Intravesical Capsaicin for Bladder Sensory Disorders
Intravesical Capsaicin as a Therapeutic Option for Bladder Sensory Disorders
Karl-Erik Andersson1,2
- Wake Forest Institute for Regenerative Medicine, Winston Salem, Winston Salem, NC, United States
- Department of Laboratory Medicine, Lund University, Lund, Sweden
OPEN ACCESS
PUBLISHED: 30 September 2025
CITATION: Andersson, KE., 2025. Intravesical Capsaicin as a Therapeutic Option for Bladder Sensory Disorders. Medical Research Archives, [online] 13(9). https://doi.org/10.18103/mra.v13i9.6940
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI https://doi.org/10.18103/mra.v13i9.6940
ISSN 2375-1924
ABSTRACT
Intravesical capsaicin has been used as a treatment for detrusor overactivity and bladder pain syndromes due to its desensitizing effect on C-fiber afferents. However, patient responses vary widely in both efficacy and tolerability. As a therapeutic option, intravesical capsaicin has largely been replaced by botulinum toxin. However, the drug may still be considered in select cases of refractory disease or within the context of research protocols.
Keywords
Intravesical capsaicin, bladder sensory disorders, detrusor overactivity, bladder pain syndromes, botulinum toxin.
Introduction
The first vanilloid (capsaicin) receptor, TRPV1, was cloned in 1997, which became the starting point for studies of the importance of this receptor for normal bladder function and dysfunction. Based on animal in vivo models, showing that intravesical capsaicin induces a reversible concentration-dependent detrusor hyperactivity that could be assessed by cystometry, intravesical capsaicin has been widely used in rats to study OAB/DO, painful bladder syndrome/interstitial cystitis (PBS/IC), neurogenic detrusor overactivity (NDO), and bladder outlet obstruction (BOO)-related OAB/DO. Even if these conditions are associated with abnormal afferent nerve activity and urgency, the underlying pathophysiology connecting these entities remains poorly understood. Still, a central hypothesis explaining the urgency and pain is the hypersensitization of bladder afferent pathways, resulting in the activation of silent C-fibers via TRPV1 receptors. The basis for the use of capsaicin in the above-mentioned disorders is the selective desensitization of C-fiber afferents via the TRPV1 receptor, making it a potential therapeutic agent when administered intravesically. Despite promising mechanistic rationale, intravesical capsaicin has yielded inconsistent clinical outcomes. Some patients experience marked symptom relief, while others report little benefit or discontinue treatment due to severe discomfort. This variability in response has decreased its clinical use. Thus, no clinical reports could be identified via PubMed or Embase over the last 10-year period. Recent systematic reviews of current intravesical NDO treatments did not include capsaicin, and the drug has been replaced by intravesical botulinum toxin. Capsaicin and botulinum toxin (BoNT) are both used for treating OAB, NDO, and IC/BPS due to their effects on sensory nerves. However, they work via different mechanisms and have distinct advantages and disadvantages.
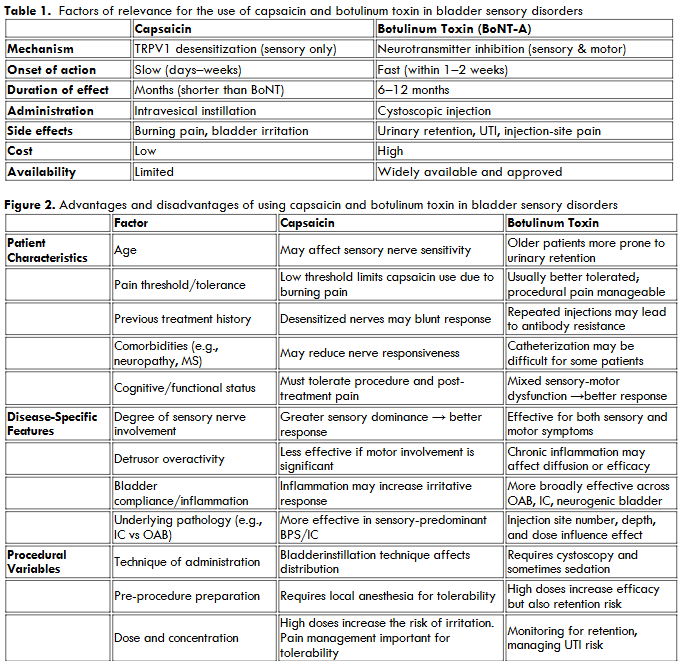
Methodology
Pubmed and Embase have been searched for articles related to the field published between 1990 and 2025. Factors influencing the response to intravesical vanilloids for more than three decades, intravesical administration of capsaicin/resiniferatoxin has been used as a treatment for bladder sensory disorders, such as NDO, OAB, and IC/BPS. However, the clinical response to vanilloids varies considerably among patients and is influenced by a combination of neurophysiological, pathological, and technical factors.
NEUROPHYSIOLOGICAL FACTORS
A key determinant of treatment response is the expression and distribution of transient receptor potential vanilloid 1 (TRPV1) receptors on C-fiber afferents in the bladder. Capsaicin/resiniferatoxin selectively target these receptors, leading to defunctionalization of sensory nerves and subsequent symptom relief. Variability in TRPV1 density, whether due to genetic polymorphisms, chronic inflammation, or disease progression, may therefore contribute significantly to the heterogeneity in therapeutic outcomes. Vanilloid treatment changes the gene expression profile in the bladder. Lepiarczyk et al. performed a transcriptomic characterization of the porcine urinary bladder trigone following intravesical administration of resiniferatoxin. Using multistep bioinformatics they identified 129 differentially expressed genes (DEGs), 54 upregulated and 75 downregulated. DEGs can be involved in nerve degeneration processes, but can also be implicated in the initiation of neuroprotective mechanisms. Analysis indicated that resiniferatoxin treatment influences the signaling pathways regulating nerve growth, myelination, axon specification, and elongation. Intravesical instillation of the drug induces changes in the expression of genes involved in synaptic plasticity and neuromodulation, including those related to 5-HT, H2S, glutamate, and GABA transmission. This suggests that resiniferatoxin may exert a therapeutic, antinociceptive effect not only by acting on TRPV1 receptors.
PATHOLOGICAL FACTORS
Patient-related factors, including pain sensitivity, age, sex, and genetic makeup, polymorphisms in the TRPV1 gene, may influence treatment responses. Elderly patients may have altered neural responsiveness or urothelial permeability, further complicating treatment outcomes. The etiology of the bladder disorder plays a critical role in the response to vanilloids. Intravesical capsaicin is most consistently effective in patients with NDO, particularly following spinal cord injury, where C-fiber hyperactivity is a well-established pathophysiological mechanism. OAB and IC/BPS, which may involve additional mechanisms such as urothelial dysfunction, mast cell activation, and central sensitization, often show more variable or limited responses. The urothelium normally acts as a protective barrier, and in individuals with intact epithelium, capsaicin penetration to the suburothelial nerve plexus may be limited, reducing efficacy. In patients with epithelial disruption—commonly seen in IC/BPS—drug permeability increases, which can enhance capsaicin’s effects but also predispose to increased discomfort or pain during instillation. If influenced by prior treatments such as botulinum toxin A, neuromodulation, or chronic inflammation, the sensitization state of afferent pathways can modulate the response to capsaicin. Pre-sensitized or desensitized nerves may respond less predictably, underscoring the importance of individualized evaluation before treatment.
TECHNICAL FACTORS
Dose, concentration, and formulation significantly affect capsaicin’s efficacy and tolerability. High doses can achieve profound desensitization, but are often associated with adverse effects such as burning, urgency, and discomfort during administration. The use of intravesical anesthetics (e.g., lidocaine) before capsaicin instillation has been shown to improve tolerability without impairing therapeutic action. Other technical aspects—including instillation technique, volume, dwell time, and prior bladder preparation—can alter drug distribution and mucosal contact time, affecting clinical results. Ensuring consistent administration protocols is essential for reducing procedural variability.
Conclusion
A multifactorial interplay of bladder pathology, neural receptor expression, individual biological characteristics, and procedural techniques can modulate the response to intravesical capsaicin. Although intravesical capsaicin has shown potential in desensitizing C-fiber afferents and alleviating symptoms of bladder hypersensitivity, its clinical use is now rare due to significant tolerability issues, limited availability, lack of regulatory support, and the emergence of more effective alternatives. Intravesical capsaicin has largely been replaced by botulinum toxin, but may still be considered in select cases of refractory disease or within the context of research protocols.
Conflicts of Interest
The author has no Conflicts of Interest
Funding
There is no funding for this article
References
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997 Oct 23;389(6653):816-24. doi: 10.1038/39807. PMID: 9349813.
- Andersson KE, Behr-Roussel D, Denys P, Giuliano F. Acute Intravesical Capsaicin for the Study of TRPV1 in the Lower Urinary Tract: Clinical Relevance and Potential for Innovation. Med Sci (Basel). 2022 Sep 10;10(3):50. doi: 10.3390/medsci10030050. PMID: 36135835; PMCID: PMC9504433.
- Kanai A, Andersson KE. Bladder afferent signaling: recent findings. J Urol. 2010 Apr;183(4):1288-95. doi: 10.1016/j.juro.2009.12.060. Epub 2010 Feb 19. PMID: 20171668; PMCID: PMC3686308.
- Andersson KE. TRP Channels as Lower Urinary Tract Sensory Targets. Med Sci (Basel). 2019 May 22;7(5):67. doi: 10.3390/medsci7050067. PMID: 31121962; PMCID: PMC6572419.
- Bielefeldt K, Lamb K, Gebhart GF. Convergence of sensory pathways in the development of somatic and visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol. 2006 Oct;291(4):G658-65. doi: 10.1152/ajpgi.00585.2005. Epub 2006 Feb 23. PMID: 16500917.
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991 Jun;43(2):143-201. PMID: 1852779.
- Giuliani S, Lecci A, Tramontana M, Maggi CA. Nociceptin protects capsaicin-sensitive afferent fibers in the rat urinary bladder from desensitization. Naunyn Schmiedebergs Arch Pharmacol. 1999 Aug;360(2):202-8. doi: 10.1007/s002109900047. PMID: 10494891.
- Cruz F. Desensitization of bladder sensory fibers by intravesical capsaicin or capsaicin analogs. A new strategy for treatment of urge incontinence in patients with spinal detrusor hyperreflexia or bladder hypersensitivity disorders. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9(4):214-20. doi: 10.1007/BF01901607. PMID: 9795827.
- Bapir R, Bhatti KH, Eliwa A, García-Perdomo HA, Gherabi N, Hennessey D, Magri V, Mourmouris P, Ouattara A, Perletti G, Philipraj J, Stamatiou K, Trinchieri A, Buchholz N. Efficacy of overactive neurogenic bladder treatment: A systematic review of randomized controlled trials. Arch Ital Urol Androl. 2022 Dec 28;94(4):492-506. doi: 10.4081/aiua.2022.4.492. PMID: 36576454.
- Yu PH, Wang CC. Adverse Effects of Intravesical OnabotulinumtoxinA Injection in Patients with Idiopathic Overactive Bladder or Neurogenic Detrusor Overactivity: A Systematic Review and Meta-Analysis of Randomized Controlled Studies. Toxins (Basel). 2024 Aug 5;16(8):343. doi: 10.3390/toxins16080343. PMID: 39195753; PMCID: PMC11359369.
- Kuo HC. Botulinum Toxin Paves the Way for the Treatment of Functional Lower Urinary Tract Dysfunction. Toxins (Basel). 2020 Jun 14;12(6):394. doi: 10.3390/toxins12060394. PMID: 32545870; PMCID: PMC7354673.
- Panunzio A, Tafuri A, Mazzucato G, Cerrato C, Orlando R, Pagliarulo V, Antonelli A, Cerruto MA. Botulinum Toxin-A Injection in Chronic Pelvic Pain Syndrome Treatment: A Systematic Review and Pooled Meta-Analysis. Toxins (Basel). 2022 Jan 1;14(1):25. doi: 10.3390/toxins14010025. PMID: 35051002; PMCID: PMC8780260.
- Nitti V, Haag-Molkenteller C, Kennelly M, Chancellor M, Jenkins B, Schurch B. Treatment of neurogenic detrusor overactivity and overactive bladder with Botox (onabotulinumtoxinA): Development, insights, and impact. Medicine (Baltimore). 2023 Jul 1;102(S1):e32377. doi: 10.1097/MD.0000000000032377. PMID: 37499088; PMCID: PMC10374192.
- de Sèze M, Wiart L, Ferrière J, de Sèze MP, Joseph P, Barat M. Intravesical instillation of capsaicin in urology: A review of the literature. Eur Urol. 1999 Oct;36(4):267-77. doi: 10.1159/000020004. PMID: 10473984.
- Juszczak K, Thor PJ. The basic neurophysiologic concept of lower urinary tract function–the role of vanilloid TRPV1 receptors of urinary bladder afferent nerve endings. Adv Clin Exp Med. 2012 Jul-Aug;21(4):417-21. PMID: 23240446.
- Holzer P, Izzo AA. The pharmacology of TRP channels. Br J Pharmacol. 2014 May;171(10):2469-73. doi: 10.1111/bph.12723. PMID: 24773265; PMCID: PMC4008994.
- Abdel-Salam OME, Mózsik G. Capsaicin, The Vanilloid Receptor TRPV1 Agonist in Neuroprotection: Mechanisms Involved and Significance. Neurochem Res. 2023 Nov;48(11):3296-3315. doi: 10.1007/s11064-023-03983-z. Epub 2023 Jul 26. PMID: 37493882; PMCID: PMC10514110.
- Ramos-Lopez O, Martinez-Aceviz Y, Sobrevilla-Navarro AA, Chavez-Mendez JR. Genetic Influence on Capsaicin Tolerance: Precision Nutrition Implications for Obesity Handling. Lifestyle Genom. 2024;17(1):57-63. doi: 10.1159/000539293. Epub 2024 May 29. PMID: 38810602.
- Lepiarczyk E, Maździarz M, Paukszto Ł, Bossowska A, Majewski M, Kaleczyc J, Łopieńska-Biernat E, Jaśkiewicz Ł, Skowrońska A, Skowroński MT, Majewska M. Transcriptomic Characterization of the Porcine Urinary Bladder Trigone Following Intravesical Administration of Resiniferatoxin: Insights from High-Throughput Sequencing. Toxins (Basel). 2025 Mar 9;17(3):127. doi: 10.3390/toxins17030127. PMID: 40137900; PMCID: PMC11946646.
- Szallasi A. Resiniferatoxin: Nature’s Precision Medicine to Silence TRPV1-Positive Afferents. Int J Mol Sci. 2023 Oct 10;24(20):15042. doi: 10.3390/ijms242015042. PMID: 37894723; PMCID: PMC10606200.
- Birder L, Andersson KE. Urothelial signaling. Physiol Rev. 2013 Apr;93(2):653-80. doi: 10.1152/physrev.00030.2012. PMID: 23589830; PMCID: PMC3768101.
- Lazzeri M, Beneforti P, Turini D. Urodynamic effects of intravesical resiniferatoxin in humans: preliminary results in stable and unstable detrusor. J Urol. 1997 Dec;158(6):2093-6. doi: 10.1016/s0022-5347(01)68164-3. PMID: 9366319.
- Hamid R, Averbeck MA, Chiang H, Garcia A, Al Mousa RT, Oh SJ, Patel A, Plata M, Del Popolo G. Epidemiology and pathophysiology of neurogenic bladder after spinal cord injury. World J Urol. 2018 Oct;36(10):1517-1527. doi: 10.1007/s00345-018-2301-z. Epub 2018 May 11. PMID: 29752515.
- Shimizu N, Saito T, Wada N, Hashimoto M, Shimizu T, Kwon J, Cho KJ, Saito M, Karnup S, de Groat WC, Yoshimura N. Molecular Mechanisms of Neurogenic Lower Urinary Tract Dysfunction after Spinal Cord Injury. Int J Mol Sci. 2023 Apr 26;24(9):7885. doi: 10.3390/ijms24097885. PMID: 37175592; PMCID: PMC10177842.
- Birder LA, de Groat WC. Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat Clin Pract Urol. 2007 Jan;4(1):46-54. doi: 10.1038/ncpuro0672. PMID: 17211425; PMCID: PMC3119256.
- Chandiramani VA, Peterson T, Duthie GS, Fowler CJ. Urodynamic changes during therapeutic intravesical instillations of capsaicin. Br J Urol. 1996 Jun;77(6):792-7. doi: 10.1046/j.1464-410x.1996.09844.x. PMID: 8705210.
- Avelino A, Cruz F, Coimbra A. Lidocaine prevents noxious excitation of bladder afferents induced by intravesical capsaicin without interfering with the ensuing sensory desensitization: an experimental study in the rat. J Urol. 1998 Feb;159(2):567-70. doi: 10.1016/s0022-5347(01)63985-5. PMID: 9649293.

