, abnormal vasculature and presence of immunosuppressive molecules. Attempts to overcome this have focused on altering tumor metabolism and modifying gene expression.
Adenosine, another byproduct of tumor metabolism, is upregulated and accumulation of extracellular quantities negatively regulates NK cell function in the TME suppressing their immune effector activity. Adenosine also activates the immunosuppressive molecules T reg cells, MDSCs and M2 macrophages further compounding this state of immunosuppression. Genetic editing has been utilized in pre-clinical studies in CAR NK cells to delete their adenosine A2A receptor to improve antitumor activity. NK cells that have high-affinity dominant-negative TGF-beta receptor that retain their potency in the milieu of TGF-beta. Laskowski et al successfully produced a CB-NK cell that was immune to TGF-beta signaling through targeted deletion of its receptor. They also provided in vivo evidence that TFB-beta signaling in a mouse model of glioblastoma safeguards the cytotoxic properties of NK cells.
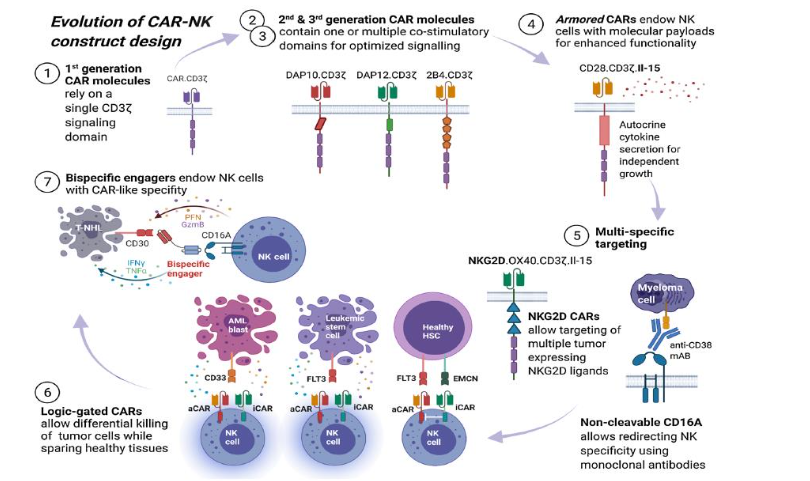
CAR-NK Engineering
NK cells have been integrated into the gene engineering pipeline of CAR T cells. CAR-NK cells engage in HLA-unrestricted killing and have the potential for off-the-shelf use. They have a favorable safety profile and do not require complex manufacturing. They also exhibit long-term persistence. CAR-NK constructs have been designed based on NK inherent activation, based on DNAX-activation protein 12 replacing CD3. Pivotal in intracellular signaling of PI3K, they activate KIRs. DAP12 also contains immune-tyrosine activating molecules, that when phosphorylated lead to the release of cytotoxic granules and pro-inflammatory cytokines such as TNFα and IFNγ. Tonic signaling in which CAR T cells demonstrate increased basal levels of activation in which antigen engagement is not involved is also a factor. The bystander effect of NK cells as a result of cytokine production activates T cell and myeloid cell effector molecules, and potentiates the antitumor response. Laskowski et al has shown that CAR NK cells armored with the cytokine 1L-15 demonstrate superior persistence in preclinical models, when compared to CAR alone, and when evaluated in a CD19+ lymphoid malignances, CAR NK cells were detected in circulation post-treatment after a year.
The NK cell specific CAR structure is based on the design of the original CAR cells comprising an extracellular antigen recognition domain, a transmembrane domain, and an intracellular signaling domain, but differs in that the intracellular domain in CAR-NK cells often includes signaling motifs from NK cell activating receptors, including DAP12 or CD3ζ. These are coupled with co-stimulatory domains from 2B4 (CD244) or DNAM-1 (CD226) to enhance activation and cytotoxic function.
According to Zhong and Liu “this configuration is crucial as it leverages the natural cytotoxic pathways of NK cells, ensuring that the engineered cells function optimally within their innate immune response mechanisms.” A number of clinical trials have been conducted evaluated CAR-NK therapies in hematologic malignancies, particularly in refractory CD19 positive cancers, such as non-Hodgkin’s lymphoma. There are number of trials that target specific tumor antigens in solid tumors.
| Trial | Target | Status |
|---|---|---|
| Trial 1 | Target 1 | Ongoing |
| Trial 2 | Target 2 | Completed |
The Immunosuppressive Tumor Environment and Targeted Approaches of CAR-NK Designed Constructs
CAR NK killing is through both CAR dependent and CAR independent means; they can eliminate cancer cells through the natural cytotoxic activity of NK cells and through certain activating KIRs as wells through CD16-mediated ADCC. The eradication of the heterogenous tumors takes place even when tumor cells do not express CAR targeted antigen, via both CAR-dependent and the NK cell receptor pathways. CAR NK therapy targets the hostile tumor microenvironment through a number of mechanisms:
- Targeted Tumor Cell Killing: CAR NK cells are engineered to express receptors that specifically recognize antigens present on tumor cells. Upon binding to these antigens, CAR NK cells become activated and kill the tumor cells through various mechanisms, including the release of cytotoxic granules (e.g., perforin and granzymes), death receptor-mediated apoptosis (e.g., FasL and TRAIL pathways), and antibody-dependent cellular cytotoxicity (ADCC).
- Cytokine Secretion: CAR NK cells secrete cytokines such as IFN-γ and TNF-α, which can enhance anti-tumor immunity by promoting the recruitment and activation of other immune cells (e.g., T cells, dendritic cells) and by inhibiting angiogenesis (formation of new blood vessels) that supports tumor growth.
- Overcoming Immunosuppression: The tumor microenvironment (TME) often includes immunosuppressive cells like regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs). CAR NK cells can alter the TME by secreting cytokines and chemokines that reduce the number and suppressive function of these cells, thereby restoring anti-tumor immunity.
- Direct Targeting of Immunosuppressive Factors: CAR NK cells can be engineered to target not only tumor cells but also components of the immunosuppressive TME. For example, CAR NK cells can be designed to target and kill cells expressing PD-L1, a checkpoint molecule that inhibits T cell activity, thereby reducing local immunosuppression.
- Metabolic Reprogramming: The TME is often characterized by hypoxia and metabolic alterations that can inhibit immune cell function. CAR NK cells can be engineered to be resistant to these harsh conditions or to modify the metabolic landscape of the TME, improving their survival and function within tumors.
- Recruitment of Other Immune Cells: By secreting various cytokines and chemokines, CAR NK cells can recruit and activate other immune cells, such as T cells and dendritic cells, creating a more robust and sustained anti-tumor immune response.
CAR-Natural Killer Cells Overcoming an Immunosuppressive Tumor Microenvironment in Solid Tumors
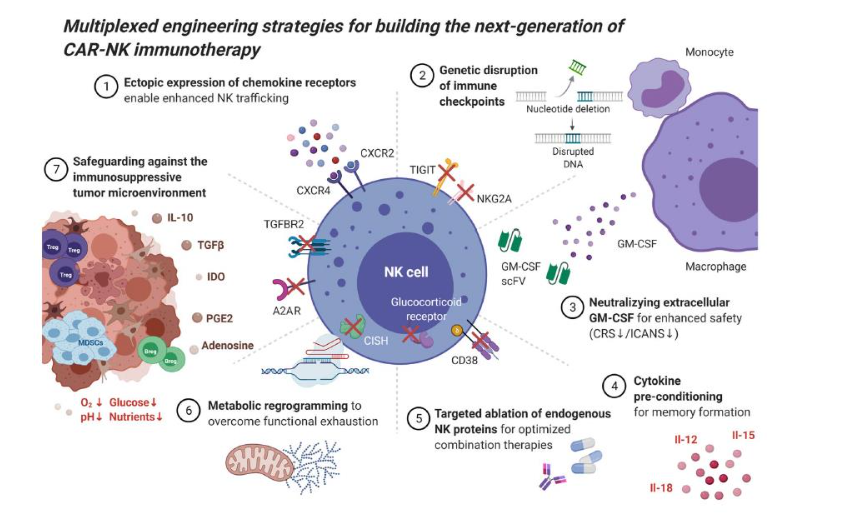
As reported by Zhong and Liu, the immunosuppressive nature of the TME leads to decreased immune cell infiltration and function, “dampening the cytotoxic response of CAR-NK cells.” IL-10 secretion by T regs and MDSCs inhibit the function of NK effector cells. CAR NK engineered cells that secrete IL-15 enhance the survival and promote the inflammatory activity of NK cells. Genetically modified CAR-NK cells that have knocked out NKG2A lead to cellular stimulation, resulting in less inhibitory signals within the TME. The NKG2A knockout CAR-NK cells displayed enhanced anti-tumor activity in a preclinical model.
Solid tumors are high in MDSCs and Tregs leading to a more resistant tumor microenvironment. DNAM overexpressing NK92 cell lines showed enhanced degranulation activity against prostate cancer, pancreatic cancer and lung cancer. A direct comparison of several CAR structures revealed a NK cell tailored CAR that resulted in superior anti-tumor effectivity than those transferred directly from CAR T. One study found that CAR NK specific cells contain NDAM1 and 2B4 co-stimulatory receptors which demonstrated high cytotoxicity against hepatocellular cancer cell lines (HEpG2, Hep3B and Huh7). PSCA-directed CAR-NK with DAP12 incorporated as co-stimulatory receptors was shown to have superior toxicity profiles in xenograft mouse models of prostate cancer.
According to Wrona et al, NK cells have been reprogramed through great efforts to overcome immunosuppressive TME. Several strategies have been used against the suppressive TME. The TME secrete TGF-beta, adenosine, IL-10, PGE2 and IDO that consist of immunosuppressive factors. TGF-beta leads to immunosuppression by reducing recruitment of NK cells and downregulates activation receptors NKG2D and DNAM1 and blocks perforin secretion. CAR NK therapy might be helped by the co-expression of chimeric co-stimulatory converting receptor (CCCR) that signals improves the effectivity of traditional CAR-NK therapy, being composed of the extracellular portion of the PD1 receptor, NKG2D and 4-1BB co-stimulators. The designed CCCR-NK structure was found to inhibit lung cancer growth in xerographic mice models by switching the immunosuppressive PD-1 signal to an activating one.
Preclinical data have been conducted on CAR-NK cells in solid tumors that targets the immunosuppressive TME. Most of the preclinical data has been in the solid tumors GBM, breast ovarian and pancreatic cancer. GBM has a considerable immunosuppressive TME, even though 89% of the GBM has naturally occurring NK cells. Pancreatic cancer is noted for its immunosuppressive stroma, reaching up to 70% of tumor volume. In vivo adoptive cells therapies report unsatisfactory outcomes. In CAR NK development, multiple attempts were conducted to overcome this hostile feature of PDAC. A study of up-regulated PDAC specific membrane markers showed that folate receptor and death receptor 4/5 were optimal targets of CAR-NK cell therapy. The study showed that high efficacy of FR-directed TRAIL induced apoptosis in in vivo xenograft models of pancreatic cancer.
Pancreatic cancer cells express ROBO-1 which CAR-NK also targets. A study with three groups of animals, an untreated group, a group receiving brachytherapy and a third group receiving brachytherapy with ROBO-1 directed CAR-NK combination infusions. Superior tumor volume reduction was observed in the third group compared to brachytherapy alone. This promising preclinical data led to “three phase I/II clinical trials aimed at assessing the safety and efficacy of ROBO-1 directed CAR-NK cell therapy in PDAC and other solid tumors depending on ROBO-1 expression on cancer cells, which are currently enrolling patients in China,” with an experimental arm of a single dose of ROBO-1 directed CAR-NK infusions with no conditioning.
Another target for CAR NK cells is mesothelian expressing ovarian cancer cell lines (OVCAR-3 and SKOV3). Mesothelin directed CAR-NK92 cells have been shown to eliminate MSLN expressing cell lines in mice models, which have been shown to have prolonged survival. CD24 is also another targeted on ovarian cancer cells in the TME, for which a dual CAR approach with NK92 cells was constructed. FR-directed CAR-NK92 cells displayed cytotoxicity in ovarian cancer models since FR antigen is expressed in 90% of the ovarian cancer cases. Stronger degranulation and cytokine secretion resulted in the TME which led to greater proliferation and longer persistence of these CAR constructs.
Efficacy of CAR-NK cells has also been demonstrated in c-Met expressing HepG2 cells in which greater cytotoxicity was observed. In prostate cancer, “knocking out PTPN11 (NK cell inhibitory receptor) in CAR-expressing NK-YT cells directed against PSMA (PSMA-directed.CD8.CD28.CD3.CAR-NK-YT) resulted in increased cytotoxicity against the Du-145 cell line, which is naturally resistant to NK cell killing.” CAR NK cell directed therapy has a unique advantages over CAR T in other TME immunosuppressive settings. Activated NK cells produce GM-CSF and IFN-gamma rather than a cytokines such as IL-10 and IL-15, making them less toxic and less likely to induce CRS and neurotoxicity. Since CAR-NK cells are generated from multiple sources such as human PBMCs and human iPSCs, they have reduced risk for alloreactivity in the allogeneic setting and off the shelf products are more readily available.
CAR directed killing is able to be enhanced by the intrinsic anti-tumor activities of natural killer cells. When genetically engineered to express a CAR molecule, which does not rely on the activity of the TCR, they have an intrinsic ability to recognize tumor cells through their native receptors. This property allows for a favorable safety profile compared to T cells that need further genetic modification to circumvent GvHD.
The first clinical studies that led to primary establishment of CAR-engineered NK cells utilized third-generation CD33-directed CAR constructing with CD28 and 4-1BB to transduce NK-92 derived NK cells that were tested in acute myeloid leukemia. However, patients did not go into remission since CAR-NK levels were below detection thresholds, which negated the intrinsic effectivity of CAR lymphocytes for in vivo expansion and long-term persistence. Much of this was attributed to the physiological life span of NK cells of only 2 weeks that precludes a sustained response, which is mitigated by the co-infusion of IL-15 cytokine support for in-vivo persistence. Another advantage of NK transduction is better safety profiles with less instances of GvHD, CRS and neurotoxicity.
The shift in CAR NK cell therapies has been in their growing use in solid tumors, which has been ameliorated by sophisticated construct designs that lead to ways of overcoming the immunosuppressive tumor microenvironment through enhancing NK cell potency and persistence. Increased antigen affinity and prolonged in vivo persistence are specific potential advantages of CAR-NK constructs when implemented in the pre-clinical and early phase clinical studies that benefit from the multiple costimulatory elements with domains derived from CD28, ICOS, the TNF receptor the TNF receptor superfamily (4-1BB, CD27, OX40, and CD40) and CD40L and toll-like receptors (TLRs).
Multiple CAR-designed NK constructs targeting a diverse set of tumor antigens are undergoing evaluation, particularly against solid tumors in order to prevent immune cell exhaustion in the tumor microenvironment. Upregulation of immune checkpoints has also been proposed including PD-1, LAG-3, TIM-3, TIGIT and KLRG1 that also confers potential for antitumor activity against solid tumors such as melanoma and non-small cell lung cancer. Genetic targeting against these receptors may evade immune blockade that results and skews the equilibrium in the TME towards anti-tumor immunity. Blockade of TIGIT has been shown to maintain anti-tumor immunity responses, another solid tumor target.
Biederstadt and Rezvani have expounded upon how CAR-NK constructs can overcome the immunosuppressive tumor microenvironment. A “hostile” TME leads to highly unfavorable tumor microenvironment for targeting immune cells, due partly to nutrient deprivation and hypoxia and regulatory T cells and MDSCs, TAMs, platelets and fibroblasts that are attracted to the milieu, along with NK-intrinsic immune checkpoints. Immunosuppressive cytokines are secreted by these molecules including TGF-beta and prostaglandin E2. The blockade of these immunosuppressive molecules has shown enhanced tumor control in xenograft mouse models of BRAF-mutated melanoma. It was also found that disruption of TGFbeta-receptor signaling preserves NK cell function in glioblastoma stem cell-engrafted mouse model.
As Biederstadt and Rezvani, conclude, “Together these studies highlight how novel genetic engineering approaches can be employed to disarm the hostile tumor microenvironment by rendering CAR-modified immune cells resistant to some of the most notorious immunosuppressive mechanisms, a theme we project will continue to have important reverberations in the treatment of human cancers, particularly when targeting solid tumors.”
However, due to solid tumor challenges of in vivo persistence and functional exhaustion, complex clonal heterogeneity and shared antigen expression in normal cells, there are express limitations of CAR-NK immunotherapy in solid tumors, the authors also note. Early phase studies are nevertheless taken place including targeting HER2 in glioblastoma, PSMA in prostate cancer and mesothelin in ovarian cancer, and next generation CAR NK therapies have been engineered enhanced ability to migrate through tumor beds and penetrate the TME barriers imposed by solid tumors, that have been described above. In renal cell carcinoma, NK cells expressing CXCR2 improved tumor trafficking and forced expression of the chemokine receptor CXCR4. EGFRvIII-directed CAR NK cells led to improved survival in an animal model of glioblastoma. In a mouse model of peritoneal ovarian cancer, ectopic expression of CAR targeting NKG2D ligands demonstrated improved immune infiltration.
Discussion
The immunosuppressive TME has demonstrated clinical resistance to adoptive cellular therapy, an example being CAR T cells, which while having superior clinical efficacy in hematologic malignancies are less effective in solid tumors with its harsh TME. The harsh TME results from the presence of regulatory T cells, MDSCs and immunosuppressive macrophages, hypoxia, PGE, adenosine molecules, the expression of cytokines IL-15, IFN-gamma and TGF-beta, and in some cases a resistant stroma such as seen in pancreatic cancer. CAR NK cellular therapies like CAR T cells have faced similar challenges in solid tumors, but have presented tumor cells with a number of adaptive mechanisms for overcoming a hostile TME. The most interesting target in tumors for CAR NK cells is HER2, and efficacy has been shown in GBM, breast, pancreatic and ovarian cancer. Preclinical and clinical studies have been reported in these tumors evaluated CAR-NK cellular therapies.
One of the aspects of CAR-NK that has been highlighted by these studies and reviews is the superior safety profile of CAR-NK when compared to original CAR T, having considerably less instances of CRS and neurotoxicity. Off the shelf therapy in the allogeneic settings is also more amenable since the sources of NK cells such as human PBMCs and iPSCs are more available when compared to autologous T cells.
Additionally, CAR T cells are being combined with other types of immunotherapies and immune effector molecules such as oncolytic viruses, macrophages, immune checkpoint inhibitors and small targeted inhibitors to overcome the hostile TME, especially in solid tumors. These agents increase CAR T efficacy by inducing cancer cell lysis such as in the case of oncolytic viruses or blocking PD-1 as in the case of immune checkpoint inhibitor pembrolizumab.
Conclusion
CAR NK cells have shown efficacy with superior safety profiles against certain targets in solid tumors in the context of an immunosuppressive tumor microenvironment. The TME contains immunomodulatory molecules, cytokines and metabolic conditions that make it resistant to this form of therapy that is elegantly designed. Future studies should expand upon the preclinical data and clinical studies to enable this therapy to be translated into the clinic.
P.H. contributed to this manuscript in its entirety including design, writing and editing.
Funding: No funding was used in the preparation of this manuscripts.
Institutional Review Board Statement: Not applicable
Informed Consent Statement: Not applicable
Data Availability: Not applicable.
Conflicts of Interest: None to disclose
References
- Figueroa JA, Reidy A, Mirandola L, Trotter K, Suvorava N, Figueroa A, et al. Chimeric Antigen Receptor Engineering: A Right Step in the Evolution of Adoptive Cellular Immunotherapy. Int Rev Immunol (2015) 34(2):154–87.
- Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and Development of Therapies Using Chimeric Antigen Receptor-Expressing T Cells. Immunol Rev (2014) 257(1):107–26.
- Perutelli F, Jones R, Griggio V, Vitale C and Coscia M (2022). Immunotherapeutic Strategies in Chronic Lymphocytic Leukemia: Advances and Challenges. Front. Oncol. 12:837531.
- Biederstädt A and Rezvani K. Engineering the next generation of CAR‑NK immunotherapies. International Journal of Hematology (2021) 114:554–571
- Adada M, Siegler EL, Kenderian SS. Combination Therapeutics with CAR-T Cell therapy in Gene and Cellular Immunotherapy for Cancer. Ghobadi A and DiPersio JF, Eds. (Switzerland: Springer Nature) 2022; pp. 69-91.
- Caligiuri MA. Human natural killer cells. Blood 2008;112(3):461–9.
- Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM. NK cell-mediated antibody dependent cellular cytotoxicity in cancer immunotherapy. Front Immunol 2015;6:368.
- Vivier E, Raulet DH, Moretta A, et al. Innate or adaptive immunity? the example of natural killer cells. Science 2011;331(6013):44–9.
- Lindner SE, Johnson SM, Brown CE, Wang LD. Chimeric antigen receptor signaling: functional consequences and design implications. Sci Adv. 2020;6.
- Kwon M, Iacoboni G, Reguera JL, Corral LL, Morales RH, Ortiz-Maldonado V. et al. Axicabtagene ciloleucel compared to tisagenlecleucel for the treatment of aggressive B-cell lymphoma. Haematologica. 2023;108:110–21.
- Zhong Y and Liu J. Emerging roles of CAR-NK cell therapies in tumor immunotherapy: current status and future directions. Cell Death Discovery (2024) 10:318.
- Laskowski TJ, Biederstädt A and Rezvani K. Natural killer cells in antitumour adoptive cell immunotherapy. Nature Reviews. 2022;22:557-587.
- Ni, J. et al. Single-cell RNA sequencing of tumor-infiltrating NK cells reveals that inhibition of transcription factor HIF-1α unleashes NK cell activity. Immunity 52, 1075–1087.e8 (2020).
- Rosario, M. et al. The IL-15-based ALT-803 complex enhances FcγRIIIa-triggered NK cell responses and in vivo clearance of B cell lymphomas. Clin. Cancer Res. 22, 596–608 (2016).
- Jin, D. et al. CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res. 70, 2245–2255(2010)
- Stagg, J. et al. Anti-CD73 antibody therapy inhibit breast tumor growth and metastasis. Proc. Natl Acad. Sci. USA 107, 1547–1552 (2010).
- Allard B, Longhi MS, Robson SC, Stagg J. The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol. Rev. 276,121–144 (2017).
- Perrot, I. et al. Blocking antibodies targeting the CD39/CD73 immunosuppressive pathway unleash immune responses in combination cancer therapies. Cell Rep. 27, 2411–2425.e9 (2019).
- Young, A. et al. Co-inhibition of CD73 and A2AR adenosine signaling improves anti-tumor immune responses. Cancer Cell 30, 391–403 (2016).
- Young, A. et al. A2AR adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment. Cancer Res. 78, 1003 (2018).
- Lupo K and Matosevic, S. Natural killer cells engineered with an inducible, responsive genetic construct targeting TIGIT and CD73 to relieve immunosuppression within the GBM microenvironment. J. Immunother. Cancer 8, A74–A75 (2020).
- Giuffrida, L. et al. CRISPR/Cas9 mediated deletion of the adenosine A2A receptor enhances CAR T cell efficacy. Nat. Commun. 12, 3236 (2021).
- Daher, M. et al. The TGF-β/SMAD signaling pathway as a mediator of NK cell dysfunction and immune evasion in myelodysplastic syndrome. Blood 130,53–53 (2017).
- Shaim, H. et al. Targeting the αv integrin/TGF-β axis improves natural killer cell function against glioblastoma stem cells. J. Clin. Investig. https://doi.org/10.1172/JCI142116 (2021).
- Daher J and Rezvani K. Outlook for new CAR-based therapies with a focus on CAR NK cells: what lies beyond CAR-engineered T cells in the race against cancer. Cancer Disco 11, 45–58 (2021).
- Lanier LL, Corliss BC, Wu J, Leong C and Phillips JH. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature 391, 703–707 (1998).
- Zhao R et al. DNAX-activating protein 10 co-stimulation enhances the anti-tumor efficacy of chimeric antigen receptor T cells. Oncoimmunology. 8, e1509173 (2018).
- Felices M et al. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight.
- Liu E et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 32, 520–531 (2018).
- Xiao X, Huang S, Chen S, Wang Y, Sun Q, Xu X, et al. Mechanisms of cytokine release syndrome and neurotoxicity of CAR T-cell therapy and associated prevention and management strategies. J Exp Clin Cancer Res. 2021;40:367.
- Lindner SE, Johnson SM, Brown CE, Wang LD. Chimeric antigen receptor signaling: functional consequences and design implications. Sci Adv. 2020;6:eaaz3223.
- Gong Y, Klein Wolterink RGJ, Wang J, Bos GMJ, Germeraad WTV. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J Hematol Oncol. 2021;14:73.
- Zhang L, Meng Y, Feng X, Han Z. CAR-NK cells for cancer immunotherapy: from bench to bedside. Biomark Res. 2022;10:12.
- Oei V, Siernicka M, Graczyk-Jarzynka A, et al. Intrinsic Functional Potential of NK-Cell subsets constrains retargeting driven by chimeric antigen receptors. Cancer Immunol Res 2018;6(4):467–80.
- Sun C, Sun H, Zhang C, Tian Z. NK cell receptor imbalance and NK cell dysfunction in HBV infection and hepatocellular carcinoma. Cell Mol Immunol 2015;12(3):292–302.
- Sun C, Sun HY, Xiao WH, Zhang C, Tian ZG. Natural killer cell dysfunction in hepatocellular carcinoma and NK cell-based immunotherapy. Acta Pharmacol Sin 2015;36(10):1191–9.
- Wu J, Mishra HK, Walcheck B. Role of ADAM17 as a regulatory checkpoint of CD16A in NK cells and as a potential target for cancer immunotherapy. J Leukoc Biol 2019;105(6):1297–303.
- Xiea G, Dong H, Liang Y, Dongjoo H, Rizwand R et al. CAR-NK cells: A promising cellular immunotherapy for cancer. EBioMedicine.
- ChatGPT, search, August 10,2024.
- Xu Y, Zeng H, Jin K, Liu Z, Zhu Y, Xu L, et al. Immunosuppressive tumor-associated macrophages expressing interlukin-10 conferred poor prognosis and therapeutic vulnerability in patients with muscle-invasive bladder cancer. J Immunother Cancer. 2022;10:e003416.
- Van den Eynde A, Gehrcken L, Verhezen T, Lau HW, Hermans C, Lambrechts H, et al. IL-15-secreting CAR natural killer cells directed toward the pan-cancer target CD70 eliminate both cancer cells and cancer-associated fibrob lasts. J Hematol Oncol. 2024;17:8.
- Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, et al. Use of CAR transduced natural killer cells in CD19-positive lymphoid Tumors. N Engl J Med. 2020;382:545–53.
- André P, Denis C, Soulas C, Bourbon-Caillet C, Lopez J, Arnoux T, et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell. 2018;175:1731–1743.e13.
- Wrona E, Borowiec M, Potemski P. CAR-NK Cells in the Treatment of Solid Tumors. Int. J. Mol. Sci. 2021, 22, 5899.
- Töpfer K, Cartellieri M, Michen S, Wiedemuth R, Müller N, et al. DAP12-Based Activating Chimeric Antigen Receptor for NK Cell Tumor Immunotherapy. J. Immunol. 2015, 194, 3201–3212.
- Lu C, Guo C, Chen H, Zhang H, Zhi L et al novel chimeric PD1-NKG2D-41BB receptor enhances antitumor activity of NK92 cells against human lung cancer H1299 cells by triggering pyroptosis. Mol. Immunol. 2020, 122, 200–206.
- Lee YE, Ju A, Choi HW, Kim JC, Kim EE, et al. Rationally designed redirection of natural killer cells anchoring a cytotoxic ligand for pancreatic cancer treatment. J. Control. Release 2020, 326, 310–323.
- He H, Hao S.-J, Yao L, Yang F, Di Y, Li J, et al. MicroRNA-218 inhibits cell invasion and migration of pancreatic cancer via regulating ROBO1. Cancer Biol. Ther. 2014, 15, 1333–1339.
- Xia N, Haopeng P, Gong JU, Lu J, Chen Z, et al. Robo1-specific CAR-NK Immunotherapy Enhances Efficacy of 125I Seed Brachytherapy in an Orthotopic Mouse Model of Human Pancreatic Carcinoma. Anticancer Res. 2019, 39, 5919–5925.
- Cao B, Liu M, Wang L, Liang B, Feng Y et al. Use of chimeric antigen receptor NK-92 cells to target mesothelin in ovarian cancer. Biochem. Biophys. Res. Commun. 2020, 524, 96–102.
- Klapdor R, Wang S, Morgan M, Dörk T, Hacker U, et al Characterization of a Novel Third-Generation Anti-CD24-CAR against Ovarian Cancer. Int. J. Mol. Sci. 2019, 20, 660.
- Ao X, Yang Y, Li W, Tan Y, Guo W, et al. Anti-FR CAR-engineered NK-92 Cells Display Potent Cytotoxicity Against FR-positive Ovarian Cancer. J. Immunother. 2019, 42, 284–296.
- Subrakova VG, Kulemzin SV, Belovezhets TN, Chikaev AN, Chikaev NA et al. shp-2 gene knockout upregulates CAR-driven cytotoxicity of YT NK cells. Vavilov J. Genet. Breed. 2020, 24, 80–86.
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–10.
- Liu E, Tong Y, Dotti G, Shaim H, Savoldo B, Mukherjee M, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia. 2018;32(2):520–31.
- Weinkove R, George P, Dasyam N, McLellan AD. Selecting costimulatory domains for chimeric antigen receptors: functional and clinical considerations. Clin Transl Immunology. 2019;8(5):e1049.
- Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu W, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol. 2018;19(7):723–32.
- Judge SJ, Murphy WJ, Canter RJ. Characterizing the dysfunctional NK cell: assessing the clinical relevance of exhaustion, anergy, and senescence. Front Cell Infect Microbiol.2020;10:49.
- Young A, Ngiow Shin F, Barkauskas Deborah S, Sult E, Hay C, Blake Stephen J, et al. Co-inhibition of CD73 and A2AR adenosine signaling improves anti-tumor immune responses. Cancer Cell. 2016;30(3):391–403.
- Young A, Ngiow SF, Gao Y, Patch A-M, Barkauskas DS, Messaoudene M, et al. A2AR adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment. Can Res. 2018;78(4):1003.
- Shaim H, Shanley M, Basar R, Daher M, Gumin J, Zamler DB, et al. Targeting the αv integrin/TGF-β axis improves natural killer cell function against glioblastoma stem cells. J Clin Invest. 2021.
- Ng YY, Tay JC, Wang S. CXCR1 expression to improve anticancer efficacy of intravenously injected CAR-NK cells in mice with peritoneal xenografts. Mol Ther Oncol. 2020;16:75–85.
- Gong Y, Klein R, Wolterink R, Wang J, Gerard MJ. Chimeric antigen receptor natural killer (CAR‑NK) cell design and engineering for cancer therapy. J Hematol Oncol. 2021;14:73.
- Pan K, Farrukh H, Reddy VCS, Xu H, Pan C-x et al. CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy. J Exp Clin Cancer Res. 2022;41:119.
- Kilgour MK, Bastin DJ, Lee S-H, Ardolino M, et al. Advancements in CAR-NK therapy: lessons to be learned from CAR-T therapy. Front Immunol. 2023;14.

