Human Health Effects of Water-Damaged Buildings: A Review
Thirty years of published research on human health effects of exposure to the interior environment of water-damaged built environments
Ritchie Shoemaker1, Scott McMahon2, Andy Heyman3, Eric Dorninger4, Ariana Thacker5, David Lark6
- Progenedx, Pocomoke, MD
- Whole World Health Care, Roswell, NM
- School of Medicine and Health Sciences, George Washington
- Roots and Branches, Boulder, Colorado
- Moldco, Boston, Mass
- NSJ EnviroSciences Pty Ltd, Newcastle, Australia
OPEN ACCESS
PUBLISHED 31 August 2025
CITATION Shoemaker, R., McMahon, S., et al., 2025. Thirty years of published research on human health effects of exposure to the interior environment of water-damaged built environments. Medical Research Archives, [online] 13(8). https://doi.org/10.18103/mra.v13i8.6769
COPYRIGHT © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI https://doi.org/10.18103/mra.v13i8.6769
ISSN 2375-1924
ABSTRACT
Learned people have known since biblical times (NKJV, Leviticus 14:33-48) that dank, moldy buildings with musty smells pose health risks to human inhabitants. The observations about sick buildings making people sick were correct, but there was no proof of causation. Older research suggested that the building-related illness involved allergies, infections, and asthma, but cellular and immune inflammatory causes awaited proof. Yes, mold was found in water-damaged buildings (WDB), but it was also present in other areas. As time passed and cytokines were discovered, the role of resident microbes found in WDB was gradually unveiled as suspected culprits in what became to be known as Sick Building Syndrome (SBS). By 1970, indoor paint was marketed that contained benomyl, a substituted benzimidazole. The paint prevented mold from growing on the paint films, but benomyl was a potent mutagen that created a roster of mutant fungi. New isolations of azole-resistant, toxin-forming fungi followed. Given the concurrent oil embargoes from oil-rich countries, the construction of energy-saving, tight buildings boomed. Tight homes reduce ventilation, raising indoor humidity. Even worse, poorly constructed basements and crawl spaces added to the moisture problems.
The recognition of toxigenic fungi in WDB exploded in the United States (US) in 1999 when a court in Texas (‘Dripping Springs’) awarded an eight-figure settlement to plaintiffs who alleged that exposure to Stachybotrys made a family ill, including dense cognitive impairments. Suddenly, “Black Mold” became a buzzword for lucrative legal awards without proof of causation; association was often enough to win a personal injury/defective construction case in some courts.
Skipping forward to 2026, current academic research has shown that SBS is a Chronic Inflammatory Response Syndrome (CIRS), with specific causation indicating that 42% of cases are due to Actinobacteria, 28% of cases are due to bacterial endotoxins, 7% are due to fungi, and 6-10% are due to beta glucans. The use of Next Generation Sequencing (NGS) has revealed the complexity of microbial residence in WDB. Transcriptomics (Gene Expression: Inflammation Explained; GENIE) provides insight into the molecular pathophysiology of CIRS, while NeuroQuant® indicates that brain injury occurs in more than 50% of cases. When you read claims on the Internet of miracle mold cures, ask to see published, peer-reviewed, data-driven papers that form the basis of those claims.
We present three publications, Human Health and the Built Environment, Part A: Part B respectively, and Part C, focusing on treatment in this Perspective. We will discuss (i) what makes wet buildings dangerous and (ii) how does a building owner correct the microbial ecosystem associated with excessive moisture; (iii) how can the CIRS patients be diagnosed with transparent, medically sound protocols; (iv) how treatment, including healing brain injury, can restore health, even after years of illness in a CIRS patient.
Keywords
Chronic Inflammatory Response Syndrome, water-damaged buildings, Sick Building Syndrome, mold, health effects, microbial ecosystems
Introduction
Recognizing the complexity of the topic of Environmental Health of the Built Environment and Human Health and then recognizing the complexity involved in presenting diagnosis and treatment of adverse health effects, leads us to describe in simple terms what Chronic Inflammatory Response Syndrome (CIRS) is in patients of all ages without differences due to race and gender. We present three papers as a series focused on (i) adverse human health effects; (ii) buildings as a potentially dangerous ecosystem; (iii) detailed discussion of the multi-faceted treatment protocol for CIRS in the built environment. Our goal is to provide solid, published evidence-based data about human health and the Built Environment. The seven sections of Part A provide a stepped approach to an extraordinarily complicated medical problem. Indeed, there is little of modern-day care that is not applicable to CIRS. Section 1 of Part A describes the underlying history of the discovery of a new illness as it makes for a compelling story, with assimilation of a multitude of symptoms and new measures of inflammation affecting all cases, but not in controls, in illnesses caused by exposure to single-celled toxigenic organisms, with individual susceptibility based on immune response genes, HLA DR. The reader will be introduced to state-of-the-art medical science, with much of our advances not part of day-to-day medical practice, nor was not in any medical textbook in 1995. The inexorable search for the hidden pathophysiological clues that have brought us to recognize that 24% of inhabitants of 50% of our buildings with moisture intrusion have an illness that exposure alone, as self-healing does not occur.
Treatment is complex but is sequential, requiring exact attention to detail. All that we advocate is published and peer-reviewed. Medical Review Archives has led the world in recognizing in print the facets of CIRS. As we know, neurodegeneration is common in CIRS patients, but the basic CIRS Protocol corrects brain injury, just as it corrects chronic fatigue and chronic pain among the multisystem, multi-symptom illness features.
Methods
Upon receiving an invitation to submit a pertinent paper to MRA for the Building Health issue, six CIRS experts agreed to write on their special interests. The paper presents a study of CIRS, incorporating research from the last thirty years. David Lark writes on the microbes of CIRS; Eric Dorninger writes on the CIRS Protocol, with a detailed look at treatment in Part C of Perspectives. Ritchie Shoemaker writes on the early pioneering days of CIRS, as well as on GENIE, the transcriptomic test that has revolutionized applications in CIRS. Scott McMahon writes on the battle for acceptance and the peer-reviewed publications that carried out the day. Andy Heyman takes a long look at the molecular biology of CIRS. Lastly, Ariana Thacker, though new to this saga, writes on her realized dream to bring CIRS medical care to vast numbers of patients.
History: How we got to CIRS
The basic scientific research that would eventually prove the causation of a complex, multisystem, multi-symptom illness by exposure to microbes, toxins, and inflammatory agents began in 1996 in the small town of Pocomoke, Maryland. Ritchie Shoemaker, MD, a Duke Medical School graduate, saw a bizarre new illness that made people sick with a multisystem illness that no one had recognized before. Fish would collect in slow-moving estuaries, where lesions on fish occurred, followed by unusual neurological disorders and death. Watermen who worked in the waters of the Pocomoke River started getting tired, with cough, abdominal pain, secretory diarrhea, muscle aches, cognitive impairment and many more symptoms, overnight. What illness was this? All standard tests were routine. No medications helped. A TV reporter sent a water sample to a research lab at NC State, where they found Pfiesteria, a dinoflagellate that sometimes made an unusual toxin. There were no previous case reports of people sickened by exposure.
On a hunch, Shoemaker gave a putative case of Possible Estuary Associated Syndrome (PEAS, as the CDC would call the illness) a prescription for cholestyramine (CSM), a binder of bile salts and cholesterol that stops secretory diarrhea rapidly. By the next day, the patient had no more diarrhea, as expected, but her headache was gone, her cognitive issues had cleared up, and her cough had stopped. Shoemaker started another patient on CSM and then administered it to three more. All improved. Shoemaker wrote two papers, one on the diagnosis and one on the treatment of Pfiesteria Human Illness Syndrome (PHIS, pronounced as FISH). Patients soon flocked to Pocomoke, but controversy quickly followed. “Pfiesteria Hysteria” was a familiar war cry, casting doubt and scorn in the same sentence. Nearly everyone had an opinion about all aspects of the illness. Looking back on the debates, what was heard was just a fraction of what would be found on social media ten years later regarding mold exposures. The public demanded that politicians act, but what we saw was a lot of posturing and not much actual progress.
Maryland sent academics from Johns Hopkins and the University of Maryland School of Medicine to review the reported cases. Surprisingly, they acknowledged Shoemaker’s findings! There was a surge in funding for academics and experts on the Chesapeake Bay, but not a single penny was directed towards treatment. Predictable! Politicians blamed the environmental cause on nutrient enrichment. Just look at all the chicken manure that was there. Shoemaker disagreed, accusing the ecological perturbation underlying the Pfiesteria blooms of being caused by two fungicides, copper and dithiocarbamates, used to control the growth of resistant blue mold that was devastating tomato fields and tobacco. Copper, a Level 1 biocide, stirred up from porewater by rain or wind events, killed off cryptomonads the preferred algal-like food for Pfiesteria in its slow-moving, amoeba-like phase. Nicknamed ‘The Cell from Hell,’ if suddenly starving from the effects of copper, the Pfiesteria could change cell forms, morphing into a fast-moving phase needed for breeding. Next, the dithiocarbamates killed off rotifers, another microbe found in river water that ate the fast-moving, toxin-forming phase of Pfiesteria. Copper induced the dinoflagellates to enter the breeding phase; dithiocarbamates triggered the bloom activity, with the toxin acting as a pheromone that attracted other Pfiesteria to the feeding and breeding cycle. Confirmation of the Copper Hypothesis came in 2006. With the removal of copper and dithiocarbamates, the Pfiesteria blooms have ceased. There were no changes in nutrient levels then or now.
In 1998, shortly after the treatment paper was published, Ken Hudnell, PhD, EPA neurotoxicologist, published his work showing that PEAS patients had a deficit in Visual Contrast Sensitivity (VCS). Controls had no VCS deficits; only the cases showed deficits. Shoemaker learned how to do the VCS test from Hudnell. They then borrowed a Heidelberg Retinal Flowmeter (HRF) that measures the velocity of red blood cell flow in the retina and neural rim of the optic nerve head. Dr Shoemaker showed that cases of PEAS had reduced flow that improved with CSM treatment. With re-exposure, the velocity showed a recrudescence; with retreatment using CSM, the velocity returned to normal. Hudnell and Shoemaker fulfilled a modified version of Koch’s Postulates!
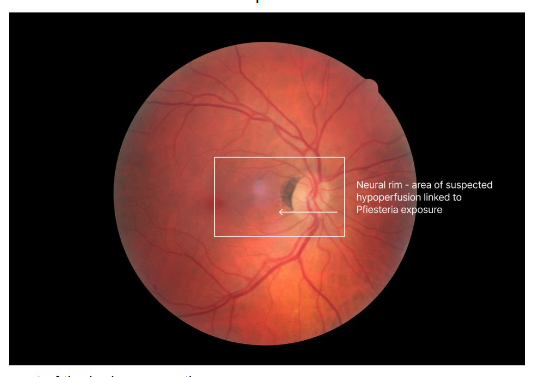
In less than a year, the mystery of PEAS was solved, but the newly emerging Biotoxin Paradigm continued to grow. Shoemaker now had the full clinical picture: exposure, symptoms, physical findings, VCS deficits, retinal flow data, differential diagnosis, pertinent negatives, and treatment response. A longer list of tests and biomarkers would follow the Pfiesteria case definition for CIRS.
Biotoxin-associated illness basic science expands
Underlying the discovery were two simple questions. What explained why some people became sick from exposure and others, with the same exposure, did not have any symptoms? As it turned out, susceptibility was linked to HLA-DR, immune response genes located on chromosome 6. We now had a biomarker! The second question still drives research: What is the mechanism of the illness? Could cytokines play a role? MMP9 appeared to be a logical candidate, as cytokines of several types triggered its expression. No local labs ran the MMP9, but Esoterix in Aurora, Colorado, would. Bingo, MMP9, was elevated in most cases but not in controls. Treatment lowered MMP9 quickly, and re-exposure made it worse. Another biomarker was added to the growing list.
Exposure to ciguatera, another dinoflagellate, also causes illness, which has an acute phase, like Pfiesteria, but also has a chronic phase that lasts for years without cure. Sure enough, acute ciguatera (less than six months) responded well to CSM, with correction of VCS and reduction of symptoms. Chronic ciguatera did not respond. What was the difference between acute and chronic ciguatera? Since the ciguatoxin is bound to a receptor, did it bind too tightly?
Unexpectantly, the answer came from untreated Lyme disease. To this day, Lyme disease remains controversial, with one group of physicians arguing passionately against the opinions of other groups. The losers in this argument were patients. What was curious was the repeated finding that a subset of Lyme patients who had finished antibiotics, but were still sick, had a horrible worsening almost immediately after taking CSM. VCS fell in Column E. The still-sick patients had an HLA DR of 15-6-51, and their MMP9 jumped up almost immediately after CSM. Was this due to the rapid separation of something made by Lyme?
END storm was blowing fifty knots. Shoemaker watched as a cascade of pear blooms poured off the tree with every gust. Imagine if the pear blossom were a metaphor for a toxin leaving its receptor. By blocking an inflammatory pathway, were we blocking the production of MMP9? Would there be such an increase in intensity from MMP9 after using a stabilizing factor? Yes, pre-treatment blocked the intensification of symptoms somewhat after giving CSM, but the key lay in the toxin’s dissociation from its receptor, triggering a cytokine storm as the freed toxin flooded the tissues. VCS fell in intensification, just as in re-exposure.
Later, Shoemaker read that the dissociation constant for ciguatoxin leaving its receptor was nearly too low to measure. Of course, no ciguatoxin storm of pear blossoms! Still, chronic ciguatera and chronic Lyme held out one more forensic clue. James Lipton was publishing papers on an obscure neuroregulatory peptide, α-melanocyte-stimulating hormone (MSH). This hormone was produced in the hypothalamus and regulates a wide range of functions, including gut health, obesity, and circadian rhythms, as well as cytokine production. When cytokines were out of control, as in Biotoxin illness, MSH should be low, and it was. How come nobody talked about MSH?
In rapid succession, the Biotoxin Pathway was soon evaluated through calls to investigate a dinoflagellate, Cryptoperidiniopsis, in the St. Lucie River in Florida, as well as multiple blooms of cyanobacteria (Microcystis, Cylindrospermopsis, and Lyngbya). The story was the same: blooms of one-celled creatures, sick people with similar symptom profiles, VCS deficits and response to CSM.
In the summer of 1998, the phone call was from a new Biotoxin patient. He had all the features, symptoms, VCS deficits, and response to CSM. Except there was no water exposure, no trips to Florida, no consumption of reef fish. His closet was full of mold. Black Mold. Shoemaker’s arguments with experts in a new field of medicine were just getting started.
The index case for mold exposure was helped, but not as much as other Biotoxin patients. Was he still exposed to the Black Mold? Time to start sampling the environment. Easier said than done. By now, it was hard to explain what was going on in new molecular biology to new patients listening to thirty minutes of jargon. Time for a graphic, with the first version coming off the presses in 2004. The Biotoxin Pathway was a big hit but nearly every day brought an exciting new paper to read and one new biomarker to confirm.
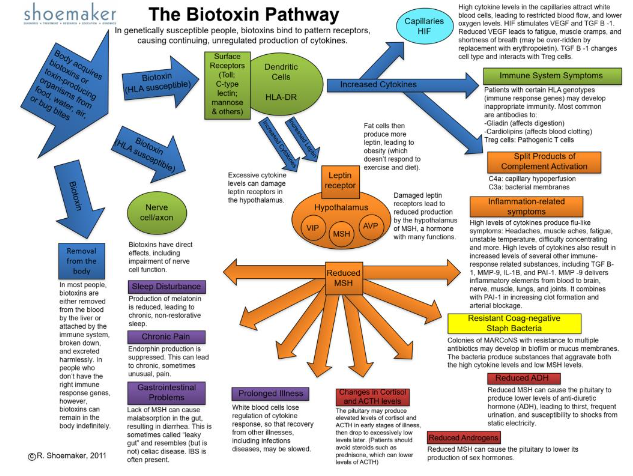
By 2003, the Biomarker list was expanding rapidly. VEGF, ACTH/cortisol, ADH/osmolality, C3a, C4a, MARCoNS, Antigliadin antibodies, Anticardiolipin antibodies. Shoemaker had a new Biotoxin Pathway and a new practice, one that focused on Biotoxin illnesses. He has published several books, including Pfiesteria: Crossing Dark Water, Desperation Medicine, and Mold Warriors, with more forthcoming. New inventions, more acronyms and steps ever closer to understanding the molecular mechanisms.
| Symptoms | Controls | Cyano | WDB-1 | WDB-2 | WDB-3 | PEAS | Ciguatera | Lyme |
|---|---|---|---|---|---|---|---|---|
| Fatigue | 6 | 100 | 89 | 83 | 100 | 70 | 91 | 94 |
| Weak | <5 | 80 | 75 | 70 | 84 | 83 | – | 89 |
| Ache | 8 | 90 | 77 | 68 | 95 | 43 | 77 | 81 |
| Cramp | <5 | 80 | 66 | 56 | 63 | 14 | 68 | 77 |
| Unusual Pains | <5 | 50 | 62 | 51 | 42 | – | 82 | 86 |
| Ice Pick Pain | <5 | 40 | 49 | 41 | – | – | 45 | 82 |
| Headache | 9 | 90 | 78 | 66 | 84 | 73 | 78 | 88 |
| Light Sensitivity | <5 | 90 | 71 | 66 | 89 | 68 | 67 | 85 |
| Red Eyes | <5 | 50 | 52 | 48 | 63 | 68 | 48 | 61 |
| Blurred Vision | <5 | 40 | 61 | 56 | 63 | – | 53 | 66 |
| Tearing | <5 | 30 | 41 | 48 | 63 | – | 28 | 55 |
| SOB | 11 | 60 | 78 | 63 | 74 | 57 | 63 | 77 |
| Cough | 7 | 50 | 72 | 53 | 53 | 43 | 62 | 71 |
| Sinus Congestion | 8 | 60 | 79 | 65 | 74 | 41 | 70 | 68 |
| Abdominal Pain | <5 | 60 | 61 | 39 | 37 | 41 | 79 | 42 |
| Diarrhea | <5 | 50 | 48 | 39 | 21 | 57 | 72 | 51 |
| Joint Pain | 11 | 70 | 75 | 53 | 84 | – | 62 | 88 |
| Morning Stiffness | 6 | 70 | 72 | 44 | – | – | 59 | 80 |
| Memory Impairment | <5 | 80 | 83 | 66 | 68 | 84 | 81 | 80 |
| Difficulty Concentrating | <5 | 70 | 81 | 62 | 53 | 35 | 83 | 82 |
| Confusion | <5 | 40 | 75 | 57 | 26 | 24 | 66 | 72 |
| Decreased Word Finding | <5 | 80 | 81 | 66 | 11 | – | 80 | 84 |
| Decreased Assimilation | <5 | 80 | 72 | 65 | 37 | – | 78 | 88 |
| Disorientation | <5 | 30 | 51 | 40 | 11 | – | 28 | 33 |
| Mood Swings | <5 | 20 | 69 | 65 | – | – | 42 | 65 |
| Appetite Swings | <5 | 50 | 58 | 58 | – | – | 61 | 77 |
| Sweats (Night) | <5 | 50 | 61 | 54 | – | – | 42 | 68 |
| Difficulty Reg. Body Temp | <5 | 50 | 63 | 60 | – | – | 67 | 72 |
| Excessive Thirst | <5 | 60 | 69 | 54 | – | – | 59 | 71 |
| Increased Urinary Freq | <5 | 60 | 66 | 58 | – | – | 66 | 75 |
| Static Shocks | <5 | 40 | 41 | 44 | – | – | 38 | 32 |
| Numbness | <5 | 40 | 48 | 44 | 37 | – | 74 | 66 |
| Tingling | <5 | 40 | 61 | 51 | 47 | – | 78 | 71 |
| Vertigo | <5 | 40 | 39 | 48 | 42 | 16 | 29 | 37 |
| Metallic Taste | <5 | 40 | 45 | 36 | 47 | – | 46 | 38 |
The lawyers started to come around; unfamiliar terms were introduced in daily readings, such as Daubert, Frye, and specific causation. Oh, how defense witnesses made up stories (discussed by Dr. McMahon) that said nothing; but the plaintiff’s claim was often not approved. What the plaintiff needed to win the case was a prospective clinical trial that was guaranteed to show risk and therefore causation. Enter, SAIIE at stage left.
Sequential Activation of Innate Immune Elements was used in a repetitive re-exposure study design, which was linked to a scoring system, all of which were presented, of course. Based on a robust literature review, SAIIE performed well.
By 2004, Stephen Vesper, PhD, an EPA scientist, published an interpretive process that answered the key question: Can we sort fungi found in wet buildings that are associated with human health effects? Finally, treating doctors could inform patients about what was causing them to feel unwell. Another fungal interpretation protocol, followed this breakthrough, called HERTSMI-2, short for Health Effects Roster of Type-Specific Formers of Mycotoxins and Inflammagens, version 2. This study, published in two versions by Shoemaker and David Lark, showed that prior CIRS patients would relapse with re-exposure to a given building when they returned, if it was not suitably remediated. However, the early history of CIRS is now in its final stages, as the next generation of Mold Warriors and researchers is here.
May they perform metaphorical autopsies on fish with lesions or conduct VCS testing on patients in their moldy buildings as Shoemaker did if they need data. While no one else is likely to repeat exactly this journey of discovery, one element stands out; the experience was like that of countless physicians. The data was consistent and incontrovertible. Presence of a unique array of symptoms told the tale: an odd diagnosis was incredibly common and beginning treatment of CIRS with a non-absorbable resin gave an effective start to healing incurable chronic illness.
But it must be said that for all the power of cutting-edge tests and technologies available to advance medical science, no tool proved more valuable in unraveling CIRS than the timeless skill of truly hearing the patient.
END NOTE. SCOTT MCMAHON MD Dr. Shoemaker’s brilliance, coupled with his single-minded tenacity and cross-disciplinary reading, has elucidated CIRS diagnostic symptoms, dozens of biomarkers, imaging abnormalities, transcriptomic signatures, and effective treatments. Dr. Shoemaker’s lifelong pursuit of answers has brought hope to millions of CIRS sufferers.
SECTION 2 DAVID LARK
Specific Microorganisms Linked to Human Health in Water-Damaged Buildings
NSJ EnviroSciences Pty Ltd, Newcastle, NSW, Australia
Abstract
Water-damaged buildings (WDB) create an ecologically unique environment conducive to the proliferation of specific microorganisms that have been repeatedly associated with adverse human health effects. Unlike the transient microbial communities encountered outdoors or in dry, well-ventilated indoor spaces, WDB ecosystems support persistent and often toxigenic microbial populations. These organisms include filamentous fungi, bacteria particularly actinobacteria mycotoxin producers, and microbes capable of biofilm formation. Recent advances in molecular diagnostics, including next-generation sequencing (NGS), have further elucidated the complexity and diversity of WDB microbial ecosystems, enabling clinicians and indoor environmental professionals (IEPs) to better understand the potential health risks associated with chronic exposure.
Introduction
This chapter explores the key microbial players in WDB, their documented health impacts, mechanisms of pathogenesis, and their relevance to chronic inflammatory conditions such as CIRS (Chronic Inflammatory Response Syndrome). The growing body of literature underscores the need to consider not only the presence of known pathogens but also the cumulative effects of microbial fragments, volatile organic compounds (mVOCs), endotoxins, and bioaerosols.
Fungi Implicated in Human Illness
Fungi remain the most visually identifiable and traditionally most studied organisms in WDB. For example, species belonging to the genera Stachybotrys, Aspergillus, Chaetomium, and Penicillium are frequently found in damp building materials.
- Stachybotrys chartarum, colloquially referred to as the black produces trichothecene mycotoxins such as satratoxins and roridins, which are capable of inhibiting protein synthesis and inducing apoptosis in mammalian cells (Etzel).
- Chaetomium globosum, another cellulolytic mould, produces chaetoglobosins, which have cytotoxic and genotoxic effects (Gareis).
- Penicillium chrysogenum and P. brevicompactum are known producers of mycophenolic acid and other immunosuppressive compounds (Li & Haugland).
- Aspergillus species play a disproportionately large role in both acute and chronic health consequences. A. flavus is a major producer of aflatoxins, known hepatocarcinogens; A. fumigatus is implicated in invasive aspergillosis and hypersensitivity pneumonitis; A. versicolor produces sterigmatocystin, a structurally similar mycotoxin to aflatoxins; A. penicillioides, although less toxigenic, is often a sentinel organism in ERMI analyses due to its prevalence in xerophilic or chronically damp environments (Straus).
These fungi release not only spores but also cell wall components, such as beta-glucans, mannans, and chitin, which activate innate immune receptors, including dectin-1 and TLRs (Reponen et al.). The exposure pathway is typically inhalational, although dermal and oral routes have also been described.
Actinobacteria and Gram-positive Soil-derived Bacteria
The actinobacteria formerly known as actinomycetes are an increasingly recognized component of the WDB microbiome. Commonly soil-derived, these filamentous bacteria can become aerosolized during or following water damage, especially in poorly ventilated spaces with cellulose-rich materials.
Species such as Streptomyces, Nocardia, and Mycobacterium have been identified in dust samples from WDB and are frequently associated with granulomatous and hypersensitivity-type pulmonary responses (Lighthart). Actinobacteria produce a wide array of secondary metabolites, including geosmin (a strong-smelling compound that is often mistakenly identified as and various antimicrobials that may paradoxically influence indoor microbial competition while posing toxicity to humans (Pascual et al.). Their small spore size (0.5 2 µm) facilitates deep lung penetration. Moreover, many actinobacteria are acid-fast and resist digestion by macrophages, resulting in chronic stimulation of innate immune responses, particularly through TLR2 and TLR4 (Shoemaker et al., 2021). In individuals with impaired detoxification genetics (e.g., HLA-DR haplotypes), this can contribute to sustained inflammation and multi-system illness.
Biofilms and Microbial Interactions
A critical yet under-recognized phenomenon in WDB is biofilm formation. Biofilms are structured microbial communities encased in extracellular polymeric substances (EPS) that confer significant resistance to cleaning agents and antimicrobials. Organisms such as Pseudomonas aeruginosa, Staphylococcus epidermidis, Aspergillus fumigatus, and actinobacteria have all been observed to form biofilms in indoor settings, especially on porous substrates like gypsum board and wood. The biofilm mode of growth facilitates horizontal gene transfer, immune evasion, and the release of quorum-sensing molecules that can independently affect human physiology, including neurological function (Donlan & Costerton).
Biofilms in HVAC systems, walls, and carpeting may be episodically disturbed, leading to pulse exposures to a mixture of live organisms, biofilm fragments, and toxins. These exposures often elude conventional air or surface sampling techniques.
Disruption of the Human Microbiome
Repeated exposure to WDB microbes has implications beyond acute illness. There is substantive, growing evidence that such exposure can alter human commensal microbiota, particularly in the respiratory and gastrointestinal tracts. Dysbiosis induced by microbial antigens and toxins may lead to reduced resilience and heightened vulnerability to autoimmune conditions and neuroinflammation.
For example, fungal metabolites like gliotoxin and bacterial lipopolysaccharides (LPS) have been shown to disrupt tight junction integrity and mucosal immunity (Rizzatti et al.). Moreover, the immune reactivity to microbial DNA and proteins in genetically susceptible individuals can lead to loss of self-tolerance, contributing to chronic conditions such as CIRS and MCAS (Shoemaker & McMahon, 2022).
Co-Exposures and Synergistic Effects
WDB exposure rarely involves a single agent. Instead, co-exposure to microbial fragments, mycotoxins, endotoxins, mVOCs, and nanoparticles (e.g., from degraded paints or plastics) can exert synergistic toxicity. These mixtures may cause oxidative stress, mitochondrial dysfunction, and persistent inflammation even after the microbial source is removed. These polymicrobic environments are often referred to as the . The immunological concept of the is applicable here, where a confluence of signals (TLR agonists, complement activators, cytokines) converges to amplify immune responses disproportionately to the initial insult. This concept is particularly relevant in the context of multiple chemical sensitivity and systemic inflammatory response syndromes.
Protective and Commensal Organisms
Interestingly, not all microbial exposures are harmful. The absence of beneficial indoor microbes, such as Lactobacillus spp. and Bifidobacteria, as well as certain outdoor-derived soil organisms, may contribute to dysbiosis and immune dysregulation in modern built environments.
Some authors have posited that microbial diversity especially in early life is protective against allergy and asthma (the However, in WDB, the microbial community is skewed toward pathogenic dominance, often characterized by the loss of protective taxa and the overrepresentation of aggressive, toxin-producing species (Hanski et al.).
Neurocognitive and Systemic Impacts
Among the most debilitating effects of WDB exposure are neurocognitive symptoms, often described as in memory deficits, poor word recall, and executive dysfunction. These are now well-characterized features of biotoxin-related illness. Mechanisms include:
- Mycotoxin-induced inhibition of protein synthesis in neurons
- Cytokine-mediated disruption of the blood brain barrier
- Activation of microglia via TLRs and NOD-like receptors
- Altered cerebral perfusion, demonstrated by NEUROQUANT imaging in CIRS patients (Shoemaker et al., 2021)
Recently published work utilizing transcriptomics has further identified upregulation of inflammatory and detoxification genes in exposed individuals, supporting a biomolecular basis for these observations.
Special Note on Cyanobacteria
Cyanobacteria, although not traditionally classified among the primary WDB organisms, have emerged as a noteworthy contributor to environmentally acquired illnesses in select indoor environments, particularly those with moisture intrusion from surface water or HVAC systems drawing from algal-contaminated sources. Some species produce BMAA (β-N-methylamino-L-alanine), a neurotoxin associated with neurodegenerative diseases. Their role in indoor air and dust contamination is the subject of ongoing investigation and will be further addressed in future publications focusing on molecular and transcriptomic analyses of WDBs.
Conclusion and Future Directions
The microbial milieu of water-damaged buildings is diverse, dynamic, and undeniably hazardous. As our detection capabilities improve, it is clear that many WDB-associated organisms exert effects through non-infectious but immunologically potent mechanisms, including mycotoxins, biofilm components, and inflammagens.
- Understanding the health consequences of WDB exposure requires:
- Molecular-level identification of microbial communities (e.g., NGS)
- Recognition of gene environment interactions (e.g., HLA-DR susceptibility)
- Multi-disciplinary collaboration between clinicians, IEPs, and researchers
Further research priorities may include:
- More longitudinal studies correlating indoor microbial profiles with health outcomes
- Exploration of protective microbiota and competitive exclusion therapies
- Better sampling methods that capture microbial fragments and toxins, not just spores and/or viable organisms
By expanding our view beyond pathogens to include the totality of microbial and chemical exposures in WDBs, we can better address the root causes of biotoxin-related illnesses and improve outcomes for affected individuals.
SECTION THREE ERIC DORNINGER ND, LAc
The CIRS PROTOCOL:
As previously stated, Chronic Inflammatory Response Syndrome (CIRS) is a complex, multisystem illness triggered by persistent innate immune activation due to biotoxins from mold, mycotoxins, actinobacteria, bacterial endotoxins, beta-glucan and others. The CIRS Protocol, evolved from Dr. Ritchie work, is a 12-step sequential treatment framework that incorporates environmental, pharmacologic, and biochemical interventions. Here we outline the clinical application of the protocol, emphasizing the importance of strict stepwise implementation for biomarker normalization and symptom resolution.
The CIRS Protocol is best visualized as a therapeutic pyramid, with foundational steps forming the base and requiring strict adherence to sequence for effective healing.
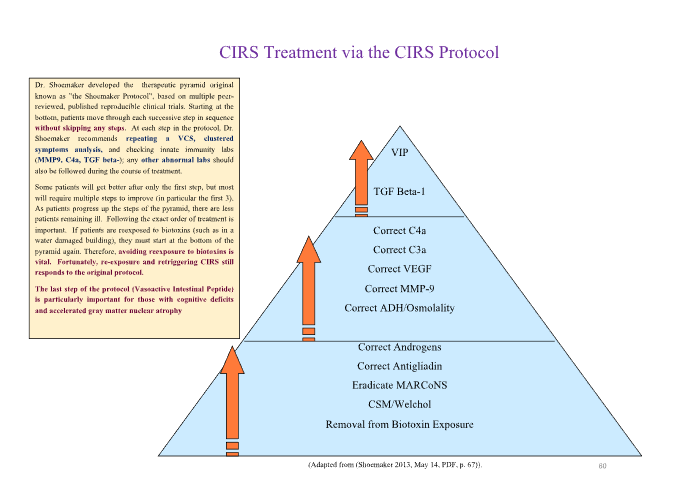
Step 1: Remove Patient from Biotoxin Exposure Biotoxins are ionophores or amphipaths/amphiphiles with hydrophilic and hydrophobic properties that can easily cross cell membranes and interact with innate immune receptors such as TLRs and C-type lectins. When screening a patient for CIRS, three cost-effective, straightforward steps are necessary to include CIRS in the differential diagnosis:
- Biotoxin exposure history (past or present)
- Clustered Symptoms Questionnaire (administered by provider or trained staff)
- Visual Contrast Sensitivity (VCS) test.
When water damage is identified and initial CIRS screenings (symptom clusters / VCS) are administered, confirmatory lab testing should follow. If confirmed CIRS, the process of identifying building-related biotoxins begins. An Indoor Environmental Professional (IEP) trained in biotoxin-related assessment should inspect the structure. Many IEPs lack CIRS specific training and use inadequate methods, impeding effective completion of Step 1. Fortunately, CIRS-aware IEPs have developed training and protocols for Medically Important Assessment and Remediation, but qualified professionals remain scarce. For effective evaluation, IEPs must assess four key biotoxin categories in a building:
- Water-damaged building (WDB) molds via ERMI/HERTSMI-2 (qPCR)
- Actinobacteria via Next Generation Sequencing (NGS)
- Endotoxins via Kinetic LAL Assay
- Beta-glucans via direct assay for (1→3)-ß-D-glucans
Microfiber samples of dust are used to measure biotoxins. Results guide further investigation (e.g., smoke testing for sewer leaks) and are compared with a scoring roster predicting statistical likelihood a building supports CIRS recovery.
Step 2: Eliminate Biotoxins Using Bile Acid Sequestrants Once out of exposure, internal reservoirs must be cleared using bile acid sequestrants like Cholestyramine (CSM) or Welchol. These agents bind negatively charged biotoxins in the gut and prevent their reabsorption. Pretreatment with high-dose fish oil (EPA 2.4g + DHA 1.8g) for 10-14 days helps modulate immune response and prevent intensification reactions. CSM is more potent but more constipating; Welchol is better tolerated. Dosing must be titrated according to patient tolerance and body weight. Adequate hydration and bowel motility support are essential. CSM dosage is 3 grams TID for ~120-pound adult, 4 grams QID for ~200-pound adult, 60 mg/kg per dose for pediatric patients. Welchol/colesevelam tablet target dose is two 625 mg TID.
Step 3: Eradicate MARCoNS Multiple Antibiotic Resistant Coagulase Negative Staph (MARCoNS) colonizes the nasopharynx in ~80% of CIRS cases. Eradication should occur only after steps 1 and 2. Treatment typically involves BEG spray (Bactroban, EDTA, Gentamicin) or similar formulas. Treatment failure is often due to ongoing exposure or failure to follow the step sequence.
Step 4: Correct Anti-Gliadin Antibodies Elevated anti-gliadin antibodies may indicate gluten sensitivity or celiac disease. Gluten removal is advised if antibodies are elevated. Restoration of immune tolerance often occurs with full protocol adherence. Reintroduction can be considered post-protocol. If celiac is confirmed, the patient should avoid gluten for life.
Step 5: Restore Androgens (DHEA/Testosterone) DHEA-sulfate is frequently depleted in CIRS patients, leading to catabolism and impaired immune function. Restoration involves titrated DHEA supplementation (10-75 mg/day) with regular monitoring. Benefits include improved energy, cognition, and hormone balance. Caution is advised in patients with a history of hormone-sensitive cancers.
Step 6: Correct ADH/Osmolality Low ADH with high serum osmolality results in excessive urination, thirst, and dehydration symptoms. Correction involves assessing serum osmolality and potentially using desmopressin in selected patients. Step 6 improves quality of life and addresses common symptoms like hypovolemic headaches, POTS, bedwetting, cramping and static shocks.
Step 7: Correct MMP-9 Matrix Metallopeptidase-9 (MMP-9) promotes blood-brain barrier permeability and systemic inflammation. Elevated levels contribute to fatigue, brain fog, and pain. Fish oil (4200 mg EPA+DHA/day) and a low-amylose diet can help reduce MMP-9.
Step 8: Correct VEGF Low Endothelial Growth Factor (VEGF) impairs oxygen delivery to tissues, promoting anaerobic metabolism and fatigue. Treatment includes high-dose fish oil and gradually increasing exercise. This step helps patients recover endurance and tolerance to physical activity.
Step 9: Correct C3a Complement 3a (C3a) is an inflammatory split product elevated in Lyme and CIRS -PLS (Post-Lyme Syndrome). High-dose water-soluble statins (e.g., rosuvastatin) are used to lower C3a, alongside CoQ10 (150 mg) and Geranyl Geraniol (150 mg) to mitigate side effects. Lyme must be ruled out before targeting elevated C3a.
Step 10: Correct C4a Complement 4a (C4a) is linked to WDB exposure and is often normalized after steps 1-3. If persistent, VIP is the preferred treatment. Elevated C4a can impair cognition and mitochondrial function.
Step 11: Correct TGF beta-1 Transforming Growth Factor beta-1 (TGF-β1) contributes to fibrosis and immune dysregulation. When elevated despite prior steps, treatment includes Losartan (ARB) at 25 mg BID or VIP. Losartan lowers TGF-β1 through a specific degradation product, EXP 3179. If hypotension is present, use VIP.
Step 12: Vasoactive Intestinal Peptide (VIP) VIP is a neuroimmune modulating peptide that downregulates inflammation and restores grey matter volume in the brain, as shown via NeuroQuant imaging. VIP nasal spray is introduced only after the previous 11 steps are completed. It has shown efficacy in reversing stubborn symptoms and restoring functional brain volume.
The CIRS Protocol provides a reproducible, evidence-based framework for resolving chronic inflammatory illness caused by biotoxins. Success depends on precise sequence, biomarker monitoring, and clinical rigor. The protocol emphasizes environmental remediation, biotoxin elimination, hormone and immune modulation, and neuronal restoration. When applied authentically, this approach offers hope to patients with treatment-resistant, multisystem illness and represents a scalable model for healthcare.
SECTION FOUR: RITCHIE SHOEMAKER MD
GENIE SHOWS DIFFERENTIAL GENE ACTIVITY OF CIRS
The story of GENIE is remarkable. First discovered by Jimmy Ryan, PhD., and patented by Ryan and Dr. Ritchie Shoemaker, GENIE (Gene Expression: Inflammation Explained) has come from a relentless pursuit of defining the molecular biology that underlies CIRS.
Dr. Ryan was using Next Generation Sequencing to put physiology and transcriptomics together to expand our insights and knowledge regarding differential gene activation and/or suppression that we see in CIRS. What Jimmy found was suppression of ribosomal genes and to a lesser extent, mitoribosomes in mitochondria. Added to reduction of ribosomal RNA are groups of genes regulating ATP synthesis and cyclooxygenase. Crucial to these different aspects of defining the physiology of CIRS are the role of translocases assisting transport of pyruvate from the cytoplasm of the cell across the outer membrane of the mitochondria into the matrix where pyruvate is then converted into ATP. If translocases are low, as is the case in approximately 85-90% of CIRS patients, pyruvate cannot cross into the matrix, ATP production is impaired, but the mitochondria are not diseased.
If translocases cannot take pyruvate to its destination, the cell will convert pyruvate into lactic acid and secrete lactic acid into capillary beds against a gradient. Excessive presence of lactic acid creates metabolic acidosis which in turn is responsible for cross-development of pulmonary hypertension, reduction of T-regulatory cells, and increased levels of grey matter nuclear atrophy, as well as insulin resistance. If we also have an increased amount of gene IRS2, there will be metabolic acidosis and proliferative physiology, which is a double hit on cell metabolism in CIRS. GENIE shows all this metabolism. No other test will; providers will not know, and patients will suffer needlessly.
Over the last eight years and for 13 separate academic publications, GENIE has revolutionized our understanding of genomics as opposed to inflammatory changes in CIRS, and metabolism as opposed to mitochondrial disease.
Metabolic acidosis is only a small parcel in the truckload of knowledge brought to us by GENIE. We use GENIE tells to determine apoptosis, understanding that caspase genes are vital to maintaining normal and predictable cell death. Fungi can interrupt this efficient pathway, marked by upregulation of two of three caspase genes and one other gene.
The ten coagulation genes show the tremendous interaction between cell function and clotting with platelet participation in both elements. GENIE shows us the way to neurodegeneration with concern for central nervous system micro clotting and micro bleeding. Simply stated, coagulation abnormalities underly neurodegenerative processes, among others.
Cytokines are shown by upregulation when there are excessive inflammatory responses including central nervous system injury from the gene RELA. The three TGF beta-1 receptors, called BR1, BR2 and BR3, if combined with activation of at least one MAP kinase gene, gives us specific causation for actinobacteria. This in turn, allows us to say with authority what the microbiological source of gene abnormalities in symptoms that we see in CIRS is.
As mentioned, if we have apoptosis and we have three MAP kinase genes activated, statistically that gives us specific causation for fungi; if we have one TGFBR 1, 2, or 3 elevated, and one MAP kinase, that gives us specific causation for Actinobacteria. Endotoxins have their own category of specific causation. If CD14, TLR2 or TLR4 are present, and the z-score greater than 1.3, there is specific causation for endotoxins. These three microbes, fungi, actinos and endos, used to be discussed as possible sources of causing CIRS. Now we know that GENIE can give us the cause of CIRS specifically.
Along the way, abnormalities in transcriptomics are found by John Aucott (Hopkins) and Charles Chiu (UCSF) to be associated with Lyme disease. A pattern of gene activation can show us if Lyme is present and not treated, as well as present and partially treated. This is a huge advance in determining yes or no for Lyme.
Ikaros can tell us about environmental susceptibility to chemicals, medications and food. We also use GENIE as a source to implicate increased risk of central nervous system injury with significant reliability.
A vital source of causation of CIRS comes from failure of T-cell synapse at the junction of antigen presenting cells and naïve antigens. What this means is defective antigen presentation leads to defective antibody production hence the illness of antibodies.
We also use GENIE to look at CIRS biomarkers understanding of the 16-genes that could be elevated, usually there is only 3 or 4. 4 genes is enough to give weight to presence of CIRS. When we first started looking at PTSD, one gene, FKBP5 stood out with its increased z-score in people who we thought had emotional or psychological problems. Now that we have FKBP5, PTSD is often both an objective diagnosis and a subjective diagnosis.
Histamine over-production is found in approximately 40% of patients with CIRS but the problem is not mast cell activation syndrome. Indeed, the genes CCL5 and HDC tell us that every cell with a nucleus is overproducing histamine!
In the world of GENIE, the function of the cytoskeleton or tubulin is important as a predictor for neurodegenerative diseases such as ALS More importantly, these genes, when active, show a decreased amounts of ions and nutrients being transported from one braithe neuronal cell body to the axon. Elevated levels of TUBA4A and TUBB1 tell us that if there is reduction in the delivery of ions in nutrients, there will be a die-back of cells in the brain itself. TUBA4A and TUBB1 are vital to understanding neurodegenerative processes as well as neurologic symptoms.
T-regulatory cells functions are dependent on adequate numbers of T-regulatory cells. Finally, we have a mechanism to tell us if there is a deficiency in T-regulatory cells or not. Similarly, TH17/Treg will tell us about T-regulatory cell imbalance. It can be manifested through cardiovascular illnesses and endothelial injury.
The new player on the block for GENIE has to do with capability of diagnosing risk for development ally in patients who have so called Triple Positives (Clusterin excess, coagulation excess and cytoskeleton excess). These are a distinct subset of genes but are vital in following correction of Triple Positives and management of neurodegenerative processes.
Finally, dispersion, which should be present at a level less than one for z-score can have increased numbers of standard deviations between dispersion in controls and dispersion in cases. As you learn more about GENIE, dispersion will become an important player to add to your database.
This has been a short run through of what GENIE is and why it is so important. For more information, look at Progenedx.com or some of the lectures given since 2016 by Dr. Ryan and Dr. Shoemaker, together with academic papers largely published for the collaboration of Shoemaker, Ryan, Scott McMahon, Andy Heyman, Eric Dorninger and David Lark.
SECTION FIVE: THE LITERATURE SPEAKS SCOTT MCMAHON MD
In toxic tort legal circles, causation is confirmed by demonstrating general causation and specific causation. General causation is established by showing that exposure to a substance or condition can cause symptoms in most or all individuals exposed to it. Peer-reviewed papers must support that exposure to the substance or condition in question leads to these symptoms, supporting general causation. Specific causation is shown by documenting that the alleged exposure to the plaintiff or plaintiffs culminated in the manifestation of some or all of these symptoms. The health of the plaintiff(s) before the exposure, any changes in health resulting from the exposure, documentation of the exposure itself, and improvements after appropriate therapy are required to demonstrate specific causation. A review of the scientific and medical literature regarding mold establishes that a general causation exists between chronic exposure to the interior of water-damaged buildings (WDB) and the development of adverse human health effects, as evidenced by reproducible symptoms and objective biomarkers.
When the interior spaces of WDB and their amplified microbes (fungi, actinobacteria and/or bacteria) and/or their products (toxins, naked DNA, fragments with cell wall components, beta-glucans and more) are exposed, the literature is rife with associated symptoms documented in humans. Hundreds of human epidemiological studies have demonstrated single and multisystem symptoms after exposure to WDB. Rather than citing these numerous articles, we refer to two summary articles to make this point.
First, the peer-reviewed Policyholders of America summary/review article referenced over 600 unique papers published in medical and environmental literature. One section summarized 14 epidemiological studies representing over 40,000 human subjects, documenting exposure to the interior of WDB and leading to illnesses affecting respiratory, immune, and other systems.
Secondly, the Dooley systematic review evaluated every peer-reviewed epidemiological study from 2011 to 2018, juxtaposing increased indoor dampness and/or microbial growth with adverse human health effects. Worldwide literature formed a near consensus.
Three key takeaways were prominent in this peer-reviewed publication, which evaluated 114 studies from more than 30 countries and represented over 273,000 subjects.
- These studies supported the role of chronic exposure to elevated indoor dampness and microbial growth in leading to human symptoms, outnumbering studies showing no effect by a count of 112 to 2. At least 98.2% of these articles supported the theory that such exposures led to human health symptoms.
- Chronic exposure to the interior of WDB led to both single-system and multisystem illness. For instance, 102 studies specifically examined respiratory symptoms, while 62 studies examined immune system symptoms. The papers focused on the respiratory System found such symptoms in 100 articles (98.0%). Fully 96.8% (60 of 62) of the studies found immune system abnormalities. See Table 1 for a more thorough breakdown by Body System.
- Over 250 associations of increased dampness/microbial growth and adverse human health effects, with odds ratios (OR) or relative risks (RR) ≥2.0, were documented in the 79 studies including either OR or RR data. Over 380 associations were found using OR or RR ≥1.5.
| Body System | Studies Supportive / Studies Reviewing that System | % of Studies Supportive of Health Effects in that System |
|---|---|---|
| Respiratory | 100/102 | 98.0 |
| Immunological | 60/62 | 96.8 |
| General | 24/24 | 100 |
| Cognitive | 16/16 | 100 |
| Ophthalmic | 16/16 | 100 |
| Neurological | 10/10 | 100 |
| Gastrointestinal | 9/9 | 100 |
| Musculoskeletal | 8/8 | 100 |
| Dermatological | 14/15 | 93.3 |
Evaluation of the percentage of articles reviewing a particular system that found adverse human health effects with chronic exposure to elevated indoor dampness or microbial growth.
Finally, adding in the peer-reviewed publications from Dr. Ritchie Shoemaker and his group, discussed individually below, there is overwhelming evidence for the general causation of the 37 diagnostic symptoms found in CIRS (chronic inflammatory response syndrome). These same studies demonstrated abnormal VCS findings, diagnostic lab abnormalities, Neuroquant (NQ) patterns of brain damage, GENIE (Gene Expression: Inflammation Explained) transcriptomic deviations and improvement documentation with appropriate therapy. There are numerous articles demonstrating the existence of reproducible, objective biomarkers capable of distinguishing CIRS cases from healthy control subjects. Finally, several articles have also demonstrated patient improvement with appropriate therapy.
All taken together, these many papers create robust evidence for the general causation of subjective symptoms and objective irregularities found reproducibly in patients chronically exposed to the interior of WDB, in general, and diagnosed with CIRS, specifically.
We will now shift this analysis to specific papers published in the CIRS corpus of literature, which support the reproducibility of symptoms, improvement with CIRS treatment strategies, VCS testing, lab abnormalities, imaging irregularities, and transcriptomic aberrations found in CIRS patients.
The first CIRS paper was published in 1997. Pfiesteria exposure to fish kills has led to a heretofore unrecognized multisystem illness in humans exposed to it. This case report was the inauspicious beginning to answer a question Dr. Shoemaker did not know was being asked. What happens to a patient when the innate immune system becomes dysregulated by chronic exposure to one or more biotoxins?
A follow-up paper added more patient experience. Cognitive issues were explored in Lancet article. The CDC featured what they called PEAS Possible Estuary-Associated Syndrome after cases were identified in 6 of the United States. They offered a two-tiered case definition, requiring the development of symptoms (memory loss or confusion, and three of headaches, skin rash at the point of water contact, burning skin, eye irritations, upper respiratory irritation, muscle cramps, and GI symptoms) within 2 weeks of the alleged exposure, as reported in a 1999 MMWR (Morbidity and Mortality Weekly Report).
Shoemaker documented the effectiveness of cholestyramine (CSM) as a treatment and introduced VCS (visual contrast sensitivity) testing as an objective biomarker. A second article touting the benefits of CSM treatment and VCS testing was published by Shoemaker and Ken Hudnell in 2001. Also in 2001, the BASE Study published by the U.S. EPA (Environmental Protection Agency), documented that 50% of U.S. residences and up to 85% of commercial buildings had evidence of historic or current water damage. In 2004, the Department of Defense declared that “several mold species commonly found indoors were identified as potential human health hazards.”
Shortly after Hurricane Katrina, Shoemaker travelled to New Orleans. He evaluated the crew of a cruise ship as the control group. Residents of flooded and destroyed homes were also on board. Later published online, Shoemaker’s letter to a variety of local and national leaders documented that 239 controls and 282 cases were evaluated using symptom clusters and VCS testing. A chi-square analysis demonstrated that using VCS with symptom clusters was able to differentiate cases from controls with 98.85% accuracy and with p-values <1 x 10-7.
In 2006, Shoemaker published a paper evaluating symptoms and VCS at five time points: before treatment, after 2 weeks of CSM therapy, 2 weeks after treatment was completed (and CSM had been stopped), after intentional re-exposure to treatment, and after re-treatment with two additional weeks of CSM. At the first point, the patients averaged 23 out of 37 diagnostic symptoms, and all participants failed the VCS tests. Measurements of MMP-9 (matrix metalloproteinase -9) and MSH (α-melanocyte-stimulating hormone) were abnormal in 84.6% and 96.2%, respectively. The average time to onset of symptoms was 11 months. After dropped to an average of 4 (82.5% reduction), with 65% improvement in their VCS scores, and MMP-9 levels decreased to control levels. At the third point of CSM therapy, patients had retained their symptom improvements, VCS results and MMP-9 levels, and were then re-exposed. Symptoms returned to pre-treatment levels, and VCS scores plunged. After retreatment with CSM for 2 weeks, symptom scores, MMP-9, and VCS improved to control levels.
A subset of this trial underwent a placebo-controlled double-blind challenge with CSM therapy. Those receiving 2 weeks of CSM experienced a reduction in symptoms from 24.7 to 2.86 (88.1% improvement), while the placebo group started with 20.8 symptoms and finished with 20.3 (2.4% improvement). The results were significantly significant with a p-value <0.001. This study also included a listing of the 37 CIRS diagnostic symptoms and the percentage of patients suffering each symptom at all 5 Time points. Shoemaker understood the pathogens were molds, bacteria and/or actinobacteria.
In 2006, Shoemaker and House also published another paper examining patients at the same five time-points. Five different WDB exposures were documented. Patients at Time point 1 had a minimum of 4 body systems involved and averaged 14.9 symptoms and failed VCS testing. After 2 weeks of CSM, symptoms reduced to an average of 1.2 (91.9% improvement), and ~50% of subjects were passing their VCS tests. MSH levels improved with therapy.
Another mold illness pioneer, Dr. Eckhardt Johanning, edited his scholarly treatise “Bioaerosols, Fungi, Bacteria, Mycotoxins and Human Health” in 2006, including Shoemaker contributing his 3-tiered case definition for CIRS. This case definition was an extension of the 1999 CDC case definition for PEAS.
William Fisk concluded that indoor dampness and mold exposure were associated with substantial and statistically significant increases in both URIs and acute bronchitis. David Mudarri estimated the U.S. cost of mold associated with allergic rhinitis, bronchitis and asthma to be $3.7, $1.9 and $15.1 billion, respectively.
In 2007, Steve Vesper, working for the EPA, published the first work on ERMI (environmental relative moldiness index). Included is a list of the geometric means of 36 molds found in 1096 U.S. homes. In 2010, he wrote that ERMI was a validated test. In 2021, he documented that the already validated ERMI test, performed by vacuuming, was equivalent to an ERMI test conducted via electrostatic cloths (Swiffer). Shoemaker wrote his first paper extolling the virtues of ERMI. Most recently, Vesper published that ERMI has been used successfully in homes, schools and large buildings.
The 2008 GAO (U.S. Government Accountability Office) report on indoor mold included a three-tiered algorithm for determining the causation of any mold-based illness, which paralleled Shoemaker’s 2006 case definition. It also highlighted the immune system’s effects on mold and suggested that this could be a potential pathway to human illness. Hundell also published a book²⁹ with a chapter suggesting cyanobacteria could cause the syndrome we now call CIRS. Shoemaker also noted that children with CFS/ME³⁰ had the same lab abnormalities as people with CIRS. CFS/ME, an illness with no biomarkers, was shown to be CIRS in these children.
The 2009 WHO (World Health Organization) report noted that non-allergic immune system health effects could be a plausible mechanism of injury leading to symptoms in humans. The Policyholders of America 2010 review article¹ first revealed the name CIRS. There were over 600 unique citations
of environmental and medical studies in this 162-page article. Fourteen worldwide studies, comprising more than 40,000 subjects, were highlighted. There was also a section rebutting junk science, written in 2002 by defense witnesses “experts” for the American College of Occupational and Environmental Medicine (ACOEM) and in 2006 for the American Academy of Asthma, Allergy, and Immunology (AAAAI). These articles are discussed in greater detail below. Cigaterra³¹ was also addressed as a CIRS.
NIOSH, a branch of the CDC, produced a brilliant article³². They compared a very water-damaged High School (LA) with a minimally damaged one (OH). Expert visual assessment and comprehensive environmental testing (including spore trap, contact, bulk, and ERMI) demonstrated the differences between these buildings quite authoritatively. Employees of the WDB school (LA) reported statistically significantly more symptoms (of 22) in all four body systems reviewed and a decreased VCS in all five columns compared to the OH school.
Shoemaker reported the lab results of multiple cohorts of cases and controls³³ in a groundbreaking study demonstrating p-values <0.001 for almost all the CIRS diagnostic tests. VIP was introduced as an effective treatment for the first time.
Shoemaker defined a pattern of brain damage (enlarged forebrain parenchyma and cortical grey matter, atrophic caudate nucleus) in the very first CIRS NeuroQuant® paper³⁴. The pattern was confirmed³⁵ as well as a new pattern revealed for CIRS after developing Lyme disease (enlarged right thalamus and atrophic putamen). This study also documented that patients treated with the CIRS protocol, sans VIP, would see their enlarged brain volumes return to control values. The caudate did not revert. However, the following year, Shoemaker showed a stoppage of caudate atrophy³⁶ with some recovery in volume using VIP therapy. Finally, Shoemaker combined the power of GENIE to define the specific causation⁹ of exposure with a new pattern of damage, characterized by atrophic cortical grey and/or enlarged superior lateral ventricles, in the context of bacterial endotoxin exposure.
Dr. Keith Berndston compiled a list³⁷ of 30 different substances found in WDB capable of activating the innate immune system. Later that year, Dr. Jimmy Ryan described CIRS transcriptomics, laid the foundations of MHM³⁸ (molecular hypometabolism), and discussed the sarcin-ricin loop and ribosome-inactivating proteins (RIPs), all of which are mycotoxins. Later that year, the first IEP/Provider Consensus statement³⁹ was released, discussing medically sound mold inspection and remediation practices.
In 2017, Dr. Shoemaker linked HERTSMI-2 scores and human health⁴⁰. Dr. Scott McMahon developed a method of approximating the two previous case definitions used for diagnosing CIRS without requiring demonstration of improvement⁴¹. This study documented that symptom clusters have high sensitivity. In contrast, multiple abnormal lab tests had high specificity for CIRS and were the first article to show the prevalence of CIRS in children at a rate of at least 7.01%. McMahon also developed VCS standards for children⁴² and conducted a second prevalence evaluation, which found at least a 7.6% prevalence in children.
A 2018 summary article⁴³ reviewed the diagnosis of CIRS, the treatment protocol, known imaging findings, physical exam abnormalities, Lyme and other CIRS triggers, ancillary testing and pulmonary hypertension.
Shoemaker⁴⁴ assailed urinary mycotoxins. A truly groundbreaking paper⁴⁵ tied GENIE findings to MHM, proliferative physiology, pulmonary hypertension, insulin resistance, T reg cell deficiency and neuronal injury. Blazing a new path more brightly, Shoemaker demonstrated that transcriptomic testing could reveal the specific causation⁴⁶ of environmental exposure. This was followed by another groundbreaking discovery, which connected GENIE, MHM, and Long-COVID through specific causation to actinobacteria⁴⁷ and bacterial endotoxin.
Dr. Aruna Bakhru co-edited (with Dr. April Vukelic) a medical textbook⁴⁸ on CIRS and ancillary treatment strategies. The first 15 chapters are peer-reviewed, discussing multiple aspects of CIRS written by several prominent authors.
Shoemaker discussed “Triple positives” and linked them⁴⁹ to Parkinson’s Disease (PD). In 2025, he identified triple positives with endotoxin-specific causationʳ causation, demonstrating large dispersion in another 24 genes as a potential early screen for PD⁵⁰. Dooley later evaluated eight studies regarding mold exposure, CIRS, and chronic fatigue⁵¹.
This summarizes the scientific and medical literature that supports the acquisition of human illness as a result of chronic exposure to the interior of WDB. There is a small corpus of publications suggesting the opposite. This review evaluated four such articles, each of which is frequently cited in legal settings and reports.
The mother of the remaining editorials was initially the position statement⁵² of the American College of Occupational and Environmental Medicine (ACOEM). The article that emerged in 2002, was revised without author attribution in 2011 and was abruptly “unsetted” (an article removed from circulation because it was no longer scientifically relevant or accurate) by the ACOEM in 2014. This publication attempted to disprove, via mathematical proof, that sufficient mycotoxins could not accumulate in the space of a WDB to cause serious human illness. In violation of epidemiological principles, the authors used six rodent studies to disprove causation in humans. All but one of these studies examined a single-dose regimen, conflating one-time dosing with chronic dosing, which violated toxicological principles. Numerous fallacious constants were used in the created mathematical model, which led to a cumulative error in the calculation of mycotoxin inhaled and their effects, on the order of billions. Additionally, the half-life of mycotoxins was reported to be in the range of days, whereas subsequent data showed that the half-life of Ochratoxin A in mouse brains⁵³ was as long as 28 years. The model also addressed only toxins acting as toxins and ignored immune stimulation from spores and spore fragments. Several other serious methodological errors were committed, relegating this article to the status of “junk science” (untested or unproven hypotheses or theories presented as scientific fact, especially in a court of law).
Three other pieces of note⁵⁴,⁵⁵,⁵⁶ all claim the premise that the ACOEM article demonstrated that it is very unlikely to accumulate enough mycotoxin to cause serious human illness. These three articles accept the mathematical model as gospel truth, even though the model is merely a hypothesis and has never been tested. This defines junk science. Indeed, a testable prediction of this model would be that humans do not show illness after chronic exposure to the interior of WDB. The evidence presented throughout this review strongly contradicts that prediction. Articles built on the chassis of junk science are also junk. None of these editorials include any human data. Humans were not “researched.” Additionally, almost all of the authors of these four articles are individuals who routinely testify as expert witnesses for the defense, i.e., on behalf of insurance companies, in lawsuits where plaintiffs allege they were injured by chronic exposure to moldy buildings. The Wall Street Journal⁵⁷ specifically named two of these articles⁵²,⁵⁴ for this reason, as well as the failure of the authors and experts to declare their potential conflicts of interest. This author testifies on behalf of plaintiffs and defendants.
In summary, when assessing the mold-based illness literature, one can reasonably conclude, to a high degree of medical certainty, that chronic exposure to the interior of water-damaged buildings leads to single-system and multisystem illnesses in humans. An example of a single-system malady would be IgE-mediated allergic rhinitis, whereas CIRS would exemplify a multisystem disease. Quantitatively, hundreds of studies have been cited or alluded to in this review, documenting chronic exposure as a leading cause of human illness, while a handful suggest the opposite.
Qualitatively, the principles of evidence-based medicine (EBM) must be invoked. Evaluating the papers referenced above, which support chronic exposure to WDB causing human illness, includes a systematic review, two randomized controlled treatment trials, two experimental studies, and a large contingent of epidemiological studies involving over 310,000 total subjects. Each of these studies is based on human data. Of the four articles cited that support the view that chronic exposure to elevated indoor dampness/microbial growth does not lead to adverse human health effects, three are opinion pieces, and one uses rodent data to disprove causation in humans. Each of these four articles lacks any human data. There is no comparison when evaluating the quality of EBM between these two corpora of mold-based illness literature. Therefore, overwhelmingly, the evidence is one-sided for the case that chronic exposure to the interior of WDB leads to single and multisystem human illness. General causation has been emphatically demonstrated. To espouse otherwise would be suggestive of ignorance of literature or frank bias.
SECTION 6 Andrew Heyman MD
CIRS, GENIE and NeuroQuant
Chronic Inflammatory Response syndrome represents a model of trained immunity, persistent inflammation and a shift towards hypometabolism (HM), aerobic glycolysis and proliferative physiology. Our data also suggests a direct association between the degree of HM and areas of nuclear atrophy demonstrated by NeuroQuant. A proposed model is emerging, drawn from AD and DM research, that aerobic glycolysis and proliferative physiology are a compensatory mechanism induced by mitochondria to protect the CNS from further injury. Additionally, modifying factors that may further accelerate atrophic changes appear to include APOe4, hyperglycemia, and reduction of NAD+ leading to oxidative stress, depleted glutathione and impaired capacity for neuronal repair.
Induction of monocytes and macrophages, via trained immunity, may be one inciting event to adopt Aerobic glycolysis (AG) as part of a broader modified adaptive immune response to tissue stress and injury. We have proposed recent research demonstrating Actinobacteria as a common microbe present in buildings with amplified microbial growth associated with CIRS. Until now, no plausible mechanism has been identified linking Actinobacteria to initiation of the inflammatory process. One alluring hypothesis is the role of liberated β-glucans from fungal cell walls produced by release of β-glucanases from Actinobacteria. This may well be one of the priming events responsible for the cascade of genomic, cellular, and tissue level consequences of CIRS via the dectin pathway.
Amplification of cytokine release due to repeated exposure to LPS, Gram -+ve bacteria, and fungal compounds leads to subsequent events that further push the mitochondrial molecular shift down the dectin-1/Akt/HIF1α pathway, resulting in destructive chronic inflammation and induction of compensatory aerobic glycolysis and proliferative physiology. More research needs to be conducted to assess this linked sequence of events. Additionally, if validated, clinical evaluation for hyperglycemia, presence of APOe4 allele, and measures of oxidative stress may be warranted for the assessment of the CIRS patient.
Trained Immunity and Priming Events
Immunological memory in vertebrates is usually attributed to T and B cell function. Recently it has been shown that innate immune responses following initial exposure may also afford protection against re-exposure, via ‘trained immunity’ by inducing memory in monocytes and macrophages. Trained immunity is the likely mechanism behind the non-specific protective effects of certain vaccines. At the same time, an increased inflammatory responsiveness of monocytes and macrophages could also play a central role in inflammatory diseases. The capacity of innate immunity to mount adaptive responses both redefines the normal host defense and identifies a novel potential therapeutic target in human diseases¹.
Trained monocytes display a specific molecular profile, including high glucose consumption, lactate production, and increased NAD⁺/NADH ratio, reflecting a shift in metabolism with an increase in glycolysis, dependent on activation of mammalian target of rapamycin (mTOR) through a dectin-1/Akt/HIF1α pathway. Furthermore, epigenetic reprogramming at the level of histone H3 methylation has been proposed as the molecular mechanism responsible for the long-term memory of innate immunity”¹.
β-Glucans
One common model to induce trained immunity involves the role of β-Glucans, the major Candida cell wall structure. Initial exposure to β-glucan induces monocytes to respond to lipopolysaccharide (LPS) with greater amplitude 7 days after “priming,” as the result of the activation of a pathway involving Akt/mTOR and HIF1α. Monocytes exposed to β-glucan for 24 h switch to aerobic glycolysis, and via epigenetic modifications sustain this metabolic choice for at least 7 days after having been “primed”¹.
“Primed monocytes are more responsive to LPS treatment as measured by the increased amount of TNFα that they secrete when compared to un-primed cells. Importantly, the switch to aerobic glycolysis during the first 24 h of priming is an absolute requirement for monocytes to undergo ‘training,’ together with epigenetic modifications and activation of the Akt/mTOR/HIF1α pathways.”¹ Additionally, the trained immune response directly primes T cells toward the Th17 lineage, IL-1 by promoting transcriptional cofactors.¹
Actinobacteria and β-Glucans
Actinobacteria are a known generator of β-glucans. The phylum Actinobacteria is recognized as the foremost taxonomic group amid the currently recognized main lineages within the Bacteria domain. The majority are free-living and are extensively disseminated in terrestrial and aquatic environments. The name Actinobacteria derived from Greek words aktis or aktin for ray and mukes for fungi, since the hyphae grow by the combination of tip extension and branching.
Conventionally, they were considered as an intermediate form between bacteria and fungi. They are aerobic, sporulating, Gram-positive bacteria, pervasive in many habitats including deep-sea vent sediments, deepest areas of Mariana Trench, and Antarctic deep-freezing soils¹.
Streptomyces is considered the “chief chemist in nature” with the ability to biotransform certain organic compounds into economically valued products. They actively participate in the putrefaction of various organic compounds and are widely exploited in food, leather, detergent, textile, pharmaceutical and medical industries¹.
Actinobacteria have been shown to produce a wide array of β-glucanases, posing an intriguing mechanism by which priming events may occur in human monocytes and macrophages, initiating trained immunity, a shift towards aerobic glycolysis and induction of chronic inflammation.
Aerobic Glycolysis: A Molecular Adaptation to Tissue Injury
The term aerobic glycolysis was coined by Otto Warburg at the beginning of the 20th century to explain the unconventional metabolism exhibited by tumor cells. Warburg noticed that malignant cells prefer to convert glucose to lactate even in the presence of oxygen, in contrast to the metabolism of healthy/differentiated cells, where glucose is usually converted into pyruvate and only converted to lactate in the absence of oxygen. The conversion of glucose to lactate in anaerobic conditions (absence of oxygen) was already known as anaerobic glycolysis and thus he defined the metabolism of cancer cells as aerobic glycolysis, to underline that the fate of glucose is not determined by the lack of oxygen.
“Aerobic glycolysis is now thought to play a larger role in tissue injury and repair. When metabolic stress is of sufficient magnitude to cause cell death, a reallocation of cellular resources for defense, damage containment, innate immunity, and repair at the expense of normal differentiated tissue function. Gap junctions between cells are decreased or lost as the tissue structure is
disrupted by injury, and cell-autonomous functions become primary and metabolic cooperation between neighboring cells is decreased or suspended. Platelets and neutrophils are recruited to sites of injury or infection. In response, aerobic glycolysis is upregulated systemically by mitochondrial alterations (M0 phenotype), to support stem cell recruitment and cell division needed for biomass replacement of lost cells. If cell loss is not replaced, then age-related atrophy and sarcopenia occur¹.
Thus, we can pose intriguing questions: can altered cellular metabolism determine seemingly unrelated cellular functions and do cells use aerobic glycolysis to accomplish and regulate these functions?
Aerobic Glycolysis: Neuroprotection and Glucose Deprivation
Studies indicate the newborn’s brain is about 13% of body mass but consumes up to 60% of total body energy, and this high level of energy utilization lasts throughout one’s entire childhood. Notably, aerobic glycolysis (AG) comprises 30% of glucose metabolism in a developing brain, compared to about 10% in an adult brain, indicating an important role of glycolysis in brain development¹.
“Moreover, during pregnancy and infancy, brain volume and weight sharply increase, with brain size reaching about 75% of an adult’s brain by 2 years old, compared to 25% at birth. Since neurogenesis mainly occurs prenatally—although some regions, such as the cerebellum, continue to generate after birth—rapid postnatal brain growth is mostly attributed to axon growth, dendritic morphogenesis, synaptic proliferation/elimination and axon myelination. This is a period when a brain meets both its highest energy demand and highest level of AG.
Elevated level of glycolysis during childhood correlates to the child’s highest rate of synaptic growth. They also discovered that in adult brain regions with the highest AG, genes that are responsible for synapse formation and growth are significantly increased. Glycolysis is important in synaptic plasticity and as a link between glycolytic function and motor adaptive learning.”¹
Even though studies are limited, the developing brain is considered to be predominantly glycolytic. This is largely due in support of developmental processes such as synaptogenesis, which ultimately leads to proper neuronal network that underlies cognitive function¹.
The Aging Brain
Accumulation of neurotoxic Aβ plaques and hyperphosphorylation of tau have long been considered the pathological hallmarks that contribute to synaptic disruption and neuronal loss in the brains of AD patients. It is reported that Aβ distributes variably among brain regions, with more deposition found in areas of high dependence on glycolysis. In a PET study of 33 neurologically healthy participants, AG was significantly elevated in the medial, lateral, and prefrontal cortices, whereas the cerebellum and medial temporal lobes exhibited lower glycolysis when compared to the mean value of the brain¹.
Follow-up studies reported that the regions with increased glycolysis in the resting state of healthy young adults closely mirror the later regional pattern where Aβ accumulates in the brains of AD patients. The correlation observed in these studies is considered a compensatory mechanism in response to Aβ toxicity and mitochondrial dysfunction at a very early stage of AD.
Further Risk: ApoE
ApoE is the primary cholesterol carrier in the brain and must be produced locally. It is predominantly synthesized by astrocytes and to a lesser extent by microglia, vascular smooth muscle cells, and the choroid plexus. In addition, studies showed that under normal conditions, neurons also produce ApoE. This may be responsible for ~20% of total ApoE protein levels in the cortex. Particularly, the intense induction of ApoE expression has been observed in injured or stressed neurons, indicating a critical role of this neuron-specific source of ApoE expression in cellular repair and maintenance.¹
References
Shoemaker
1. Diagnosis of Pfiesteria-human illness syndrome. Md Med J 1997 521-523L
2. Shoemaker R. Treatment of persistent Pfiesteria-human illness syndrome. Md Med J 1998; 47:64-66.
LARK
1. Donlan, R. M., & Costerton, J. W. “Biofilms: Survival mechanisms of clinically relevant microorganisms.” Clinical Microbiology Reviews, vol. 15, no. 2, 2002, pp. 167–193.
2. Etzel, R. A. “Stachybotrys.” Pediatric Clinics of North America, vol. 48, no. 5, 2001, pp. 1189–1201.
3. Gareis, M., et al. “Toxic effects of mycotoxins in humans.” Archives of Toxicology, vol. 64, 1990, pp. 117–122.
4. Hanski, I., et al. “Environmental biodiversity, human microbiota, and allergy are interrelated.” PNAS, vol. 109, no. 21, 2012, pp. 8334–8339.
5. Li, D. W., & Haugland, R. A. “Gene sequences reveal fungal identities in building-related illnesses.” Mycopathologia, vol. 156, 2003, pp. 129–136.
6. Lighthart, B. “Bioaerosols associated with plants.” Applied and Environmental Microbiology, vol. 63, no. 5, 1997, pp. 1839–1845.
7. Pascual, J., et al. “Actinobacteria in special habitats and their metabolic potential.” Frontiers in Microbiology, vol. 6, 2015, p. 1198.
8. Reponen, T., et al. “Viable mold propagules in indoor air.” Environmental Health Perspectives, vol. 105, no. 6, 1997, pp. 633–640.
9. Rizzatti, G., et al. “Intestinal microbiota: Involvement in human health and disease.” Frontiers in Cellular and Infection Microbiology, vol. 7, 2017, p. 140.
10. Shoemaker RC, House DE. Sick building syndrome (SBS) and exposure to water-damaged buildings: Time series study, clinical trial and mechanisms. NTT 2006; 28: 573-588.
11. Straus, D. C. “Molds, mycotoxins, and sick building syndrome.” Toxicology and Industrial Health, vol. 25, no. 9–10, 2009, pp. 617–635.
McMahon References
1. Policy Holders of America: Research Committee Report on Diagnosis and Treatment of Chronic Inflammatory Response Syndrome Caused by Exposure to the Interior Environment of Water-Damaged Buildings (2010).
2. Dooley M, McMahon SW. A comprehensive review of mold research literature from 2011 – 2018. Internal Medicine Review. 2020;3(1):1-39.
3 Shoemaker R. Residential and recreational acquisition of possible estuary-associated syndrome: A new approach to successful diagnosis and treatment. Environmental Health Perspectives 2001;109:791-796
4 Shoemaker R, Hudnell K. Possible Estuary-Associated Syndrome: Symptoms, vision, and treatment. Environmental Health Perspectives 2001;109:539-545.
5 Shoemaker R, House D, Ryan J. Vasoactive intestinal polypeptide (VIP) corrects chronic inflammatory response syndrome (CIRS) acquired following exposure to water-damaged buildings. Health 2013;5(3):396-401.
6 Shoemaker R. House D, Ryan J. Structural brain abnormalities in patients with inflammatory illness acquired following exposure to water damaged buildings: A volumetric MRI study using NeuroQuant®. Neurotoxicology Teratol. 2014;45:18-26.
7 McMahon SW, Shoemaker RC, and Ryan. Reduction in forebrain parenchymal and cortical grey matter swelling across treatment groups in patients with inflammatory illness acquired following exposure to water-damaged buildings. J Neurosci Clin Res 1:1. April 12, 2016
8 Ryan C, Shoemaker RC. RNA-Seq on patients with chronic inflammatory response syndrome (CIRS) treated with vasoactive intestinal peptide (VIP) shows a shift in metabolic state and innate immune functions that coincide with healing. Medical Research Archives, Volume 4, Issue 7. November 2016.
9 Shoemaker R, Heyman A, Lark D. Transcriptomics and brain volumetrics define the causes of cognitive impairment in patients with CIRS and support the use of VIP in treatment. Medical Research Archives. March 2023. 11(3):online.
10 Shoemaker RC, House DE. Sick building syndrome (SBS) and exposure to water-damaged buildings: Time series study, clinical trial and mechanisms. NTT 2006; 28: 573-588.
11 Shoemaker RC, Katz D, Ackerley M, Rapaport S, McMahon SW, Berndston K, Ryan JC. Intranasal VIP safely restores volume to multiple grey matter nuclei in patients with CIRS. Internal Medicine Review. 2017;1-14.
12 Shoemaker R. Diagnosis of Pfiesteria-human illness syndrome. Maryland Medical Journal 1997; 521-523.
13 Shoemaker R. Treatment of persistent Pfiesteria-human illness syndrome. Maryland Medical Journal 1998; 47: 64-66.
14 Grattan L, Oldach D, Perl T, Lowitt M, Matuszak D, Dickson C, Parrott C, Shoemaker R, Kauffman L, Wasserman M, Hebel R, Charache P, Morris G. Learning and memory difficulties after environmental exposure to waterways containing toxin-producing Pfiesteria or Pfiesteria-like dinoflagellates. The Lancet 1998; 352: 532-539
15 US Environmental Protection Agency. Indoor air quality in buildings: Building assessment survey and evaluation (BASE) study. www.epa.gov/iaq/base
16 Taylor K, Clifford R, Kollmeyer B, Pielak J. Toxic mold assessment, mitigation, and prevention at DoD facilities. Federal Facilities Environmental Journal. July 2004. https://doi.org/10.1002/ffej.20014
17 Shoemaker R, Lipsey R. Results of health screening and visual contrast testing. St. Bernard’s Parish, Louisiana. 2006. published on-line.
18 Shoemaker RC, House DE. Sick building syndrome (SBS) and exposure to water-damaged buildings: Time series study, clinical trial and mechanisms. Neurotoxicology and Teratology. 2006;28:573-588.
19 Shoemaker R, House DE. A time-series study of sick building syndrome: chronic, biotoxin-associated illness from exposure to water-damaged buildings. Neurotoxicology and Teratology. 2005;27:29-46.
20 Shoemaker RC, Rash JM, Simon EW. Sick Building syndrome in water damaged buildings: generalization of the chronic biotoxin associated illness paradigm to indoor toxigenic fungi. Bioaerosols, fungi, bacteria, mycotoxins and human health. Dr med Eckardt Johanning MD editor 2006.
21 Fisk W.J, Eliseeva EA, Mendell MJ. Association of residential dampness and mold with respiratory tract infections and bronchitis: a meta-analysis. Environ Health 2010. 9(72). https://doi.org/10.1186/1476-069X-9-72
22 Mudarri DH. Valuing the economic costs of allergic rhinitis, acute bronchitis, and asthma from exposure to indoor dampness and mold in the US. Journal of Environmental and Public Health. 2016. https://doi.org/10.1155/2016/2386596
23 Vesper S, McKinstry C, Haugland R, Wymer L, Bradham K, Ashley P, Cox D, Dewalt G, Friedman, Warren PhD. Development of an Environmental Relative Moldiness Index for US Homes. Journal of Occupational and Environmental Medicine 49(8):p 829-833, August 2007. | DOI: 10.1097/JOM.0b0 13e3181255e98
24 Reponen T, Vesper, Levin L, Johansson E, Ryan P, Burkle J, Grinshpun SA, Zheng S, Bernstein D I, Lockey J, Villareal M, Khurana Hershey GK, LeMasters G. High environmental relative moldiness index during infancy as a predictor of asthma at 7 years of age. Annals of Allergy, Asthma & Immunology. 2011;107(2):120-126. https://doi.org/10.1016/j.anai.2011.04.018
25 Vesper S, Wymer L, Cox D, Dewalt G, Pinzer E, Friedman W, Ashley PJ. Comparison of ERMI results from dust collected from homes by an electrostatic cloth and by the standard vacuum method. Journal of Occupational and Environmental Hygiene. 2021;18(9):423–429. https://doi.org/10.1080/15459624.2021.1946254
26 Shoemaker R, Lin K. Inside indoor air quality: Environmental relative moldiness index (ERMI). Filtration News. 2007;32:36.
27 Vesper SJ. The development and application of the environmental relative Moldiness index (ERMI). Critical Reviews in Microbiology. 2025;51(2):285–295. https://doi.org/10.1080/1040841X.2024.2344112
28 GAO Report to the Chairman, Committee on Health, Education, Labor and Pensions, U.S. Senate, Indoor Mold. In: 2008.
29 Hudnell HK. Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs. Springer. New York, New York. 2008.
30 Shoemaker R, Maizel M. Exposure to interior environments of water-damaged buildings causes a CFS-like illness in pediatric patients: a case/control study. Bulletin of the IACFS. 2008.
31 Shoemaker R, House D, Ryan J. Defining the neurotoxin derived illness chronic ciguatera using markers of chronic systemic inflammatory disturbances: A case/control study. Neurotoxicology and Teratology 2010; 633-639.
32 Thomas G, Burton NC, Mueller C, Page E, Vesper S. Comparison of work-related symptoms and visual contrast sensitivity between employees at a severely water-damaged school and a school without significant water damage. American Journal of Industrial Medicine. 2012;55(9):844-854.
33 Shoemaker R, House D, Ryan J. Vasoactive intestinal polypeptide (VIP) corrects chronic inflammatory response syndrome (CIRS) acquired following exposure to water-damaged buildings. Health 2013;5(3):396-401.
34 Shoemaker R. House D, Ryan J. Structural brain abnormalities in patients with inflammatory illness acquired following exposure to water damaged buildings: A volumetric MRI study using Neuroquant®. Neurotoxicology Teratol 2014;45:18-26.
35 McMahon SW, Shoemaker RC, and Ryan. Reduction in Forebrain Parenchymal and Cortical Grey Matter Swelling across Treatment Groups in Patients with Inflammatory Illness Acquired Following Exposure to Water-Damaged Buildings. J Neurosci Clin Res 1:1. April 12, 2016.
36 Shoemaker RC, Katz D, Ackerley M, Rapaport S, McMahon SW, Berndston K, Ryan JC. Intranasal VIP safely restores volume to multiple grey matter nuclei in patients with CIRS. Internal Medicine Review. 2017;1-14.
37 Medically sound investigation and remediation of water-damaged buildings in cases of chronic inflammatory response syndrome. Berndtson K, McMahon S, Ackerley M, Rapaport S, Gupta S, Shoemaker R. January 19, 2016.
38 Ryan JC, Shoemaker RC. RNA-Seq on patients with chronic inflammatory response syndrome (CIRS) treated with vasoactive intestinal peptide (VIP) shows a shift in metabolic state and innate immune functions that coincide with healing. Medical Research Archives. 2016;4(7):online.
39 Indoor Environmental Professionals Panel of Surviving Mold CONSENSUS STATEMENT Medically sound investigation and remediation of water-damaged Buildings in cases of CIRS-WDB. Larry Schwartz CIEC, BSME, MBA, Greg Weatherman CMC, Michael Schrantz CIEC, CMI, BPI-BA/EP, Will Spates CIAQP, CIEC, Jeff Charlton, ACIEC, AACIEH, Keith Berndtson MD, Ritchie Shoemaker MD April 12, 2016.
40 Shoemaker, RC and Lark, D – 2016, HERTSMI-2 and ERMI: “Correlating Human Health Risk with Mold Specific qPCR in Water-Damaged Buildings” #658 in Proceedings of the 14th International Conference on Indoor Air Quality and Climate, International Society for Indoor Air Quality and Climate, Ghent, Belgium.
41 McMahon, SW. An Evaluation of Alternate Means to Diagnose Chronic Inflammatory Response Syndrome and Determine Prevalence. Medical Research Archives, 2017;5(3):1-18.
42 McMahon SW, Kundomal KA, Yangalasetty S. Pediatrics Norms for Visual Contrast Sensitivity Using an APT VCS Tester. Medical Research Archives 2017;5(5):1-9.
43 Shoemaker RC, Johnson K, Jim L, Berry Y, Dooley M, Ryan J, McMahon SW. Diagnostic Process for Chronic Inflammatory Response Syndrome (CIRS): A Consensus Statement Report of the Consensus Committee of Surviving Mold. Internal Medicine Review, 2018;5(4):1-47.
44 Shoemaker RC. Urinary mycotoxins: A review of contaminated buildings and food in search of a biomarker separating sick patients from controls. Internal Medicine Review. 2019;5(6):1-35.
45 Shoemaker RC, Metabolism, molecular hypometabolism and inflammation: Complications of proliferative physiology include metabolic acidosis, pulmonary hypertension, Treg cell deficiency, insulin resistance and neuronal injury. Trends in Diabetes and Metabolism. 2020;3:1-15.
46 Shoemaker R, Neil V, Heyman A, van der Westhuizen, McMahon S, Lark D. Newer molecular methods bring new insights into human- and building-health risk assessments from water-damaged buildings: defining exposure and reactivity, the two sides of causation of CIRS-WDB illness. Medical Research Archives. 2021;9(3):1-36.
47 Shoemaker R, McMahon S, Heyman A, Lark D, van der Westhuizen M. Treatable metabolic and inflammatory abnormalities in post COVID syndrome (PCS) define the transcriptomic basis for persistent symptoms: Lessons from CIRS. Medical Research Archives. 2021;9(7):1-18.
48 Nutrition and Integrative Medicine for Clinicians Volume Two. Edited Aruna Bakrhu. CRC Press. 2023.
49 Shoemaker R, Ryan J, Heyman A, Dorninger E, McMahon S, Lark D. A Transcriptomic fingerprint for Parkinson’s Disease found in patients with Chronic Inflammatory Response Syndrome: Implications for diagnosis, treatment and prevention. Medical Research Archives. 2023;12(10):online.
50 Shoemaker R, Ryan J, Heyman A, Dorninger E, McMahon S, Lark D. A Novel therapeutic approach to Parkinsons Disease: Using transcriptomics to identify unique patterns of gene expression including triple positives, Toll receptors, and endotoxin exposure. Medical Research Archives. 2025 Accepted but not in print.
51 Dooley M. McMahon, S. Fatigue and exposure to mold and/or dampness: A systematic review of the literature from 2011-2018. EAHT. 2025;40:online.
52 Hardin B, Kelman B, Saxon A. Adverse Human Health Effects Associated with Molds in the Indoor Environment. Journal of Occupational and Environmental Medicine. 2003;45(5):470-478.
53 Sanchez-Ramos J. Brain’s DNA Repair Response to Neurotoxicants. Tampa University, Florida. Annual report. 1 Jul 2004-30 Jun 2005. Available at: http://www.dtic.mil/dtic/tr/fulltext/u2/a452374.pdf
54 Bush RK, Portnoy J, Saxon A, Terr AI, Wood RA. The Medical Effects of Mold Exposure. The Journal of allergy and clinical immunology. 2006; 117(2):326-323.
55 Chang C, Gershwin ME. Mold Hysteria: Origin of the Hoax. Clinical & Developmental Immunology 2005;12(2):151-158.
56 Chang C, Gershwin ME. The Myth of Mycotoxins and Mold Injury. Clinical Reviews in Allergy & Immunology 2019;57(48). DOI:10.1007/s 12016-019-08767-4.
57 Armstrong D. Amid Suits over Mold Experts Wear Two Hats. Wall Street Journal. 1/9/2007.
Heyman References
1.Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JH, Rao NA, Aghajanirefah A, Manjeri GR, Li Y, Ifrim DC, Arts RJ, van der Veer BM, Deen PM, Logie C, O’Neill LA, Willems P, van de Veerdonk FL, van der Meer JW, Ng A, Joosten LA, Wijmenga C, Stunnenberg HG, Xavier RJ, Netea MG. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014 Sep 26;345(6204):1250684. doi: 10.1126/ science.1250684. Erratum in: Science. 2014 Nov 7;346(6210):aaa1503. van der Meer, Brian M J W [corrected to van der Veer, Brian M J W]. PMID: 25258083; PMCID: PMC4226238.
Ibid 1
Ibid 1
Ibid 1
Ibid 1
2. Barka EA, Vatsa P, Sanchez L, Gaveau-Vaillant N, Jacquard C, Meier-Kolthoff JP, Klenk HP, Clément C, Ouhdouch Y, van Wezel GP. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol Mol Biol Rev. 2015 Nov 25;80(1):1-43. doi: 10.1128/MMBR.00019-15. Erratum in: Microbiol Mol Biol Rev. 2016 Nov 9;80(4):iii. doi: 10.1128/MMBR.00044-16. PMID: 26609051; PMCID: PMC4711186.
Ibid 5
3..Naviaux RK. Incomplete Healing as a Cause of Aging: The Role of Mitochondria and the Cell Danger Response. Biology (Basel). 2019 May 11;8(2):27. doi: 10.3390/biology8020027. PMID: 31083530; PMCID: PMC6627909.
4. Zhang X, Alshakhshir N, Zhao L. Glycolytic Metabolism, Brain Resilience, and Alzheimer’s Disease. Front Neurosci. 2021 Apr 28 ;15 :662242. Doi: 10.3389/fnins.2021.662242. PMID : 33994936 ; PMCID : PMC8113697.
Ibid 11
Ibid 11
Ibid 11
5.Zhao N, Liu CC, Qiao W, Bu G. Apolipoprotein E, Receptors, and Modulation of Alzheimer’s Disease. Biol Psychiatry. 2018 Feb 15;83(4):347-357. doi: 10.1016/j.biopsych.2017.03.003. Epub 2017 Mar 14. PMID: 28434655; PMCID: PMC5599322.
Ibid 12
IBID
Thacker References
1.American Community Survey: 1-year estimates. (2023). U.S. Census Bureau. Census Reporter. http://censusreporter.org/profiles/01000US-united-states/
2. Berndtson, K., McMahon, S., Ackerley, M., Rapaport, S., Gupta, S., & Shoemaker, R. C. (2015). Consensus statement – Part 1: Medically sound investigation and remediation of water-damaged buildings in cases of CIRS-WDB. Center for Research on Biotoxin Associated Illness. https://www.survivingmold.com/MEDICAL_CONSENSUS_STATEMENT_10_30_15.PDF
3 Breathe Cities. (n.d.). Who we are. Clean Air Fund, C40 Cities & Bloomberg Philanthropies. Retrieved July 2, 2025, from https://breathecities.org/who-we-are/
4.Boyle, P. (2022, January 13). A data-based look at America’s physicians and medical students, state-by-state. AAMCNews. https://www.aamc.org/news/data-based-look-america-s-physicians-and-medical-students-state-state
5. Cho, S. H., Reponen, T., Bernstein, D. I., Olds, R., Levin, L., Liu, X., & LeMasters, G. (2005). The effect of home characteristics on dust antigen concentrations and loads in homes. Science of the Total Environment, 346(1–3), 131–144. https://doi.org/10.1016/j.scitotenv.2004.11.021
6. Dooley, M., & McMahon, S. W. (2020). A comprehensive review of mold research literature from 2011–2018. Internal Medicine Review, 6(1), 1–38. https://www.internalmedicinereview.org/index.php/imr/article/view/836
7. Fisk, W. J., Eliseeva, E. A., & Mendell, M. J. (2010). Association of residential dampness and mold with respiratory tract infections and bronchitis: A meta-analysis. Environmental Health, 9, Article 72. https://doi.org/10.1186/1476-069X-9-72
8. Heyman, A., & Shoemaker, R. (2025). Clinical management of Chronic Inflammatory Response Syndrome [Online continuing education course]. George Washington University School of Medicine and Health Sciences. https://cme.smhs.gwu.edu/a4m-mmi-/content/clinical-management-chronic-inflammatory-response-syndrome
9. Institute of Inspection, Cleaning and Restoration Certification. (n.d.). ANSI/IICRC S520: Standard for professional mold remediation. IICRC. Retrieved July 2, 2025, from https://iicrc.org/s520/
10. Insurance Information Institute. (2023). Facts + statistics: Homeowners and renters’ insurance. https://www.iii.org/fact-statistic/facts-statistics-homeowners-and-renters-insurance
11. Ivanova, I., & Layne, R. (2020, June 30). 15 million U.S. homes are at risk of flooding — 70% higher than FEMA estimates. CBS News MoneyWatch. https://www.cbsnews.com/news/15-million-homes-at-risk-of-flooding-new-data-from-first-street-foundation/
12. Jacobs, D. E. (2011). Environmental health disparities in housing. American Journal of Public Health, 101(S1), S115–S122. https://doi.org/10.2105/AJPH.2010.300058
13. Mayo Clinic. (1999, September 10). The study of the Mayo Clinic implies fungus as the cause of chronic sinusitis. ScienceDaily. https://www.sciencedaily.com/releases/1999/09/990910080344.htm
14. McMahon, S. W. (n.d.). About [Web page]. Retrieved July 2, 2025, from https://scottmcmahon.doctor/about

