Efficacy of Vildagliptin vs Sitagliptin in T2DM Care
Patient-centered Assessment of the Efficacy and Safety of Vildagliptin Sustained-release Tablet (100 mg) Relative to Sitagliptin (100 mg) in Patients with Type 2 Diabetes Mellitus Inadequately Controlled on MEtformin (PRIME-Vilda Trial)
Abdul Hamid Zargar ¹, Sunil Gupta ², Balaji Jaganmohan ³, Sona Warrier ⁴, Faraz Farista ⁵, Chetan Patil ⁶, Sachin Yadav ⁷
OPEN ACCESS
PUBLISHED: 31 May 2025
CITATION Zargar, AH., Gupta, S., et al., 2025. Patient-centered Assessment of the Efficacy and Safety of Vildagliptin Sustained-release Tablet (100 mg) Relative to Sitagliptin (100 mg) in Patients with Type 2 Diabetes Mellitus Inadequately Controlled on MEtformin (PRIME-Vilda Trial). Medical Research Archives, [online] 13(5). https://doi.org/10.18103/mra.v13i5.6599
COPYRIGHT © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.18103/mra.v13i5.6599
ISSN 2375-1924
ABSTRACT
Dipeptidyl peptidase-4 inhibitors (DPP4Is) were introduced for the management of type 2 diabetes mellitus (T2DM) due to their insulinotropic effects, lack of inherent hypoglycemia risk, and neutral impact on body weight. Vildagliptin is a dipeptidyl peptidase-4 inhibitor that improves glycemic control by increasing the incretin levels and prolonging the effect of glucagon-like peptide 1 (GLP- 1). Due to its unique mechanism of action for glycemic control and its advantages, DPP-4 inhibitors have been widely used alone in monotherapy or combined with metformin. The present study aimed to evaluate the efficacy and safety of Vildagliptin 100 mg sustained release tablet once daily (SR tablet OD) compared to Sitagliptin 100 mg tablet in patients with Type 2 diabetes mellitus not optimally controlled on Metformin alone. A total of three sites were involved in the enrollment of 128 Type 2 diabetes mellitus patients, with 64 patients in each treatment arm. 50 patients were selected to monitor the average blood glucose levels by continuous glucose monitoring system (CGMS) measurements in a subset of population with 25 patients in each treatment arm. Eligible patients were randomly assigned to receive (orally) Vildagliptin sustained release tablets 100 mg or Sitagliptin tablets IP 100 mg with metformin once daily for 84 days. The reduction in HbA1c, fasting blood glucose (FBG) levels, postprandial blood glucose (PPBG) levels and the average glucose values measured using the Continuous Glucose Monitoring system (CGMS) from baseline to the end of treatment (Day 84±3) were found to be -0.22%, -1.43 mg/dL, 2.55 mg/dL, and -19.14 mg/dL respectively indicating that Vildagliptin 100 mg Sustained Release Tablet administered once daily in patients with Type 2 diabetes mellitus who are not optimally controlled on metformin alone is beneficial for better patient compliance towards effective glycemic control while reducing the frequency of dosing.
Keywords
Efficacy, safety, sustained-release tablet, Vildagliptin
INTRODUCTION
Dipeptidyl peptidase 4 (DPP-4) inhibitors are among the most important treatment options for type 2 diabetes mellitus (T2DM). DPP-4 inhibitors improve glycemic control by increasing the incretin levels and prolonging the effect of glucagon-like peptide 1 (GLP-1). Due to its unique mechanism of action for glycemic control and its advantages of a low risk of hypoglycaemia and weight-neutral effects, DPP-4 inhibitors have been widely used alone in monotherapy or combined with metformin.
Vildagliptin, a commonly used DPP-4 inhibitor, showed significant glucose-lowering efficacy as an initial monotherapy and add-on therapy in several clinical studies in patients with T2DM. It decreases the mean amplitude of glycemic excursion efficiently and reduces oxidative stress and inflammation. Vildagliptin’s overall safety and tolerability profile was comparable to placebo throughout clinical studies, and real-world data in a large group of T2DM patients corroborated this finding. The nature of diabetes and its management are complex, requiring compliance-oriented care to simplify treatment. The appeal of single pills as OD formulation has increased as the burden of diabetes regimens increases, thus increasing adherence to the administration of antidiabetic agents.
The sustained-release (SR) formulation of Vildagliptin 100 mg was designed to maintain consistent drug levels over an extended period by utilizing a polymer matrix system that ensures controlled and gradual release of the active pharmaceutical ingredient (API). This matrix-based approach helps deliver the full dose of Vildagliptin steadily throughout the day, supporting once-daily (OD) dosing.
The core mechanism of this formulation relies on a hydrophilic matrix, where drug release primarily occurs through diffusion rather than tablet erosion. Upon ingestion, the tablet interacts with gastrointestinal fluids, forming a gel layer on its surface. This gel acts as a diffusion barrier, allowing the drug to slowly dissolve and permeate through the matrix. As hydration continues, the gelation process penetrates deeper into the tablet core, sustaining the release over time.
The matrix polymer employed in this SR formulation is Microcrystalline Cellulose (MCC) PH 112, a well-established excipient known for its role in controlled-release systems. MCC is favored for its robustness, safety profile, and its ability to support zero-order drug release kinetics, where the drug is released at a constant rate regardless of concentration. Notably, MCC-based systems demonstrate minimal sensitivity to gastric pH variations and gastrointestinal motility, ensuring consistent performance across a wide range of physiological conditions.
By providing a steady and predictable release profile, the SR formulation significantly reduces peak-to-trough fluctuations seen in immediate-release (IR) formulations, thereby minimizing the risk of side effects and enhancing patient tolerability. The optimized delivery profile also aligns with the therapeutic goal of maintaining uniform glycemic control throughout the day.
The current study is a prospective, multi-center, parallel-group study designed to evaluate the efficacy and safety of Vildagliptin 100 mg SR Tablet OD compared to Sitagliptin 100 mg tablet in patients with type 2 diabetes mellitus not optimally controlled on metformin alone. In this study, we aimed to compare the efficacy and safety of Vildagliptin 100 mg SR Tablet OD compared to Sitagliptin 100 mg Tablet once daily in patients with Type 2 diabetes mellitus not optimally controlled on metformin alone.
Subjects and Methods
STUDY DESIGN AND SUBJECTS
The study was planned as a prospective, multi-center, parallel-group study to evaluate the efficacy and safety of Vildagliptin 100 mg SR tablet compared to Sitagliptin 100 mg tablet administered once daily in patients with Type 2 diabetes mellitus not optimally controlled on Metformin alone. A total of three sites were planned to enroll the patients.
The trial was conducted in three healthcare centres from Pune, Nashik and Hyderabad in India. The total duration of the study for each subject was up to maximum of 98 Days. The first Patient first visit was on 27 Jul 2023 and the last patient last visit was on 07 May 2024.
A total of five visits to the investigator site were scheduled; [screening period (visit 1: Day – 14 to -1), treatment period (visit 2: Day 1) (randomization/ baseline visit), (visit 3: Day 21±3) (visit 4: Day 63±3) and (visit 5: Day 84±3) (End of study)]. The total duration of the study for each subject was up to maximum of 100 Days including 14 day of screening period, 84 days of treatment period.
Eligible patients were randomly assigned to administer (orally) with either Vildagliptin 100 mg SR tablet (test) or Sitagliptin 100 mg tablet (reference) with or without Metformin once daily for 84±3 days.
The participants provided written informed consent before any study-related procedures. The study inclusion criteria include:
- Male or female patients of >18 years age diagnosed with type 2 diabetes mellitus using American Diabetes Association Criteria; Treated with metformin monotherapy at a stable dose (≥1,000 mg per day) for at least one month before randomization were selected to participate in this study.
- Eligibility was also based on previous medical and medication history, general, systemic and physical examination, vital signs, 12-lead electrocardiography (ECG), and clinical laboratory tests including serum pregnancy test, urinalysis (color, appearance, pH, specific gravity, white blood cells, red blood cells, epithelial cells, pus cells, casts, crystals, protein, glucose, bilirubin, urobilinogen, ketones, blood, nitrites, bacteria), hematology (hemoglobin, total and differential WBC, absolute neutrophil count (ANC), red blood cells (RBC) count, platelet count), liver function tests (total bilirubin, direct bilirubin, indirect bilirubin, blood urea nitrogen (BUN), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total protein, albumin and globulin), renal function tests (estimated glomerular filtration rate (eGFR), urea, creatinine and uric acid.), HbA1c test, fasting blood glucose test (FBG), postprandial blood glucose test (PPBG), lipid parameters (HDL-C, LDL-C, VLDL-C, total cholesterol and triglycerides), serology (HIV 1 & 2, HBs (Ag), HCV) tests were performed at baseline of the study.
- Patients who are willing to comply with Continuous Glucose Monitoring system (CGMS) were included in CGMS subgroup.
- Patients who are uncontrolled on metformin with HbA1c of >7%.
- Ability to understand and the willingness to sign and date a written informed consent document at the screening visit before any protocol specific procedures are performed.
- Patients with body mass index (BMI) 18.0 to 35.0 kg/m2.
Patients were excluded from the study if they meet one of the below exclusion criteria:
- Patients with Type-1 diabetes mellitus.
- Patients who were on more than two oral anti-diabetic drugs or on insulin.
- Uncontrolled hypertension (systolic blood pressure >160 mm Hg or diastolic blood pressure > 100 mm Hg).
- Symptomatic heart failure.
- Severe hepatic dysfunction (alanine aminotransferase or aspartate aminotransferase levels more than three times the normal upper limit).
- Patients with known hypersensitivity to Vildagliptin or Metformin HCl or Sitagliptin to any of the excipients.
- Patients with severe renal impairment.
- Patients with acute or chronic metabolic acidosis, including lactic acidosis or diabetic ketoacidosis, with or without coma.
- Subjects who are pregnant or are currently breastfeeding.
- Patient who is an employee of the Investigator or the Institution, or a patient who has direct involvement with the trial or other trials under the direction of the Investigator.
- Patient who has been treated with other investigational agent or devices within the previous 30 days, has planned use of drugs or devices, or has been previously randomized in this trial.
- Laboratory value abnormalities as defined by the protocol.
- Evidence of serious diabetic complications.
- Patients who are Hepatitis B or C or HIV positive.
- Patients who have undergone pancreatectomy or pancreas/islet cell transplant.
The study protocol and informed consent form were approved by the Royal Pune Independent Ethics Committee, FS Endocrine & Diabetic Center to the FS Independent Ethics committee, Muktai Hospital Institutional Ethics Committee. The study was registered with the clinical Trial Registry -India (CTRI/2023/06/053737). This study was conducted in compliance with Declaration of Helsinki (Ethical principles for medical research involving human patients, revised by the 64th WMA General Assembly, Brazil, October 2013), ICH [E6 (R3)] ‘Guidance on Good Clinical Practice’, “ICMR Ethical Guidelines for Biomedical Research on Human Subjects” 2017, New Drugs and Clinical Trials Rules 2019 G.S.R. 227(E) by CDSCO.
EFFICACY ANALYSIS
The efficacy analyses include changes in HbA1c, fasting blood glucose (FBG) levels, and postprandial blood glucose (PPBG) levels at baseline and at the end of the treatment on day 84±3. In addition, Continuous Glucose Monitoring system (CGMS) was performed in 25 patients in each treatment arm to assess average blood glucose levels.
SAFETY ANALYSIS
Safety and tolerability were evaluated throughout this study based on adverse events (AEs), physical examinations, vital signs, 12-lead ECG, and clinical laboratory tests.
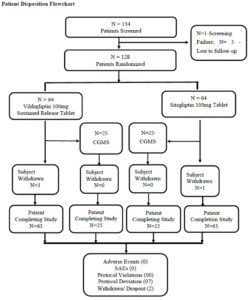
Sample Size and Statistical Analysis
The sample size was determined based on the published clinical trials of Vildagliptin for the treatment of Type 2 diabetes mellitus, by assuming standard deviation (SD) of change in HbA1c at baseline and week 12 as 0.45 with 80% power to establish non-inferiority between the groups using one sided test at 95% CI, it was estimated that 60 evaluable patients would need to be enrolled in each treatment group. However, 6 extra patients, accounting for 66 patients per group in total were enrolled in the study with an aim to compensate for a dropout rate of 10%. Thus, a total number of 132 patients were randomly assigned to either of the two treatment groups in the study in the ratio of 1:1 (i.e., 66 patients assigned to each of the 2 treatment arms).
Descriptive statistics were used to summarize the demographic, baseline characteristics, safety and efficacy data. Demographic data include age, gender, race, body weight (kg), height (cm) and body mass index (BMI) (kg/m2). Further baseline data was summarized using standard descriptive statistics and frequency tables, respectively: HbA1c values, fasting blood glucose values (FBG) and postprandial blood glucose values (PPBG), lipid parameters, hematology and Continuous Glucose Monitoring System (CGMS).
All statistical analyses were conducted using SAS®, Version 9.4 or higher. The Comparison among treatment groups at day 84 (or week 12) for primary and secondary efficacy variables were analyzed using analysis of covariance (ANCOVA) model. The ANCOVA model includes treatment, visit as fixed effect, and baseline score as co-variate. Change was estimated using the least-square means derived from the ANCOVA model. Comparison between each treatment groups was made using the difference in least-square means and p-values.
Results
STUDY POPULATION
In this study, 128 patients were enrolled, 126 of whom completed the study. Patients include both males (52.3%) and females (47.7%) with the age range between 28 to 75 years belonging to the race, Asians. Demographic characteristics including age, height, weight, and body mass index showed no significant differences between the Vildagliptin 100mg SR tablet and Sitagliptin 100mg tablet arms.
EFFICACY
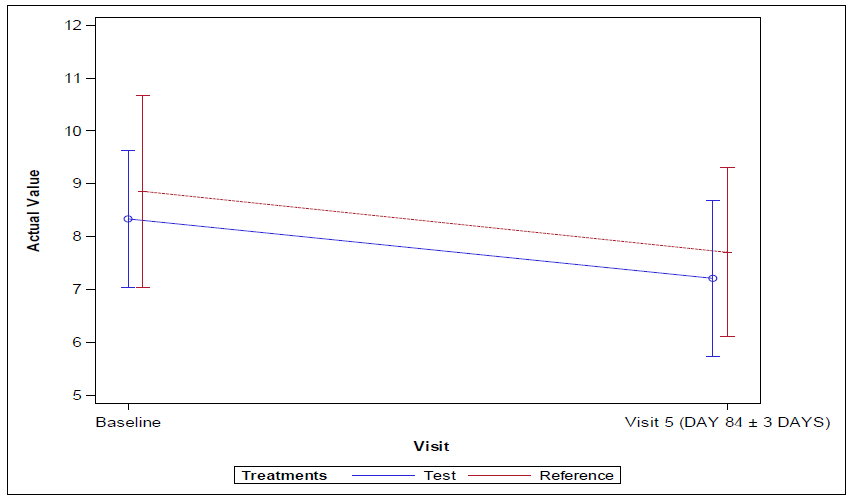
The 95% confidence interval lower limit for the mean difference for Vildagliptin 100mg SR tablet and Sitagliptin 100mg tablet groups for change in HbA1c levels from baseline visit 1 (Day -14 to -1) to end of the treatment at visit 5 (Day 84±3) for per protocol (PP) and full analysis set population was found to be -0.52 and the p-value was found to be 0.152 which was greater than 0.05 indicating no statistical significance between the arms.
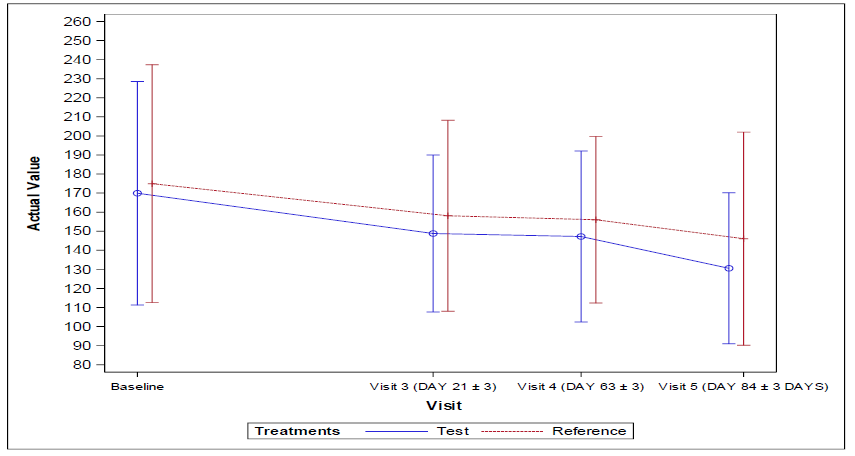
The 95% confidence interval lower limit for the mean difference of change in percentage reduction of fasting blood glucose (FBG) level and Postprandial fasting blood glucose (FBG) level and Postprandial blood glucose (PPBG) from baseline visit 1 (Day -14 to -1) to end of the treatment at visit 5 (Day 84±3) for per protocol (PP) and full analysis set population were found to be -8.06, -7.12 respectively and the corresponding p values were found to be 0.719 and 0.525 respectively indicating no statistical significance between the arms.
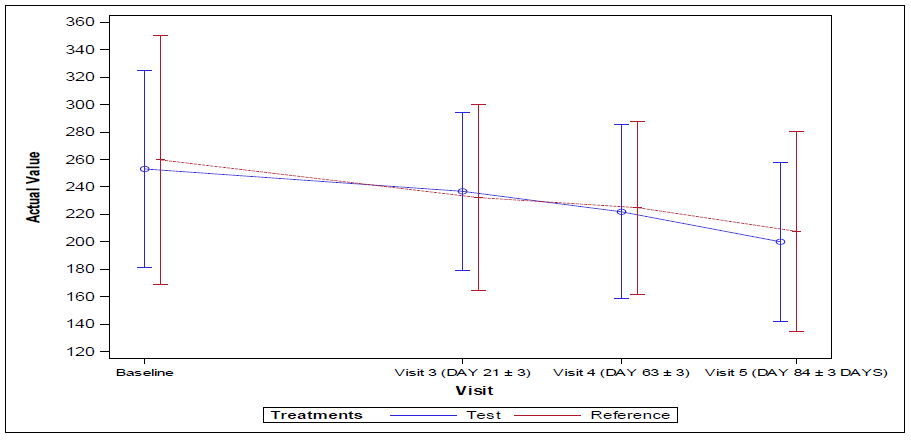
The 95% confidence interval lower limit for the mean change in average blood glucose levels by Continuous Glucose Monitoring system (CGMS) levels from baseline visit 1 (Day -14 to -1) to end of the treatment at visit 5 (Day 84±3) for both per protocol (PP) and full analysis set population was found to be -58.40 and the p-value 0.332 indicating no statistical significance between the arms.
SAFETY
During the study period no treatment related or treatment emergent adverse events were reported. No clinically significant changes from baseline in laboratory tests, ECG, vital signs, and physical examination results were observed. The treatments were safe and tolerable with no differences in the safety profiles among the two groups.
HYPOGLYCEMIA
In the present study, Vildagliptin 100 mg SR Tablet OD or Sitagliptin 100 mg Tablet in patients with Type 2 diabetes mellitus not optimally controlled on metformin alone no hypoglycemia episodes (day time and nocturnal hypoglycemia) that required assistance were reported during the study.
RENAL LABORATORY PARAMETERS
There was no difference in the mean serum creatinine levels between the test and reference groups at baseline (mean = 0.90 mg/dL). At end of the treatment (visit 5), the test treatment showed a slight decrease in creatinine levels of -0.02 mg/dL, while no change was observed in the reference treatment. Overall, the creatinine levels remained stable and within normal range.
The mean uric acid levels at baseline were 4.56 mg/dL for the test treatment and 4.62 mg/dL for the reference treatment. At end of the treatment (visit 5), there were no significant changes in uric acid levels (T=4.38; R=4.72), with changes of -0.18 for the test group and 0.10 for the reference group. These slight change in uric acid levels remains within normal ranges.
The mean urea levels at baseline were 25.13 mg/dL for the test treatment and 25.20 mg/dL for the reference treatment. At end of the treatment (visit 5), there were no significant changes in urea levels (T=22.86; R=23.89), with changes of -2.27 for the test group and -1.31 for the reference group. These slight change in urea levels remains within normal ranges.
Regarding the estimated glomerular filtration rate (eGFR), baseline values were 82.71 mL/min/1.73 m² for the test treatment and 87.04 mL/min/1.73 m² for the reference treatment. By end of the treatment (visit 5), there was a slight increase to 88.56 mL/min/1.73 m² for the test treatment, while the reference treatment experienced a slight decrease to 86.14 mL/min/1.73 m². The increase in eGFR for the test treatment indicates an improvement in kidney function over the study period.
HEPATIC LABORATORY PARAMETERS
Total bilirubin levels remain stable over the study period i.e. 0.68 mg/dL for test and 0.66 mg/dL reference groups.
Direct bilirubin levels at the baseline were found to be 0.27g/dL and 0.26g/dL for test and reference groups and at end of the treatment (visit 5) values obtained were found to be 0.26g/dL and 0.25 g/dL respectively. No significant change was observed for both the groups i.e., mean difference of -0.01 indicating direct bilirubin levels were stable and within normal limits.
Indirect bilirubin levels at baseline were found to be 0.41g/dL and 0.40g/dL for test and reference groups and at end of the treatment (visit 5) values obtained were found to be 0.42g/dL and 0.41 g/dL for test and reference groups respectively. Negligible increase of 0.01 for test and for reference indicates indirect bilirubin levels remaining stable.
Aspartate aminotransferase (AST) levels at the baseline were found to be 26.60 U/L and 26.73 U/L for test and reference groups and at end of the treatment (visit 5) values obtained were found to be 27.25 U/L and 25.41 U/L respectively. There was a slight increase for test mean of 0.65 and decrease for reference mean -1.33 indicating AST levels were relatively stable.
Alanine aminotransferase (ALT) levels at the baseline were found to be 26.76 U/L and 27.00 U/L for test and reference groups and at end of the treatment (visit 5) values obtained were found to be 27.28 U/L and 26.36 U/L respectively. There was a slight increase for test mean 0.52 and decrease for reference mean -0.64 indicating ALT levels remained stable, with a minor increase in the test group is clinically not significant.
Alkaline phosphatase (ALP) levels at the baseline were found to be 101.90 U/L and 111.79 U/L for test and reference groups and at end of the treatment (visit 5) values obtained were found to be 96.59 U/L and 95.49 U/L respectively. There was a slight decrease for test mean = -5.31 and for reference mean -16.30. ALP levels decreased, indicating stable liver function.
At baseline, blood urea nitrogen (BUN) values were 12.14 mg/dL for the test group and 11.73 mg/dL for the reference group. At end of the treatment (visit 5), there was a slight decrease in BUN levels, with values of 10.52 mg/dL for the test group and 10.84 mg/dL for the reference group. The changes of -1.62 mg/dL for the test group and -0.89 mg/dL for the reference group remained within normal limits.
Total protein levels were also within normal limits, measuring 7.15 g/dL for the test group and 7.23 g/dL for the reference group at baseline, and 7.18 g/dL and 7.24 g/dL, respectively, at end of the treatment (visit 5).
The albumin, baseline values were 4.04 g/dL for the test group and 4.07 g/dL for the reference group, while end of the treatment (visit 5) values were 4.10 g/dL and 4.20 g/dL, respectively. There was minimal change in albumin levels, with changes of 0.01 for the test group and 0.06 for the reference group, indicating albumin levels were stable.
Globulin levels showed no significant change, with baseline values of 3.13 g/dL for the test group and 3.15 g/dL for the reference group, and end of the treatment (visit 5) values of 3.13 g/dL and 3.12 g/dL, respectively. The changes were 0.00 for the test group and -0.03 for the reference group, indicating stability throughout the study.
Total bilirubin levels remained stable during the study period, at 0.68 mg/dL for the test group and 0.66 mg/dL for the reference group.
Baseline direct bilirubin levels were 0.27 g/dL for the test group and 0.26 g/dL for the reference group, at end of the treatment (visit 5) values of 0.26 g/dL and 0.25 g/dL, for test and reference groups respectively. There were no significant changes in mean = -0.01 for both groups, indicating that direct bilirubin levels were stable and within normal limits.
Indirect bilirubin levels at baseline were 0.41 g/dL for the test group and 0.40 g/dL for the reference group, while end of the treatment (visit 5) values were 0.42 g/dL for the test group and 0.41 g/dL for the reference group respectively. The slight increases of 0.01 for the test and for the reference group indicate minimal changes, remaining stable.
LIPID PROFILE
At baseline, total cholesterol levels were 176.17 mg/dL for the test group and 174.15 mg/dL for the reference group. At end of the treatment (visit 5), these values were 176.45 mg/dL for the test group and 170.33 mg/dL for the reference group. The mean change was negligible for the test group at 0.28 mg/dL, while the reference group experienced a decrease of 3.81 mg/dL. Both groups maintained values within normal range throughout the study.
Baseline triglyceride levels were 193.00 mg/dL for the test group and 203.18 mg/dL for the reference group. At end of the treatment (visit 5), the test group’s mean decreased to 169.67 mg/dL, and the reference groups mean was 178.05 mg/dL. The mean changes were -23.33 mg/dL for the test group and -25.13 mg/dL for the reference group, indicating a reduction in triglyceride levels for both groups.
At baseline, HDL-cholesterol levels were 40.47 mg/dL for the test group and 40.19 mg/dL for the reference group. By end of the treatment (visit 5), these values increased to 41.51 mg/dL for the test group and 40.67 mg/dL for the reference group. The mean change was 1.04 mg/dL for the test group and 0.48 mg/dL for the reference group, indicating a slight increase in HDL levels.
Baseline LDL-cholesterol levels were 97.05 mg/dL for the test group and 92.04 mg/dL for the reference group. At end of the treatment (visit 5), these values decreased to 96.22 mg/dL for the test group and 87.06 mg/dL for the reference group. The mean change was -2.27 mg/dL for the test group and -2.31 mg/dL for the reference group, indicating a slight decrease in LDL levels.
At baseline, VLDL-cholesterol levels were 39.39 mg/dL for the test group and 42.58 mg/dL for the reference group. At end of the treatment (visit 5), these values were 39.89 mg/dL for the test group and 42.44 mg/dL for the reference group. The mean changes were -1.16 mg/dL for the test group and -0.07 mg/dL for the reference group, suggesting relative stability in VLDL levels across both groups.
Overall, the lipid profile data indicate that while there were slight changes in total cholesterol, LDL, and VLDL levels, both groups exhibited stability in their HDL levels. Notably, triglyceride levels significantly decreased in both groups, which indicates improved lipid metabolism. The hematological data reflect no significant hematological abnormalities during the study period.
ECG
In this study during the screening period some of the participants had abnormal ECGs due to either abnormal left axis deviation, sinus bradycardia, left atrial enlargement, T-wave abnormalities, mild left axis deviation, anterior wall ST-T changes, LV strain pattern, Left anterior fascicular block, Left ventricular hypertrophy (LVH) with repolarization abnormality etc. However, all these abnormalities were clinically non-significant as per the principal investigator discretion. At the end of the study at end of the treatment (visit 5) a significant number of the participants demonstrated improvements in their ECG readings, transitioning from abnormal to normal results.
Discussion
This 12-week, randomized, open-label, parallel-group study assessed the efficacy and safety of two DPP-4 inhibitors- Vildagliptin 100mg SR and Sitagliptin 100mg once daily in Indian patients with type 2 diabetes mellitus (T2DM) who were uncontrolled on Metformin monotherapy. Conducted in a real-world setting, the study also examined clinical outcomes, and the determinants influencing glycemic control.
Vildagliptin 100mg SR provided a robust and clinically relevant reduction in HbA1c i.e., change in HbA1c from baseline visit 1 (Day -14 to -1) to visit 5 (EOS) (Day 84±3) of -1.35 and Sitagliptin offered a decrease of -1.13 in HbA1c in patients with Type 2 diabetes mellitus. About 45% of patients achieved HbA1c goals of <7% in the study in which Vildagliptin treated patients were of 58% and Sitagliptin treated patients were of 42%.
Patients treated with either Vildagliptin 100mg SR experienced significant improvements in glycemic parameters. These reductions translated into a notable increase in the proportion of patients achieving glycemic targets, underscoring the clinical effectiveness of Vildagliptin 100mg SR therapy. These findings are consistent with previous studies of Vildagliptin 50mg BID demonstrating the glycemic-lowering efficacy of DPP-4 inhibitors, both as monotherapy and in combination regimens. However, to the best of our knowledge, this is the first head-to-head comparison of Vildagliptin 100mg SR and Sitagliptin 100mg as add-on therapy in patients already on background metformin therapy, highlighting vildagliptin’s superior efficacy in this clinical scenario.
The study also evaluated the safety of these DPP-4 inhibitors when used in combination with metformin. The present study demonstrates that both Vildagliptin 100 mg SR and Sitagliptin 100 mg, when added to metformin in patients with type 2 diabetes mellitus (T2DM), exhibit a favorable safety profile across a broad range of clinical and laboratory parameters over the treatment period. Importantly, no episodes of daytime or nocturnal hypoglycemia requiring assistance were reported in either treatment arm, reinforcing the known glucose-dependent mechanism of DPP-4 inhibitors and their low intrinsic risk of hypoglycemia, even when used in combination with metformin.
From a renal safety perspective, there were no significant adverse changes in serum creatinine, urea, uric acid, or eGFR. In fact, the slight increase in eGFR observed in the Vildagliptin group suggests a potential renal protective trend, although the changes were not clinically significant. These findings affirm the renal tolerability of both agents in patients with preserved renal function. Hepatic safety parameters including total, direct, and indirect bilirubin levels as well as AST, ALT, and ALP remained stable across the study, with only minimal intra-group variations that were clinically insignificant. Likewise, total protein, albumin, and globulin levels remained within normal limits, confirming hepatic safety throughout the treatment duration.
The lipid profile results were also reassuring. Both groups demonstrated a reduction in triglyceride levels and minor improvements in HDL-cholesterol. LDL and total cholesterol remained largely stable or slightly reduced, indicating neutral to slightly beneficial effects on lipid metabolism. Hematological parameters revealed no abnormalities or trends of clinical concern, further supporting the hematological safety of both treatments. Notably, while some participants exhibited baseline ECG abnormalities, these were deemed non-significant by investigators. Interestingly, several patients showed improvement in ECG readings by the end of the study, suggesting possible indirect cardiovascular benefits or the resolution of transient abnormalities.
Overall, this comparative safety assessment confirms that both vildagliptin and sitagliptin are well-tolerated as once-daily agents in the management of T2DM. The observed safety trends, particularly in renal and hepatic markers, support their continued use in real-world settings, with Vildagliptin 100mg SR showing comparable, if not slightly favorable outcomes in certain parameters.
One limitation of our study is its relatively short 12-week duration. Longer study up to 24-week will be required to assess the durability effect of Vildagliptin. However, durable glycemic control with Vildagliptin has been demonstrated previously. Vildagliptin demonstrated robust glucose-lowering efficacy and good safety with low risk of hypoglycemia and weight gain. This makes Vildagliptin an attractive treatment option for patients with uncontrolled T2DM.
Conclusion
In conclusion, overall, the Vildagliptin 100mg SR tablet OD showed effective glycemic control while reducing the frequency of dosing. None of the patients were withdrawn from the treatment due to lack of effective glycemic control. This confirms that the indication of Vildagliptin 100 mg SR Tablet OD in patients with Type 2 diabetes mellitus who are not optimally controlled on metformin alone is quite rationale for better patient compliance towards effective glycemic control while reducing the frequency of dosing.
Based on the assessment of clinical laboratory evaluations, it was concluded that the Vildagliptin SR Tablet 100 mg of USV Private Limited, India was well tolerated and found to be safe in patients with Type 2 diabetes mellitus who were not optimally controlled on metformin alone.
Acknowledgments
The authors acknowledge Mr. Jenil Choksi, Mr Alireza Doctor and Ms. Monal Patil from USV Pvt Ltd for their assistance in carrying out the project. The authors also thank the staff of Jeevan Scientific Technology Limited, Telangana and USV Private Limited, Mumbai – 400 088, India for the conduction of the real-world study and medical writing support.
Conflicts of Interest
The authors report no conflicts of interest associated with this work.
Funding
The study was sponsored by USV Private Limited, Mumbai – 400 088, India.
References
- Ceriello A, Sportiello L, Rafaniello C, Rossi F. DPP-4 inhibitors: pharmacological differences and their clinical implications Expert Opin Drug Saf. 2014; 13(sup1):57- 68.
- Das S, Gupta A, Bandyopadhyaya B, et al. Data on vildagliptin and vildagliptin plus metformin combination in type-2 diabetes mellitus management Bioinforma- tion. 2021; 17(3):413.
- Mohan V, Zargar A, Chawla M, et al. Efficacy of a combination of metformin and vildagliptin in comparison to metformin alone in type 2 diabetes mellitus: a multi-centre, retrospective, real-world evidence study Diabetes Metab Syndr Obes Targets Therapy. 2021; 14:2925.
- Rizzo MR, Barbieri M, Marfella R, Paolisso G. Reduction of oxidative stress and inflammation by blunting daily acute glucose fluctuations in patients with type 2 diabetes Diab Care. 2012; 35(10):2076-2082.
- GR Sridhar, Kaushik Pandit, Sona Warrier, Ashish Birla. Sustained-Release Vildagliptin 100 mg in Type 2 Diabetes Mellitus: A Review. Cureus. 2023 May 18; 15(5):e39204.
- Timmins P, Desai D, Chen W, et al.: Advances in mechanistic understanding of release rate control mechanisms of extended-release hydrophilic matrix tablets. Ther Del. 2016, 7:553-72.
- Al-Hashimi N, Begg N, Alany RG, et al.: Oral modified release multiple-unit particulate systems: compressed pellets, microparticles and nanoparticles. Pharmaceutics. 2018, 10:176.
- Conley R, Gupta SK, Sathyan G: Clinical spectrum of the osmotic-controlled release oral delivery system (OROS), an advanced oral delivery form. Curr Med Resc Opin. 2006, 22:1879-92.
- Verma RK, Krishna DM, Garg S: Formulation aspects in the development of osmotically controlled oral drug delivery systems. J Cont Rel. 2002, 79:7-27.
- Sahoo CK, Sahoo NK, Rao SR, Sudhakar M, Satyanarayana K: A review on controlled porosity osmotic pump tablets and its evaluation. Bull Faculty Pharm. 2015, 53:195-205.
- Aroda VR, Henry RR, Han J, Huang W, DeYoung MB, Darsow T, Hoogwerf BJ. Efficacy of GLP-1 receptor agonists and DPP-4 inhibitors: meta-analysis and systematic review. Clin Ther. 2012;34(6):1247–58.
- Fakhoury WK, Lereun C, Wright D. A meta-analysis of placebo-controlled clinical trials assessing the efficacy and safety of incretin-based medications in patients with type 2 diabetes. Pharmacology. 2010;86(1):44–57.
- Tang YZ, Wang G, Jiang ZH, Yan TT, Chen YJ, Yang M, Meng LL, Zhu YJ, Li CG, Li Z, Yu P. Efficacy and safety of vildagliptin, sitagliptin, and linagliptin as add-on therapy in Chinese patients with T2DM inadequately controlled with dual combination of insulin and traditional oral hypoglycemic agent. Diabetology & Metabolic Syndrome. 2015 Dec;7:1-9.
- Matthews DR, Paldánius PM, Proot P, Chiang Y, Stumvoll M, Del Prato S. Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): a 5-year, multicentre, randomised, double-blind trial. The Lancet. 2019 Oct 26;394(10208):1519-29.

