Emerging Neuroengineering Technologies in Rehabilitation
Looking through the mirror of neuroengineering: Emerging technologies in neurorehabilitation
Isil Saadet Yenice Balevi1, MD, Yaacov Anziska1, MD
Abstract
Neurorehabilitation is an evolving area that seeks to improve life standards with functional restoration in neurological conditions such as stroke, traumatic brain injury (TBI), spinal cord injury (SCI), neurodegenerative, and neuromuscular diseases. In this field, neurorehabilitation devices, which essentially target stimulation of the neural pathways to promote recovery and increase patient engagement, have been getting primary attention. Emerging technologies in neurorehabilitation, particularly those that incorporate artificial intelligence (AI) and machine learning, provide a targeted and personalized approach that traditional rehabilitation methods struggle to achieve. Recent advancements in this field, such as but not limited to robotics (Robotic Assisted Therapy [RAT]) and exoskeletons, brain-computer interfaces (BCIs) via direct neural control or neurofeedback, virtual reality (VR), augmented reality (AR), non-invasive brain stimulation techniques (transcranial magnetic stimulation [TMS]), transcranial direct current stimulation [tDCS]), and remote monitoring through wearable sensors, are revolutionizing traditional methods. The integration of variable sensors for real-time monitoring enables dynamic therapy adjustments, further enhancing the potential of these technologies. However, despite the variety and high potential features, challenges remain in adopting emerging technologies in neurorehabilitation. These include the difficulty of access, training requirements for patients and providers, and the crucial need for establishing reliable protocols and regulations before these technologies can be integrated into daily practice. This review underscores the novelties and advancements in neurorehabilitation technologies, accentuating their potential to not just reshape but inspire the landscape and future of neurological recovery.
Keywords
neurorehabilitation, neuroengineering, brain–computer interfaces (BCI), exoskeleton
Introduction
Neurorehabilitation is essential to improve function and reduce the burden of care in patients with neurologic diseases. After the damage to the central nervous system, neural plasticity enables the brain to adapt in response to motor learning and to achieve this, it is recommended to perform a highly intensive, repetitive, and task-specific rehabilitation. With technological advances, these rapidly evolving and promising novel treatment approaches are becoming part of the therapeutic tools to improve recovery and functional gain via providing individual and task-specific treatment. Some therapeutic options include but are not limited to robot-assisted therapy (involving the use of robotic exoskeletons to supplement patients’ movement) and neural interfaces, which include brain-computer interfaces (BCIs) and myoelectric interfaces, allow the control of neuroprosthesis or functional electrical stimulation (FES) and enable patients with little or no residual limb functions to mobilize the paralyzed limb through imagined or attempted movements. This review aims to highlight the contemporary conditions in novel technologies in neurorehabilitation to increase awareness in medical practice by mentioning some examples.
1. NEURAL INTERFACES
Neural interfaces, which include brain-computer interfaces (BCIs) and myoelectric interfaces, allow the control of neuroprosthesis or functional electrical stimulation (FES) and enable patients with little or no residual limb functions to mobilize the paralyzed limb through imagined or attempted movements. Importantly, neural interfaces also allow closed-loop, functionally contingent proprioceptive feedback. Neural interfaces can be linked to various end effectors to form a closed system. These include virtual reality−based systems, visual feedback systems (e.g., through an avatar limb shown on a screen), or other nonanthropomorphic signals (e.g., moving a screen cursor sideways). These systems augment stroke recovery by facilitating use-dependent neuroplastic changes.
a. Brain-computer interfaces (BCIs)-assisted rehabilitation systems
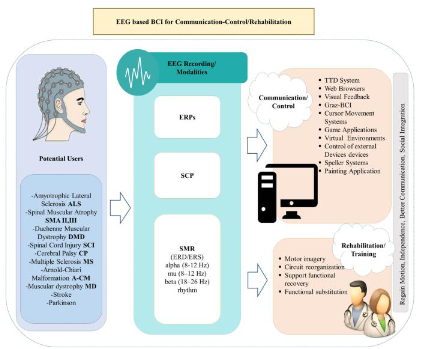
Brain-computer interfaces (BCIs) are a technology transforming signals from the brain to actions through bypassing the peripheral nerves or muscles and has been actively explored and utilized in neurorehabilitation with several different operational ways and for various indications which the examples will be discussed in this section of the review (Figure 1). Brain-computer interface (BCI) systems comprise three components: signal acquisition, signal processing, and application, which are interconnected, allowing brain signals to flow to the target BCI application such as a robotic arm system and, in some settings, control signals from the target can be sent back to the brain to stimulate sensory inputs such as vision and hearing (Figure 2). Signal acquisition involves capturing electrophysiological signals representing specific brain activities (e.g., movement, speech, hearing, and vision). Current BCI systems obtain signals through invasive recordings such as implantable electrodes or grid recordings from the cortex, local field potentials (LFPs), multi-unit activity (MUA) or single-unit activity (SUA), as well as non-invasive methods e.g. EEG, MEG (magnetoencephalography), NIRS (near infrared spectroscopy), functional MRI (fMRI) and among these techniques EEG recordings have been more thoroughly studied and used due to their practical implementations, non-invasiveness, and portability and adaptability. In addition to EEG, EMG feedback-based BCIs were shown to be used in patients with more active movements as opposed to stroke survivors with severe motor impairments.
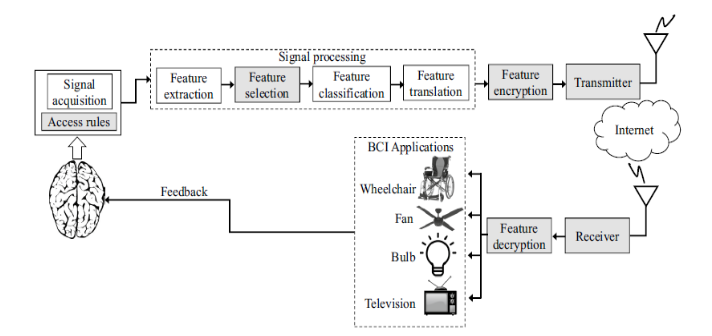
In the signal processing step, which consists of feature extraction, feature classification, and feature translation, the BCI extracts critical electrophysiological features from the acquired signals to define brain activities, encoding the user’s intent. The extracted features, which are brain activities intended for desired actions, go through the classification process, which helps to recognize patterns of the features corresponding to these actions. This stage is followed by feature translation described as classified features are translated and transformed into actual commands to operate an external device i.e. BCI application such as commanding cursor movements, writing a text or volume control of an audio device. Additionally, the algorithm for this stage can be adaptable, i.e., tracking the changes of the features and generating appropriate output. In the last component of the BCI system, commands to control external devices (BCI application), such as a wheelchair or a robotic arm, are generated through feature translation.
Brain-computer interfaces (BCIs) have been used to help restore motor function in stroke, amyotrophic lateral sclerosis (ALS) and spinal cord injury (SCI) by translating brain signals into movement, either by controlling external devices like robotic arms, exoskeletons, or by stimulating muscles using functional electrical stimulation (FES). FES has been used as a hybrid system via combination with the BCI system and operates by stimulating the specific muscle to contract by FES. At the same time, BCI interprets the user’s brain activity to trigger these stimulations.
Brain-computer interfaces (BCIs) are also being used to promote motor recovery and neuroplasticity in stroke patients in conjunction with physical therapy, as shown by a recent systematic review through a combination of traditional physical therapy and robotic assistive orthotic devices.
Neuro, auditory, and visual feedback systems also aim to improve motor and cognitive function (Figure 2). They use BCIs to monitor brain activity and provide real-time feedback, allowing the patient to regulate specific brainwave patterns consciously. For example, they show the patient their brain activity on a screen, and through training, they learn to modulate these signals.
In addition, brain-computer interface systems (BCIs) can be used for sensory recovery in post-stroke patients. These systems rely on repeated neuromuscular electrical stimulation and aim for targeted closed-loop sensory cortex plasticity modulation.
Brain-computer interfaces (BCIs) can also be used for speech rehabilitation for aphasic patients and other speech impairments, as shown by a recent study. A speech synthesizer that produces intelligible speech in real-time as a synthesizer converts movements of the main speech articulators (tongue, jaw, velum, and lips) into intelligible speech, which is opening the future of BCI speech systems using such articulatory-based speech synthesizer.
BCI systems are an emerging field, given the recent advancements in virtual reality, robotics, and sensors. They work with a mechanism that allows the control of robotic devices by translating the brain’s neural and/or physiological activity into a signal, completely bypassing lesion.
Activity-based therapies, which will be later mentioned in this article, require some residual movement of the affected limb, unlike brain-computer interface (BCI) assisted systems. Valuable for individuals with severe motor impairments, particularly for stroke recovery, through computer interfaces, controlling robotic limbs, and even the possibility of speaking through thought-based commands. Brain-computer interfaces (BCIs) aim to enhance neuroplasticity with motor imagery and sensory feedback by closing the gap in the motor intention, execution, and feedback loop. Thus, BCI-based neurorehabilitation engaged users better than alternative therapy. The rehabilitative (i.e., neurofeedback) BCIs aim to recognize patients’ intention of movement/task with brain signals captured with scalp-recorded electroencephalogram (EEG), then provide user perceivable feedback using a feedback mechanism (combination of several examples such as visual displays, robotic devices, exoskeletons).
Most studies assessed rehabilitative brain-computer interfaces (BCIs) for upper extremity motor recovery in stroke. BCIs improved the functions of proximal joints, which could have also led to greater functional use of the hand and wrist. Vice versa was also true because several studies also reported that training of the wrist and fingers also led to improvement in more proximal joints. In this group of patients with residual limb functions (which have plateaued with other rehabilitation therapies), BCI training could bring about more subtle but clinically meaningful benefits in other aspects of upper-limb functions beyond just improvements in motor impairment per se. Regarding stroke chronicity, the time since stroke appeared to have no particular effect on treatment outcome. There is limited evidence for using rehabilitative BCIs for lower extremity recovery due to difficulties in decoding algorithms of lower extremity movement kinematics using non-invasive recordings. However, literature is promising for BCIs’ use for cognitive and speech rehabilitation in other brain disorders like cognitive impairments, attention deficit disorders, and traumatic brain injury.
In terms of neuroplasticity in brain-computer interface (BCI)-driven neurorehabilitation, a closed-loop system has been postulated to facilitate cortical reorganization, and this phenomenon appears to involve a system of parallel networks in both hemispheres. Hebbian plasticity, a process in which synaptic strength is strengthened when presynaptic (i.e., cortical) and postsynaptic neurons (i.e., spinal cord) fire in synchrony, may underlie this process. Motor recovery is correlated with the activation and improved functional connectivity of the ipsilesional somatosensory cortex, dorsal premotor, and supplemental motor cortices, as well as inhibition of the competing contralesional hemisphere. There is also evidence of involvement of large-scale functional networks like the dorsal attention network. Often, though, the cortical network still retains some residual function after stroke and is not entirely injured, as seen in diffusion tensor imaging studies, and can relay sufficient information to control the neural interfaces.
In brain-computer interface (BCI) technology, despite its broad applications, limitations, and possible threats, including convenience, ease-of-use, privacy, security, and safety to humans (especially for the invasive BCI systems), need to be addressed. Several studies show that these applications may extract sensitive information from users without their knowledge. Thus, standards should be well-defined regarding acquisition methods, access control protocols, and encryption techniques. Given the increasing demand for BCI-internet communications, this system creates opportunities for cyber attackers to intervene in the everyday operations of the BCI application by altering commands, thus causing adverse effects to the target subject. Most BCI applications require calibration data to reverse undesirable changes caused by neural plasticity or micro-movements of the electrode arrays, which require frequent decoder retraining, which is an inconvenient and time-consuming process. Also, the affordability of BCIs by the public because of their prohibitively high costs is still an issue, followed by the difficulty of portability due to the complex and bulkier construction of current systems.
2. ROBOTICS
a. Robot-assisted therapy (RAT)
Robot-assisted therapy (RAT) assists patients in performing repetitive movements and is particularly effective when combined with brain-computer interfaces (BCIs). Neural plasticity and the brain’s ability to adapt require extensive time and personnel investments. Patient attention and motivation are also crucial to achieving high training dosages, and robot-assisted therapeutic devices and gaming approaches address those challenges. Several commercially available robotic devices for the upper limb are currently Armeo Power (Hocoma AG, CH) or AMADEO (Tyromotion GmbH, AT), which supplement neurorehabilitation. Robot-assisted therapies (RAT) provide patients with robot devices for upper or lower limb therapy. These devices aid in achieving standardized high-intensity therapy, which is crucial for motor cortex reorganization and is also thought to promote recovery. These combined therapies aim to increase the motor control capacity and strength of the paretic arm or leg and thus promote basic activities of daily living.
b. Exoskeleton
Conventional rehabilitation hospital treatments suffer from issues of limited accessibility, high cost, and complex equipment operation, leading to suboptimal rehabilitation outcomes for patients. Thus, the need for wearable devices that are portable and user-friendly, enabling patients to engage in self-directed training, has been emerging. Exoskeletal robots, rooted in the principles of robotics, are robotic systems designed to be worn by individuals, allowing them to accomplish specific tasks through user-controlled movements. They are mainly designed for individuals with lower limb disabilities. This system enables patients to maintain specific positions and empowers them to perform movements in their joints during daily activities, thereby mitigating functional impairments resulting from joint stiffness and muscle contracture. Also, it facilitates functional training, enhances patients’ self-care capabilities, and improves their quality of life. Currently, this field is rapidly evolving in the rehabilitation of spinal cord injuries. However, despite all the benefits, challenges continue to exist in the practical implementation of these systems, such as sensory and motor impairment, as shown in patients with spinal cord injury (SCI). Some studies have shown the utility and effectiveness of a particular exoskeleton robot. However, research on developing lower extremity medical exoskeletons, such as robotic gait trainers, that can be used for SCI is quite limited. Multi-joint assisted lower limb training robots are categorized into first, second, and third generations. Examples for each generation can be described as follows:
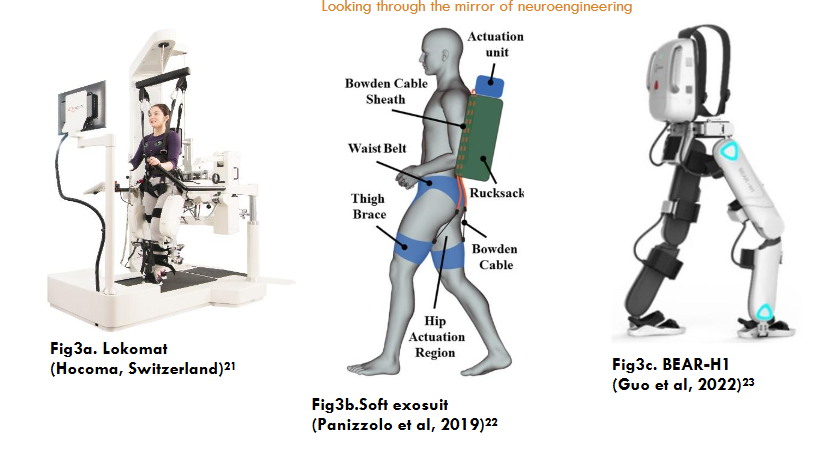
The first generation consists of weight-reducing exoskeleton robots that utilize suspended belts and sports running tables, such as Lokomat. It is designed for in situ gait rehabilitation on a treadmill. It influences the patient’s gait and modulates walking patterns. The second generation comprises gait-assisted exoskeleton robots capable of walking on flat ground, such as ReWalk, Ekso, HAL, and Soft Exosuit. The third generation features intelligent powered exoskeleton robots integrating technologies such as artificial intelligence (AI), augmented reality (AR), virtual reality (VR), and the Internet of Things (IoT). These robots provide assisted walking along with additional capabilities, such as FourierX2, UGO, BEAR-H1, and others. AI utilizes intelligent learning to personalize gait and training styles for individuals, collect and analyze data to monitor rehabilitation progress and enhance human-computer interaction and gait training.
When exoskeletons are employed for assisted rehabilitation, patients can access a range of motor feedback information, including leg and plantar loads and joint movements, focusing on the hip, which promotes neurological recovery and mitigates complications through various biological pathways. Exercise-facilitated neural repair after spinal cord injury (SCI) relies on neuroplasticity, encompassing both spontaneous plasticity directly induced by the injury and plasticity dependent on training tasks. Moreover, motor feedback impacts neural activity within the brain. Following body weight-supported exercise training, there is enhanced activation in somatosensory cortical areas (S1, S2) and cerebellar regions. In addition to spinal cord injury (SCI), lower extremity exoskeleton robots hold the potential for the rehabilitation of various other diseases, such as stroke, multiple sclerosis, traumatic brain injury, Alzheimer’s disease, Parkinson’s disease, cerebral palsy, and other neurological disorders resulting in minor lower extremity paralysis, e.g., Ekso GT can be suitable for stroke patients. At the same time, Ekso NR is designed for patients with acquired brain injury.
Robotics in neurorehabilitation showed promising results. However, they come with several limitations, such as high cost, limited availability, weight, and limited personalization, as many robotic systems have pre-programmed movements that may not have full adaptation abilities, complexity of setup, and reduced engagement due to decreased active participation as the patients may become passive during robotic therapy.
3. COGNITIVE BASED THERAPIES
Examples are virtual reality (VR) and augmented reality (AR), which provide an interactive and immersive experience. Patients can participate in therapeutic exercises and improve motor and coordination skills as well as cognitive functions.
a. Virtual Reality (VR)
Virtual reality (VR) therapies use an interactive simulation where users can engage in an environment like the real-world using controllers. While therapy is tailored to individuals’ goals and capabilities, it is often constrained by the physical world’s limitations. This system offers interactive and engaging exercises that can be individualized to meet patients’ needs, promoting neuroplasticity and motor learning, also allowing patients to visualize muscle activity and movement patterns in real-time through virtual reality-based biofeedback facilitating better control and coordination of affected muscles. VR is an emerging source in rehabilitating progressive neuromuscular disorders due to its ability to promote independence and improve training outcomes. Through VR, a higher dose of rehabilitation can be achieved by enabling patients to practice functional tasks in simulated environments, which yielded positive results in recent studies that can be attributed to higher doses of therapy, thus suggesting a dose-effect relationship. Virtual reality-based rehabilitation has been studied in various neurological and neuromuscular disorders, including stroke, cerebral palsy, spinal cord injury, amyotrophic lateral sclerosis, and Duchenne and Becker muscular dystrophies (DBMD). In amyotrophic lateral sclerosis, it was studied across various domains, including communication, cognition, eye movements, neurological evaluation, and neurorehabilitation, which was found safe and feasible, yielding improvements. It was also promising in diabetic peripheral neuropathies and chemotherapy-induced peripheral neuropathies to aid in rehabilitation for balance, postural stability, and gait. VR can also be used to assess and diagnose neuromuscular conditions by capturing detailed movement data and enabling the remote monitoring of patients.
There are three main virtual reality systems available: non-immersive, semi-immersive, and fully immersive simulations.
i. Non-immersive virtual reality systems
A two-dimensional computer-generated environment is created through consoles and computer displays, where patients can control an avatar using a control tool, similar to common video games.
ii. Semi-immersive virtual reality systems
These systems provide a three-dimensional environment with a fixed visual perspective.
iii. Fully immersive virtual reality systems
Patients can experience the most realistic simulation environment in fully immersive systems through a head-mounted display and extensive motion sensors. This multimodal sensory information promotes body movement rehabilitation. A recent meta-analysis comparing immersive and non-immersive virtual reality for upper extremity motor recovery showed superior effects with immersive virtual reality systems, and the superiority can be attributed to higher user perception, sensory feedback, and facilitation of patients’ engagement in task-oriented activities. Despite variations in the virtual reality technologies and outcome measures used across studies, there was a collective demonstration of improvements in function, quality of life, and motivation through virtual reality. When combined with augmented reality, exoskeletons, and other environmental interfaces, virtual reality holds the eventual potential to enhance the capacity of patients to control their external environments in ways beyond the limitations of their physical bodies.
b. Augmented Reality (AR)
Augmented reality (AR) merges computer-generated models with actual scenes, providing an interactive and visually engaging training experience and freeing patients’ hands to a certain extent. Head-mounted displays as the gaming environment represent a virtual, realistic third dimension; however, using fully immersive virtual reality glasses may induce motion sickness, as there is a complete loss of reference to the real world. Augmented reality (AR) head-mounted displays, such as HoloLens (Microsoft, US), seem to overcome this issue. HoloLens with a head-mounted AR display has only been used with neurological patients in a few exploratory trials, e.g., to improve the pantomime performance of patients with apraxia and to support ADL tasks in patients with dementia, patient education before surgery of epilepsy patients, gait training in patients after stroke or with Parkinson’s Disease or during the evaluation of vision in patients after stroke. However, due to the concern of patients with cognitive, sensory, and visual impairments (which might occur due to stroke) facing difficulties in perceiving the three-dimensionality of an AR environment, the combination of AR with a robotic device to administer a serious gaming therapy was investigated by de Crignis et al. (2023).

4. NON-INVASIVE BRAIN STIMULATION AND NEUROPLASTICITY
These techniques are named neuromodulation through electrical or magnetic stimulation, as the aim of brain stimulation techniques is to modulate neural networks specifically and selectively through enhancing adaptive patterns and suppressing maladaptation, which will be explained further in this section. Currently, available non-invasive techniques are transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS), which can be used for following indications of stroke, traumatic brain injury (TBI), major depression, obsessive-compulsive disorders, and migraines which the latter three have FDA approval for repetitive transcranial magnetic stimulation (rTMS) modality. These two main modalities will be discussed further in this section.
In stroke recovery, one of the most studied indications, reorganization of the lesional area into the non-lesional cortex, is shown by the functional MRI (fMRI) studies, which is associated with increased bilateral activation in the acute phase followed by lateralization to the non-lesional hemisphere in chronic state and eventually leading to maladaptive interhemispheric imbalance interfering with recovery. In the post-stroke period, high-frequency EEG waves (≥ 8 Hz, (alpha and beta)) and intra-hemispheric connectivity reduce, resulting in an imbalance between lesioned and non-lesioned hemispheres. Thus, recovery mechanisms focus on enhancing interhemispheric balance restoration and increasing intrahemispheric connection through neuromodulation techniques. The hypothetical model of brain stimulation modulated the imbalanced interhemispheric inhibition via activating the lesioned hemisphere while inhibiting the non-lesioned hemisphere to enhance adaptive plasticity with other treatment modalities applied simultaneously. Of the several types of non-invasive neuromodulation techniques, the most studied ones, transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS), target either the dorsolateral prefrontal cortex or the motor cortex (M1).
Neuromodulation techniques are also studied in the treatment of disorders of consciousness, commonly caused by traumatic brain injury (TBI), cardiac arrest, and stroke with intracerebral hemorrhage. Current practice suggests amantadine is the treatment option. However, TMS, tDCS, tACS (transcranial alternating current stimulation), and tRNS (transcranial random noise stimulation) could be possible options for restoring consciousness through neural restoration within cortical-thalamo-cortical networks, although studies yielded limited results.
The primary targets of non-invasive brain stimulation (NIBS) continue to focus on supratentorial regions, such as the primary motor cortex (M1), for enhancing motor function and the Broca area for speech improvement. Over the past thirty years, the cerebellum has been confirmed to regulate motor and non-motor function. Cortico-cerebellar circuits are associated with motor learning and feedforward control, as well as cognitive regulation, based on providing unique plasticity mechanisms and have vast connections to cortical areas; it has been served as a target of NIBS, which was supported by the recent review by Qi et al. (2024) observed positive effects on motor functions like gait and balance and some efficiency in dysphagia rehabilitation in stroke patients.
a. Transcranial Magnetic Stimulation (TMS)
Transcranial magnetic stimulation (TMS) is a non-invasive brain stimulation technique that applies magnetic fields to the cerebral cortex in single or pulsed forms. TMS can be used for both diagnosis and treatment in stroke patients. Neuromodulation of cortical excitability is through the scalp using a coil based on the principle of electromagnetic induction. An electromagnetic field induces a focal electric current in the brain, which can cause transient depolarization in neurons. In terms of diagnosis, TMS can be given as a single or paired-pulse to assess brain functioning. In contrast, repetitive application, known as repetitive TMS (rTMS), can be used for treatment as cortical excitability can be modulated through this approach. Also, when used with proper protocol, rTMS is considered safe. The excitability through neuromodulation depends on the frequency of stimulation, e.g., high frequency (≥5 Hz) rTMS induces excitatory effects while low frequency (≤1 Hz) rTMS induces inhibitory effects. The effects of high and low-frequency modalities have been mainly studied for upper extremity impairment in stroke, with limited data for lower extremity functional recovery. This data suggests that low-frequency rTMS use over the contralesional hemisphere (non-lesioned) and high-frequency rTMS over the ipsilesional (lesioned) hemisphere in the subacute phase is potentially effective. In contrast, limited evidence of the benefit of use in the chronic phase has been reported. However, current data still provides conflicting results given the heterogeneity of strokes involving motor deficits and the contribution of structural reserve after the stroke to recovery models.
Also, based on the interhemispheric inhibition hypothesis, studies investigated aphasia recovery using repetitive transcranial magnetic stimulation (rTMS). In chronic aphasia patients, a perilesional activation was shown; in addition, right sided contralesional activation during the incorrect naming responses was shown, which was thought to be maladaptation rather than compensation, and that led the most rTMS studies to be focused on delivering low-frequency inhibitory stimuli over the right hemisphere in combination with speech therapy, showing promising results especially on chronic stage Broca aphasia with inhibitory stimuli applied to right inferior frontal gyrus. Other indications which provided promising are hemispatial neglect treatment using low frequency repetitive transcranial magnetic stimulation (rTMS) and continuous theta burst stimulation (cTBS) over the contralesional-hemisphere which both have excitability reducing features, however the non-controlled design of these studies should be kept in mind. A better improvement at visuospatial neglect was shown at high-frequency rTMS to the ipsilesional hemisphere in a study with a design of low-frequency rTMS of the contralesional hemisphere, high-frequency rTMS of the ipsilesional hemisphere, and sham stimulation. Improvement in this field is also shown with another modality, including cTBS over the left posterior parietal cortex. However, response to this modality is shown to be associated with the integrity of interhemispheric connections within the corpus callosum, thus requiring an intact corpus callosum.
b. Transcranial Direct Current Stimulation (tDCS)
Transcranial direct current stimulation (tDCS) is essentially the application of steady, low-amplitude, direct current via electrodes placed over the scalp penetrating to the underlying brain tissue, providing a sub-threshold stimulus that modulates neuronal transmembrane potentials and influences the level of polarization and the likelihood of neuronal firing. There are two modalities available: anodal stimulation increases excitability, and cathodal stimulation decreases the excitability of the cortex, and if applied for sufficient duration and intensity, prolonged clinical effects through long-term potentiation and depression can be obtained. Unlike transcranial magnetic stimulation (TMS), tDCS stimulates a broader area of the cortex with less targeted specificity, which was suggested to be beneficial by stimulating additional regions. Also, this modality provides a better safety profile. However, typical side effects of mild, temporary headache, skin irritation, redness and tingling, and itching sensation during ramp up and down were reported. The parameters considered in tDCS are electrode location (montage and stimulation target), electrode size (current density), current intensity, frequency, and duration. Similar to TMS, motor recovery through interhemispheric inhibition modulation by increasing the lesioned hemisphere’s excitability or decreasing the non-lesioned hemisphere’s excitability is studied chiefly. Upper extremity motor function improvement lasting several weeks and associated with increased cortical excitability in the lesioned hemisphere was shown in several studies; however, given the significant variety in stimulation parameters and study designs, the studies yielded mixed results. The current literature suggests a possible role of tDCS as an adjuvant therapy for poststroke upper extremity motor recovery, mainly when applied in chronic stroke patients.
Like transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS) use as an adjunct was also investigated in aphasia recovery and showed feasibility and possible clinical improvement in speech production (naming) in chronic stroke patients with anodal tDCS over language cortex during outpatient speech therapy guided by functional MRI (fMRI) which determined the stimulation target. Another study with a similar stimulation way with fMRI guidance was conducted with subacute stroke patients; however, it yielded no significant difference in naming function, which was attributed to the high variability of improvement in the subacute stroke patient population in the primary analysis in secondary analysis, significant improvement was found in tDCS group after five weeks therapy. Despite the side effects stated earlier, no detrimental effects of stimulating over more extended periods, even up to 20 weeks, were shown.
In addition to transcranial direct current stimulation (tDCS), two other electric stimulation methods are studied more in different indications, such as behavioral and movement disorders: transcranial alternating current stimulation (tACS) and transcranial random noise stimulation (tRNS) based on delivering weak alternating currents, with transcranial alternating current stimulation (tACS) sinusoidal current (0.1-80Hz) is delivered. In contrast, transcranial random noise stimulation (tRNS) delivers 0.1-640 Hz frequency currents in a random order, modulating neuronal activity by influencing brain oscillations. Also, there are studies yielding possible effects in the post-stroke chronic phase with these modalities. Currently, clinical evidence is limited for use in clinical practice.
5. WEARABLE SENSORS AND MONITORING
Monitoring through wearable sensors offers precise, real-time data to enhance treatment outcomes and provide more personalized therapy. Several types are available in neurorehabilitation settings and are more commonly used for stroke, spinal cord injury (SCI), traumatic brain injury (TBI), Parkinson’s disease (PD), and multiple sclerosis (MS). Examples of application sites are gait and balance training in stroke and SCI patients, upper limb rehabilitation in stroke and TBI patients, cognitive and motor assessment, and postural control and stability. Types of wearable sensors are accelerometers, inertial measurement units (IMUs) (generally placed on the wrist uni or bilaterally, forearms, upper arm, and on chest as a reference to compensate for trunk movements, for UE and mainly include 3-axis accelerometer, 3-axis gyroscope and 3-axis magnetometer and can reconstruct orientation in 3D world), ego-centric camera (placed on forehead), EMG sensors (surface EMG), force sensors and EEG sensors. A review regarding monitoring individuals with neurological impairments (mostly stroke then, MS, SCI, PD) using their upper extremities during their everyday activities investigated the most common types of wearable sensors used to monitor upper extremities during daily activities and reported sensor-measured upper extremities performance improved in the first 12 weeks after stroke based on the data from unsupervised conditions showing spontaneous capacity to improve post stroke. Another study showed that upper extremities performance reaches a plateau 3-6 weeks after stroke, and the availability of data in the early phases of the post-stroke period highlights the importance of monitoring as it can address a critical phase in recovery.
After discharge, rehabilitation progression would be challenging to keep track of; however, wearable sensors allow the patients to perform exercises at home while being monitored by a therapist and ensure the gains continue to progress effectively in real-world settings. Sensors and monitoring are ideal fields to implement AI and machine learning to interpret vast amounts of data regarding extremity location and contact state information and summarize into simple measures for tracking extremity function progress, subtle improvements or regressions, and to create personalized treatment plans based on an individual’s specific needs, adjusting therapy automatically based on the patient’s evolving condition. However, the accuracy and reliability of data from the sensors in diverse environments would be a challenge, which requires regular calibration, power consumption, data storage, and user comfort to facilitate continuous monitoring, and providing affordable tools is still an issue yet to be resolved. Power efficiency, data compression techniques, and cloud-based storage can ease some difficulties. Another challenge is the concern for privacy, especially in the setting of video monitoring, which needs to be addressed, and data should be encrypted with user-controlled data access.
Conclusion
This review discussed the wide range of novel neurorehabilitation technologies presenting a transformative approach to managing and treating neurological diseases. Various contemporary approaches, including BCIs, robotics, VR/AR systems, non-invasive brain stimulation techniques, and wearable sensors, have demonstrated the potential to enhance traditional rehabilitation methods’ efficacy, engagement, and outcomes. The adaptability and individualized nature of these technologies provide a more targeted therapy that caters to each patient’s unique needs, thereby facilitating improved functional recovery and quality of life. Despite promising results, challenges such as accessibility, training requirements, high costs, technical complexities, and the need for robust clinical protocols and regulatory frameworks are yet to be addressed to ensure widespread adoption and integration into routine clinical practice. However, studies underscore the potential of these technologies to reshape the future landscape of neurorehabilitation, offering hope for more effective treatment strategies in neurologic diseases. As technology continues to evolve, a multidisciplinary and collaborative effort will be crucial to harness these innovations’ full capabilities and establish them as essential components in the neurorehabilitation continuum.
Conflicts of Interest Statement
There is no conflict of interest regarding the publication of this review.
Funding Statement
No external funding is received.
Acknowledgments
1) The image used to describe Figure 1. Example of BCI system and indications is obtained from the article cited as “Lazarou I, Nikolopoulos S, Petrantonakis PC, Kompatsiaris I, Tsolaki M. EEG-based brain–computer interfaces for communication and rehabilitation of people with motor impairment: a novel approach of the 21st century. Front Hum Neurosci. 2018;12:14”, published by Frontiers Media SA and distributed under Creative Commons Attribution License (CC BY) (https://creativecommons.org/licenses/by/4.0/) and no change was made on the image.
2) The image used to describe Figure 2. Example of neurofeedback in BCI system is obtained from Maiseli B, Abdalla AT, Massawe LV, et al. Brain–computer interface: trend, challenges, and threats. Brain Inform. 2023;10(1):20, published by Springer Nature and distributed under Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/) and no change was made on the image.
3) The images used to describe Figure 3(a,b,c). Multi joint assisted lower limb training robots are obtained from following references: a. Figure 3a. Lokomat Picture: Hocoma Switzerland: https://hocoma.b-cdn.net/wp-content/uploads/2020/07/6_LokomatNanos.jpg; and no change was made on the image. b. The image used to describe Figure 3b. Soft Exosuit is obtained from Panizzolo FA, Freisinger GM, Karavas N, et al. Metabolic cost adaptations during training with a soft exosuit assisting the hip joint. Sci Rep. 2019;9(1):9779 published by Springer Nature and distributed under Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/) and no change was made on the image. c. The image used to describe the Figure 3c. BEAR H1 is obtained from Guo Z, Ye J, Zhang S, et al. Effects of individualized gait rehabilitation robotics for gait training on hemiplegic patients: Before-after study in the same person. Front Neurorobot. 2022;15:817446. doi:10.3389/fnbot.2021.817446 published by Frontiers Media SA and distributed under Creative Commons Attribution License (CC BY) (https://creativecommons.org/licenses/by/4.0/) and no change was made on the image.
4) The image used to describe Figure 4. Example of Augmented Reality (AR) is obtained from Crignis ACd, Ruhnau S-T, Hösl M, et al. Robotic arm training in neurorehabilitation enhanced by augmented reality: a usability and feasibility study. J NeuroEngineering Rehabil. 2023;20(1):105 published by Springer Nature, and distributed under Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/) and no change was made on the image.
References
- Lo YT, Lim MJR, Kok CY, et al. Neural interface-based motor neuroprosthesis in post-stroke upper limb neurorehabilitation: an individual patient data meta-analysis. Arch Phys Med Rehabil. 2024;4.
- Crignis ACd, Ruhnau S-T, Hösl M, et al. Robotic arm training in neurorehabilitation enhanced by augmented reality: a usability and feasibility study. J NeuroEngineering Rehabil. 2023;20(1):105.
- Gunduz ME, Bucak B, Keser Z. Advances in stroke neurorehabilitation. J Clin Med. 2023;12(21):6734.
- Patil V. Advancements in neurorehabilitation devices: pioneering recovery and independence. Healthcare News. 2023.
- Baniqued PDE, Stanyer EC, Awais M, et al. Brain–computer interface robotics for hand rehabilitation after stroke: a systematic review. Journal of NeuroEngineering and Rehabilitation. 2021;18(1):15.
- Elashmawi WH, Ayman A, Antoun M, et al. A Comprehensive Review on Brain–Computer Interface (BCI)-Based Machine and Deep Learning Algorithms for Stroke Rehabilitation. Appl Sci. 2014;14(14):6347.
- Maiseli B, Abdalla AT, Massawe LV, et al. Brain–computer interface: trend, challenges, and threats. Brain Inform. 2023;10(1):20.
- Lazarou I, Nikolopoulos S, Petrantonakis PC, Kompatsiaris I, Tsolaki M. EEG-based brain–computer interfaces for communication and rehabilitation of people with motor impairment: a novel approach of the 21st century. Front Hum Neurosci. 2018;12:14.
- Vourvopoulos A, Pardo OM, Lefebvre S, et al. Effects of a Brain-Computer Interface With Virtual Reality (VR) Neurofeedback: A Pilot Study in Chronic Stroke Patients. Front Hum Neurosci. 2019;13:460405.
- Mane R, Wu Z, Wang D. Poststroke motor, cognitive, and speech rehabilitation with brain–computer interface: a perspective review. Stroke Vasc Neurol. 2022;7(6):564-572.
- Lorach H, Galvez A, Spagnolo V, et al. Walking naturally after spinal cord injury using a brain–spine interface. Nature. 2023;618(7963):126-133.
- Vaughan TM. Brain-computer interfaces for people with amyotrophic lateral sclerosis. Handb Clin Neurol. 2020;168:33-38.
- Ren C, Li X, Gao Q, et al. The effect of brain-computer interface controlled functional electrical stimulation training on rehabilitation of upper limb after stroke: a systematic review and meta-analysis. Front Hum Neurosci. 2024;18:143809.
- Remsik A, Young B, Vermilyea R, et al. A review of the progression and future implications of brain-computer interface therapies for restoration of distal upper extremity motor function after stroke. Expert Rev Med Devices. 2016;13(5):445-454.
- Mang J, Xu Z, Qi Y, Zhang T. Favoring the cognitive-motor process in the closed-loop of BCI mediated post-stroke motor function recovery: challenges and approaches. Front Neurorobot. 2023;17:1271967.
- Franc SL, Altamira GH, Guillen M, et al. Toward an adapted neurofeedback for post-stroke motor rehabilitation: state of the art and perspectives. Front Hum Neurosci. 2022;16:917909.
- Savić AM, Marija Novičić, Miler-Jerković V, Djordjević O, Konstantinović L. Electrotactile BCI for top-down somatosensory training: clinical feasibility trial of online BCI control in subacute stroke patients. Biosensors. 2024;14(8):368.
- Bocquelet F, Hueber T, Girin L, Savariaux C, Yvert B. Real-time control of an articulatory-based speech synthesizer for brain-computer interfaces. PLoS Comput Biol. 2016;12(11).
- Peksa J, Mamchur D. State-of-the-art on brain-computer interface technology. Sensors. 2023;23(13):6001.
- He Y, Xu Y, Hai M, et al. Exoskeleton-Assisted Rehabilitation and Neuroplasticity in Spinal Cord Injury. World Neurosurg. 2024;5.
- Picture: Hocoma, Switzerland. Lokomat Nanos [product image]. Hocoma website. Published July 2020. Accessed October 29, 2024. https://hocoma.b-cdn.net/wp-content/uploads/2020/07/6_LokomatNanos.jpg.
- Panizzolo FA, Freisinger GM, Karavas N, et al. Metabolic cost adaptations during training with a soft exosuit assisting the hip joint. Sci Rep. 2019;9(1):9779.
- Guo Z, Ye J, Zhang S, et al. Effects of individualized gait rehabilitation robotics for gait training on hemiplegic patients: Before-after study in the same person. Front Neurorobot. 2022;15:817446. doi:10.3389/fnbot.2021.817446.
- Louie DR, Eng JJ. Powered robotic exoskeletons in post-stroke rehabilitation of gait: a scoping review. J NeuroEngineering Rehabil. 2016;13:1-0.
- Mehrholz J, Pohl M, Platz T, Kugler J, Elsner B, Group. CS. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst Rev. 2015;10:14651858.
- Rodríguez-Fernández A, Lobo-Prat J, Font-Llagunes JM. Systematic review on wearable lower-limb exoskeletons for gait training in neuromuscular impairments. J NeuroEngineering Rehabil. 2021;18(1):22.
- Vaughan-Graham J, Brooks D, Rose L, Nejat G, Pons J, Patterson K. Exoskeleton use in post-stroke gait rehabilitation: a qualitative study of the perspectives of persons post-stroke and physiotherapists. J NeuroEngineering Rehabil. 2020;17(1-5).
- Duvuru R, Hobart-Porter L, Veerapandiyan A. Revolutionizing neuromuscular disorders rehabilitation: The virtual reality edge. Muscle Nerve. 2024;22.
- Liu Q, Liu Y, Zhang Y. Effects of cerebellar non-invasive stimulation on neurorehabilitation in stroke patients: an updated systematic review. Biomedicines. 2024;12(6):1348.
- Li H, Guan C, Fang D, et al. Research hotspots and global trends in transcranial magnetic stimulation for stroke neurorestoration: A 30-year bibliometric analysis. J Neurorestoratol. 2024;100148.
- Proietti T, Bandini A. Wearable Technologies for Monitoring Upper Extremity Functions During Daily Life in Neurologically Impaired Individuals. IEEE Trans Neural Syst Rehabil Eng. 2024;29.
- Porciuncula F, Roto AV, Kumar D, et al. Wearable movement sensors for rehabilitation: a focused review of technological and clinical advances. PM R. 2018;10(9).

