Placental Tissue Therapies for Diabetic Foot Ulcers
The exploration of the use of placenta in Diabetic Ulcer Disease: A Systematic Review
Daniela J. Arezina, Dan Li, PhD1
- Yale School of Public Health, 60 College Street, New Haven CT 06511
OPEN ACCESS
PUBLISHED: 31 December 2024
CITATION: Arezina, DJ., and Li, D., 2024. The exploration of the use of placenta in Diabetic Ulcer Disease: A Systematic Review. Medical Research Archives, [online] 12(12). https://doi.org/10.18103/mra.v12i12.5978
COPYRIGHT: © 2024 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI https://doi.org/10.18103/mra.v12i12.5978
ISSN 2375-1924
Abstract
This paper seeks to provide a comprehensive review of the utilization of placental tissue therapies in addressing the challenges posed by diabetic foot ulcers (DFUs). A systematic review approach was employed, utilizing the PubMed database to identify relevant research papers. Following the initial search, a rigorous screening process was undertaken, comprising two rounds of screening to ensure the inclusion of relevant studies. The review showed that placental tissue therapies are effective for diabetic foot ulcers (DFUs). They can be applied topically, injected, or implanted, leading to faster wound healing and tissue regeneration. Studies found quicker closure of wounds, shorter healing times, and better outcomes with these treatments. Placental tissues contain mesenchymal stem cells and growth factors, making them effective for diabetic foot ulcers management. They offer a promising way to speed up wound healing and enhance patient outcomes. Further research is warranted to validate these findings and explore optimal treatment protocols for maximizing the therapeutic benefits of placental tissue therapies in diabetic foot ulcers management.
Keywords
placenta, diabetic foot ulcers, placental tissue therapies, wound healing, mesenchymal stem cells
Introduction
Diabetes is a prevalent health concern affecting an estimated 537 million people worldwide, which poses a substantial risk for complications such as Diabetic Foot Ulcers (DFUs). Between 19-34% of individuals with diabetes will experience DFUs during their lifetime, or approximately 150 million globally. Factors causing DFUs include: neuropathy, poor circulation, foot deformities, and trauma. The consequences of these ulcers range from infection to potentially amputation.
Despite the widespread occurrence of Diabetic Foot Ulcers, a gap exists for effective treatment strategies, with amputation often presented as the best “solution”. The current approach lacks a concrete method for addressing DFUs, because DFU can recur after treatment in approximately 40% of patients treated within 1 year and 65% in 5 years. The presence of recurring infection shows the need for an alternative treatment.
Integrating placenta research offers potential solutions to mitigate the recurrence and severity of DFUs, providing a more sustainable approach to addressing this condition. Previous studies have demonstrated the efficiency of placental tissue therapies in wound healing and tissue regeneration. Placental tissues contain an abundance of mesenchymal stem cells and growth factors, which play crucial roles in promoting tissue repair and modulating the inflammatory response. These tissues possess unique immunomodulatory capabilities, which can regulate the inflammatory response and promote tissue regeneration in various pathological conditions. The extracellular matrix components in placental tissues maintain structural integrity and aid in cell functions like migration, proliferation, and differentiation during healing.
Furthermore, bioactive molecules like cytokines and growth factors in placental tissues coordinate signaling pathways, promoting tissue repair and remodeling. This approach to healing makes placental tissue therapies promising candidates not only for DFUs but also for a wide range of wound types and tissue injuries.
This paper aims to provide a systematic review for a novel and effective treatment for diabetic foot ulcers. By offering a comprehensive analysis and integrating placenta-derived therapies, as an alternative treatment approach, we can improve outcomes for patients suffering from DFUs. This paper could be used as a guideline for healthcare professionals offering insight into a more efficient and sustainable method for managing DFUs. The implications of this research, beyond individual patient care, address the importance of placental tissue in healing as well as the need for an effective treatment for DFUs.
Despite limitations, including the scope of this review and potential biases in the selected articles, this paper synthesizes current evidence and provides valuable insights for clinicians and insurance companies. In our result section we have analyzed and reviewed the mechanism, procedures, methods, long-term effects, complications, and ethics of the use of placental treatment on DFUs.
Research Methodology:
RESEARCH QUESTION OVERVIEW
Our research questions include: 1) How can placental tissues be used in order to treat Diabetic Ulcer Disease? 2) What does the procedure of placental healing entail? 3) What are the long term effects and outcomes of using placental tissues in the treatment of Diabetic Ulcer Disease? 4) What, if any, complications exist in the use of placental tissue to treat Diabetic Ulcer Disease?
PUBMED SEARCH STRATEGY:
The search strategy we utilized was (“Placenta”[MeSH Terms] OR “placenta*”[Title/Abstract]) AND (“ulcer*”[Title/Abstract] OR “Ulcer”[MeSH Terms]).
SCREENING PROTOCOL
We went through two rounds of screening. The first round of screening we only read the title and abstract of the paper. Then we chose yes or no and grouped whether we should keep it or not. For the second round of screening we took the papers we said yes to and read the full text of the manuscript retrieved by our search strategy to determine if we will continue to use this paper. We conducted two rounds of screening – we screened the title and abstract, as well as the full text of the manuscript retrieved by our search strategy.
INCLUSION CRITERIA
The population for this review is people who suffer from Diabetic Ulcer Disease. The concept of this review is being able to use the placenta tissue in order to treat DFUs. This systematic review is not limited to a specific geographical location. Our source of evidence is the Pubmed database.
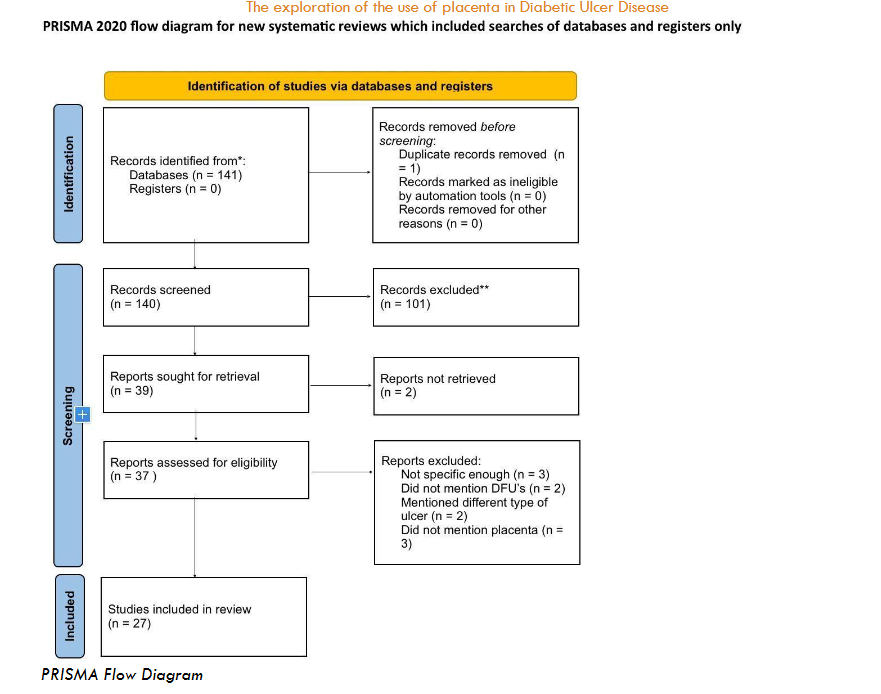
FLOWCHART/DATA EXTRACTION
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart was used to visualize the numerical outputs from reviews and the inclusion decision process. In our flow chart, we clearly illustrated the process for finding studies, removing duplicates, selecting the right research, retrieving the full article from the library and presenting the final analysis. We developed a charting template to facilitate the extraction of data.
We started the first round of screening with 141 articles from a database using a search term. We removed one record before the screening; one duplicate but no ineligible entries were identified. We screened each title and abstract of the articles on the database, and through the first round of screening we excluded 101 articles. For the second round of screening, we used 39 articles for retrieval. We screened the entire paper for 37 of the retrieved and eliminated 10 reports in this second screening. These were excluded due to their lack of specificity or failure to align with the criteria of the systematic review. We used the remaining 27 articles for systematic review.
Results Section
1. THE MECHANISM OF WHY WE CAN USE PLACENTAL TISSUES IN ORDER TO TREAT DIABETIC ULCER DISEASE
Placental tissues are highly effective in treating diabetic foot ulcers (DFUs) due to their regenerative capacity, structural composition, and abundance of growth factors. These tissues contain mesenchymal stem cells (MSCs) that promote cell proliferation, angiogenesis, and immune modulation, essential processes for wound healing.
The amniotic membrane, with its structural similarity to skin layers, provides a natural scaffold for cellular proliferation and tissue regeneration, making it ideal for DFU treatment. Rich in growth factors such as Epidermal Growth Factor (EGF), Transforming growth factor-β (TGF-β), Vascular endothelial growth factor (VEGF), and Platelet-derived growth factor (PDGF), placental tissues support cell proliferation, angiogenesis, extracellular matrix synthesis, and tissue remodeling.
1.1 General
Placental tissues exhibit remarkable similarities to skin in terms of their regenerative capacity, structural composition, and abundance of growth factors. This regenerative ability enables them to promote tissue repair and wound healing in diabetic foot ulcers (DFUs). The presence of mesenchymal stem cells (MSCs) within placental tissues plays a crucial role in facilitating regeneration by promoting cell proliferation, angiogenesis, and immunomodulation. The structural composition of placental tissues closely resembles that of skin, providing a natural scaffold for cellular proliferation and tissue regeneration. The amniotic membrane consists of distinct layers, including an epithelial layer, basement membrane, and mesenchymal layer, mirroring the structure of epidermis, dermis, and subcutaneous tissue found in skin. This structural similarity allows for seamless integration and interaction with host tissues, facilitating the healing process. Placental tissues are rich in growth factors essential for wound healing, similar to those present in healthy skin. These growth factors include the epidermal growth factor (EGF), transforming growth factor-beta (TGF-β), vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF). The presence of these growth factors promotes cell proliferation, angiogenesis, extracellular matrix synthesis, and tissue remodeling, key processes in wound repair. Placental tissues retain their biological activity even after processing, making them valuable therapeutic agents for DFU treatment. Specialized preservation techniques, such as lyophilization and gamma irradiation, help maintain the integrity of the extracellular matrix and preserve the viability of cells and growth factors within the tissue. This ensures that placental tissues retain their regenerative potential and efficacy when used clinically to treat DFUs.
1.2 Regenerative property
Placental tissues offer a promising avenue for treating diabetic foot ulcers (DFUs) due to their regenerative properties. Mesenchymal stem cells (MSCs) derived from placental tissues promote essential processes like angiogenesis and immune modulation, crucial for wound repair. Additionally, the angiogenic factors present in placental tissues, along with the secretion of angiogenic factors by MSCs, contribute to improved wound healing outcomes, highlighting the interconnectedness of regenerative mechanisms in DFU management.
Mesenchymal stem cells (MSCs) derived from placental tissues are pivotal in promoting diabetic foot ulcer (DFU) healing due to their multifaceted regenerative properties. These cells have been shown to promote angiogenesis, re-epithelialization, and immune modulation, essential processes in wound repair. Placental MSCs exhibit a higher proliferation capacity compared to those from other sources, enhancing their therapeutic potential in DFU management. Their ability to differentiate into various cell types, including fibroblasts and endothelial cells, contributes to tissue regeneration and angiogenesis, ultimately facilitating wound closure. Moreover, MSCs derived from placental tissues play a regulatory role in modulating inflammatory and immune responses associated with DFUs, promoting a favorable microenvironment for wound healing.
Angiogenesis, the process of new blood vessel formation, is critical for supplying oxygen and nutrients to the wound site, facilitating tissue repair in DFUs. Placental tissues, particularly the amniotic membrane, contain factors that stimulate angiogenesis, including vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF). These growth factors promote endothelial cell proliferation, migration, and vessel formation, enhancing blood flow to the wound area. Additionally, MSCs derived from placental tissues secrete angiogenic factors, further promoting the formation of new blood vessels in the wound bed. Studies have demonstrated that placental-derived MSCs enhance angiogenesis in DFUs, leading to improved wound healing outcomes. Overall, the angiogenic properties of placental tissues, mediated by MSCs and growth factors, play a crucial role in promoting tissue revascularization and wound closure in DFUs.
1.3 Similarity to skin
The amniotic membrane (AM) presents an ideal option for treating diabetic foot ulcers (DFUs) due to its structural and compositional similarities to skin components. With layers resembling those of the dermis and epidermis, including vital proteins like collagen and elastin, the AM provides a conducive environment for cellular adherence and migration during wound healing. Moreover, its rich content of growth factors and cytokines, akin to those in healthy skin, fosters cell proliferation, angiogenesis, and matrix remodeling, expediting the wound healing process. Consequently, the structural parallels between the AM and skin underscore its potential as an effective biomaterial for managing DFUs.
The amniotic membrane (AM) shares several key components with the skin, making it an ideal candidate for diabetic foot ulcer (DFU) treatment. Structurally, the AM consists of an epithelial layer, a basement membrane, and a stromal layer rich in extracellular matrix (ECM) proteins such as collagen and elastin. This composition resembles that of the dermis and epidermis of the skin, providing structural support and promoting cellular adherence and migration during wound healing. Furthermore, the AM contains various growth factors and cytokines, including vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and transforming growth factor-beta (TGF-β), which play pivotal roles in tissue repair and regeneration. These bioactive components stimulate cell proliferation, angiogenesis, and matrix remodeling, accelerating the wound healing process. The presence of these growth factors in the AM mirrors those found in healthy skin, highlighting its therapeutic potential in DFU management.
The structure of the skin, particularly the dermis and epidermis, shares striking similarities with the components of the amniotic membrane (AM), underscoring its regenerative capacity for diabetic foot ulcer (DFU) treatment. The dermis, comprising collagen fibers, elastin, and fibroblasts, provides structural integrity and elasticity to the skin, akin to the stromal layer of the AM. This resemblance facilitates cell migration and tissue remodeling, crucial processes in wound repair. Similarly, the epidermis of the skin, composed of epithelial cells and a basement membrane, functions as a protective barrier against pathogens and dehydration, a role mirrored by the epithelial layer of the AM. Moreover, both skin and AM contain resident stem cells and immune cells that contribute to tissue homeostasis and immune surveillance, further enhancing their regenerative potential. The structural similarities between the AM and skin underscore its suitability as a biomaterial for DFU management, offering a supportive matrix rich in growth factors and cytokines essential for wound healing.
1.4 Growth and Inflammatory factors
There are several different techniques that can be employed to preserve the growth factors present in placental tissues, each affecting the efficacy of treatment modalities for diabetic foot ulcers (DFUs). Cryopreserved placental membrane (vCPM) retains the integrity of the extracellular matrix (ECM) and viable cells while preserving growth factors through cryopreservation, ensuring their bioactivity upon application. This technique maintains the regenerative potential of mesenchymal stem cells (MSCs) and other bioactive factors, including vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and basic fibroblast growth factor (bFGF), essential for angiogenesis and tissue repair. Conversely, devitalized/dehydrated amniotic membrane (dHAMA) lacks viable cells but retains the ECM and growth factors, providing structural support and promoting wound healing through paracrine signaling. While devoid of living cells, dHAMA still exhibits anti-inflammatory and pro-regenerative properties attributed to its preserved growth factor content.
Moreover, decellularized human amniotic membrane (dHAM) eliminates immunogenicity by removing cellular components, leaving behind the ECM scaffold enriched with growth factors such as epidermal growth factor (EGF), transforming growth factor-beta (TGF-β), and interleukins. This technique ensures biocompatibility and reduces the risk of adverse immunological reactions, enhancing the safety and efficacy of placental tissue-based therapies for DFUs. Lyophilization and gamma irradiation are utilized to sterilize and preserve the structural and biochemical integrity of amniotic membranes, ensuring their suitability for clinical applications without compromising growth factor activity. Collectively, these techniques facilitate the sustained release of growth factors and cytokines, orchestrating the complex cascade of events necessary for effective wound healing in DFUs.
2. PROCEDURES FOR UTILIZING PLACENTAL TISSUES IN DIABETIC ULCER HEALING
The use of placental tissues in DFU requires meticulous preparation and preservation to ensure their safety and effectiveness. There are essential steps involved in preparing these tissues for clinical application, followed by an examination of various preservation methods that sustain their viability and therapeutic potential.
2.1 Preparation:
These therapies, derived from prepared placental membranes, are cultured and loaded onto scaffolds in vitro, ensuring optimal safety and efficacy. Studies such as Oesman et al. have demonstrated diligent preparation methods for placental membrane treatments. The preparation process involves washing the fresh amniotic membrane with sterile saline, followed by thorough sterilization using 0.05% sodium hypochlorite solution and gamma irradiation. This method ensures the removal of blood particles and pathogens, rendering the membrane safe for clinical application. These prepared placental tissues are then applied to debrided wound beds and covered with non-adherent dressings. These therapies are biodegradable and do not require removal before repeat application, minimizing patient discomfort and simplifying treatment. Moreover, Luck et al. discussed a similar preparation process for placental products highlighting the necessity of culturing and loading onto scaffolds in vitro before application to the wound bed. Their research highlights the significance of preserving the integrity and viability of placental tissues in order to maximize their efficacy. This emphasizes how crucial it is to have a “standard preparation protocol” in order to improve placental tissue therapy for DFUs in terms of clinical effectiveness.
Additionally, the harvested placental tissues were extensively washed with ice-cold phosphate-buffered saline (PBS) and then manually minced. The tissues were digested in 0.1% collagenase IV at 37°C for 1 h followed by a cell strainer filtration to eliminate undigested debris. Primary human dermal fibroblasts were co-cultured with PMSCs in separate six-well plates in a transwell cell culture system. Fibroblast cells were serum starved for 24 h prior to any experiment, and after serum starvation, fibroblast cells were stimulated with LPS.
In the majority of cases, sharp debridement was performed to expose viable bleeding tissue in the ulcer bed, and negative pressure wound therapy was used as necessary. Dehydrated Human Umbilical Cord (DHUC) was rehydrated for approximately 10 seconds in a saline bath, and the DHUC EX was cut to fit the desired area after rehydration of the product. It is important to ensure the safety as well as the effectiveness of placental tissue therapies in the management of DFUs. These studies have provided effective preparation methods that guarantee the safety and effectiveness of placental tissue therapies, laying the groundwork for effective DFU management through application procedures.
2.2 Preserving Methods
In utilizing placental tissues for therapeutic purposes, three main preserving methods are commonly employed: cryopreservation, room temperature preservation, and dehydration. Each method presents its unique advantages and challenges, directly influencing the viability of placental tissue therapies. Understanding these preservation techniques is paramount for optimizing their application in managing diabetic foot ulcers (DFUs). Placental membrane treatments, such as dehydrated amniochorionic (dHACM) and viable cryopreserved human placental membrane (vCHPM), as well as lyophilization (vLPM), have demonstrated significant success rates in achieving complete wound closure within relatively short treatment durations.
The process of preserving placental tissues through cryopreservation involves freezing them at ultra-low temperatures to maintain their viability and biological activity for extended periods. Similarly, viable cryopreserved human placental membrane (vCHPM) applications have resulted in rapid granulation and closure rates, with 96.3% of patients achieving 100% granulation of their index wound within 16 weeks post-application. The study using Grafix PL PRIME illustrated the beneficial healing rates associated with placental tissue therapies.
Additionally, studies have reported the immunomodulatory properties of placental-derived mesenchymal stem cells (MSCs) in promoting wound healing. MSCs harvested from healthy donors induce proliferation of fibroblasts and endothelial cells and accelerate wound healing. The study by Frykberg et al. used MSCs in diabetic mice and improved the activation of vascular endothelial cells and induced angiogenesis through paracrine vascular endothelial growth factor (VEGF) signaling. These cryopreserved placental tissues show the therapeutic potential in healing DFUs, offering accelerated wound closure and improved patient outcomes.
There are certain challenges that arise when storing placental tissue at room temperature, mostly in maintaining their continuity and integrity over time. While studies evaluating the effectiveness and limitations of room temperature preservation methods are limited, further research is needed to explore the efficiency of these methods. However, recent advancements in lyophilization techniques have enabled the storage and preservation of living tissue at room temperature. Studies evaluating the effectiveness of viable lyopreserved placental membrane (vLPM) for the treatment of chronic wounds have demonstrated promising outcomes. Maguire et al. reported significant improvements in wound healing outcomes in patients treated with vLPM, with a substantial proportion achieving complete wound closure after treatment. Transplantation of placental-derived MSCs has shown promise in promoting wound healing by suppressing pro-inflammatory cytokine secretion and increasing the production of anti-inflammatory cytokines such as interleukin-10 (IL-10) at the wound site. These findings indicate the potential of room temperature-preserved placental tissues in diabetic ulcer management, but further research and investigation must be conducted on this preservation method to fully utilize their clinical utility.
Dehydration, on the other hand, is a method utilized for preserving placental tissues, eliminating water content while retaining their structural and biological properties. Notably, patients treated with dehydrated human amnion/chorion membrane (dHACM) achieved a 97% rate of complete wound closure within a 12-week study period, surpassing standard wound care and other cellular tissue products (CTPs). This finding elicits the potential of dehydrated placental tissue products in accelerating the healing process of DFUs. Similarly, Shah et al. demonstrated the benefits of dehydrated amniotic membrane (dHACM) in promoting wound closure in patients with chronic diabetic foot ulcers (DFUs), with 92% of wounds treated with dHACM, reaching complete closure within 6 weeks. Dehydrated placental tissue products offer advantages such as prolonged shelf life and ease of storage compared to fresh or cryopreserved tissues, making them a practical option for clinical use in diabetic ulcer management. These studies suggest the significant potential for dHACM in expediting DFU healing, with placental tissue therapies emerging as clinically effective alternatives for enhancing DFU healing.
2.3 Special techniques and Considerations
Among the variety of techniques for treating diabetic foot ulcers (DFUs), the human amnion/chorion membrane (HACM) allograft emerges as a promising avenue. Derived from placental membranes, HACM retains crucial growth factors and ECM components vital for wound healing, showcasing efficacy in promoting re-epithelialization and inflammation resolution. Furthermore, its low immunogenicity allows for allogeneic transplantation without human leukocyte antigens (HLA) matching, reducing treatment barriers and facilitating widespread clinical use. Alongside human amnion/chorion membrane (HACM), advanced techniques like lyophilization, decellularization, and cryopreservation further enhance the therapeutic potential of placental tissues, offering innovative solutions for challenging DFU cases and improving patient outcomes.
Among the special techniques utilized for treating diabetic foot ulcers (DFUs), the human amnion/chorion membrane (HACM) allograft stands out as a promising option. Derived from the placental membrane, the HACM allograft retains the structural integrity and bioactivity of the native tissue, comprising growth factors, cytokines, and extracellular matrix (ECM) components critical for wound healing. Clinical studies have demonstrated the efficacy of HACM allografts in promoting re-epithelialization, angiogenesis, and inflammation resolution in DFUs, resulting in accelerated wound closure and improved clinical outcomes. Moreover, the low immunogenicity of placental tissues allows for allogeneic transplantation without the need for HLA matching, facilitating widespread clinical use and reducing treatment barriers.
In addition to human amnion/chorion membrane (HACM) allografts, other advanced techniques such as lyophilization, decellularization, and cryopreservation are employed to enhance the therapeutic properties of placental tissues for DFU management. Lyophilization preserves the structural and biochemical integrity of amniotic membranes, enabling their storage at room temperature while maintaining growth factor activity. Decellularization removes cellular components from placental tissues, minimizing immunogenicity and optimizing biocompatibility for allograft transplantation. Cryopreservation ensures the long-term viability of mesenchymal stem cells (MSCs) and growth factors in placental tissues, preserving their regenerative potential for DFU treatment. Collectively, these special techniques harness the regenerative capacity of placental tissues, offering innovative solutions for challenging DFU cases and improving patient outcomes.
3. METHODS OF UTILIZING PLACENTAL TISSUE IN DIABETIC ULCER TREATMENT
The use of placental tissue in treating DFUs involves various preparation and application methods to maximize their regenerative properties. These methods include preparing the tissue to retain its bioactive components and employing pharmacological and practical application techniques to enhance healing outcomes.
3.1 Types of preparation of tissue
Due to its rich environment of growth hormones and regenerative qualities, placental tissue has been prepared in various ways to treat diabetic foot ulcers (DFUs). Techniques like the preparation of dehydrated human amnion/chorion membrane (DHACM), such as EpiFix, involve dehydration while preserving structural integrity and bioactive components. Cryopreserved products like Grafix retain native components through cryopreservation, maintaining crucial growth factors and cellular elements for wound healing. Advanced techniques such as decellularization and lyophilization remove cellular components while preserving the extracellular matrix and growth factors. These methods highlight the versatility of placental tissue in DFU management, offering various therapeutic options for patients with chronic wounds.
3.2 Pharmacological preparation of the patient
The pharmacological preparation of patients receiving placental tissue-based therapies is crucial for optimizing treatment outcomes. Pharmacological interventions aim to create an environment conducive to wound healing and enhance the therapeutic efficacy of placental products. For instance, patients undergo systemic or local pharmacological interventions in order to modulate inflammatory responses, promote angiogenesis, and improve tissue regeneration. In preclinical studies, local injection and intravenous infusion of mesenchymal stem cells (MSCs) have been shown to accelerate wound healing in animal models of type 2 diabetes mellitus (T2DM), highlighting the potential of pharmacological interventions to enhance the regenerative effects of placental tissue. Moreover, the administration of growth factors or cytokines can complement placental-based therapies, further augmenting tissue repair processes and promoting wound closure. Thus, pharmacological preparation techniques are essential for maximizing the therapeutic benefits of placental tissue-based therapies.
3.3 Application of Placenta:
Placental tissue can be applied to DFUs through various modalities, including topical application, injection, and implantation, each offering unique advantages and mechanisms of action.
Topical application involves the direct placement of placental membrane products onto the wound surface, facilitating the delivery of bioactive factors and creating a conducive environment for wound healing. This method typically begins with thorough cleaning and debridement of the ulcer to remove necrotic tissue and debris, ensuring optimal contact between the placental membrane and the wound bed. The placental membrane, such as dehydrated human amnion/chorion membrane (DHACM) or cryopreserved placental membrane products like Grafix, is then carefully applied to cover the ulcer area, conforming to the wound’s contours. In some cases, the membrane may be secured with sutures or adhesive dressings to prevent displacement and ensure continuous contact with the wound surface throughout the healing process. Topical application provides sustained release of growth factors and cytokines, and a way for those factors to directly seep into the wound, promoting tissue regeneration and wound closure over time.
Injection involves delivering placental-derived cells or growth factors directly into the ulcer site, targeting specific areas of tissue damage and promoting angiogenesis, re-epithelialization, and immune regulation. This method typically begins with the preparation of the placental-derived material, which may involve isolating mesenchymal stem cells (MSCs) or extracting growth factors and cytokines from placental tissue. The prepared solution is then injected into the periphery of the ulcer, either intradermally or subcutaneously, using a fine-gauge needle. Careful attention is paid to the injection technique to ensure precise placement of the solution within the wound margins, maximizing its therapeutic effects. By avoiding potential obstacles to wound healing and expediting tissue repair processes, injection-based therapies provide specific administration of bioactive materials.
Implantation techniques involve the surgical placement of placenta, such as dehydrated human amnion/chorion membrane (DHACM) or amnion-based grafts, directly onto the wound bed. This method typically begins with the preparation of the placental membrane, which may involve cutting or sizing the material to fit the dimensions of the ulcer. The prepared membrane is then placed over the wound surface, ensuring complete coverage and contact with the underlying tissue. In some cases, the membrane may be secured with sutures or surgical staples to prevent displacement and promote adherence to the wound bed. Implantation-based therapies provide structural support to the wound, facilitating tissue regeneration and promoting wound closure over time. The selection of the optimal application method depends on various factors, including the severity of the ulcer, patient comorbidities, and treatment goals, highlighting the importance of personalized approaches in the management of DFUs.
4. LONG-TERM EFFECTS
There are several long-term effects of placental treatment that can span between weeks, months, and years. The amount of time leads to different effects in the aftermath of treatment.
4.1 Weeks
Within the initial 11 weeks of treatment, patients undergoing placental tissue therapies experience major improvements in wound healing and symptom relief. Studies have shown that patients achieving complete wound healing within this timeframe often maintain their “healing status” in the subsequent weeks, indicating the durability of the treatment. The mean time to heal with placental tissue interventions is notably shorter compared to standard of care (SOC), with patients experiencing faster closure rates and reduced healing times. For instance, a study done on patients with chronic DFUs indicated that the mean time to heal was significantly faster for the group treated with dehydrated human amnion/chorion membrane (dHACA) compared to SOC, with a mean healing time of 37 days versus 67 days. Additionally, the mean time to heal in wounds treated with viable human amniotic membrane allografts (vHAMAs) was 46.6 days, indicating a relatively rapid healing process. The use of placental tissue products, such as Grafix, has shown a high probability of wound closure, with a mean time to closure of 42 days in treated patients. These findings, collectively, highlight the overall success of placental tissue therapies in the early stages of diabetic ulcer management, with patients experiencing accelerated wound closure and improved healing outcomes in an extremely short period of time.
4.2 Months
Placental treatment progresses from 12 weeks to 6 months, placental tissue therapies continue to demonstrate sustained efficacy in promoting wound healing and patient outcomes. Studies reveal that a significant proportion of patients achieve complete ulcer healing within this timeframe, with favorable long-term results observed. For example, in randomized controlled trials (RCTs), significant improvements within the intervention group were reported, having up to 92.5% of patients achieving complete wound healing by 12 weeks. Follow-up data further indicated that patients who experience ulcer closure within 3 months often maintain durable healing from 6 months up to 1 year post-treatment, emphasizing the enduring benefits of placental tissue interventions. These retrospective studies demonstrate a median time of healing of 26 weeks, further supporting the long-term effectiveness of these therapies in the management of DFUs. These findings collectively show the benefits of placental tissue therapies in the time frame of 3-6 months of healing.
4.3 Years
Beyond the 6-month mark, placental tissue therapies continue to exert lasting benefits on DFUs. Studies examining mortality outcomes at 365 days post-treatment reveal superior survival rates for patients receiving placental tissue therapy compared to other interventions, examining the long-term effects of these treatments. Specifically, the application of viable cryopreserved human placental membrane (vCPM) indirectly results in an average mortality rate of 12.3%, significantly lower than mortality rates associated with other cellular and acellular interventions. Furthermore, patients treated with placental tissue therapies exhibit enhanced tissue regeneration, reduced mortality rates, and improved quality of life. For example, at the 6-month mark following treatment, patients exhibited no recurrence, with health education interventions playing a significant role in preserving patient well-being and averting additional complications. Additionally, the structural integrity and biochemical activity of dehydrated human amniotic membrane tissue, which can be stored at ambient conditions with a shelf-life of up to 5 years, contribute to reduced mortality rates and enhanced wound healing. These results demonstrate the promise of placental tissue therapies in managing diabetic foot ulcers, providing patients with a viable option for long-term treatment aimed at achieving sustained healing.
5. COMPARING THE BENEFITS OF PLACENTAL TISSUE TO OTHER TREATMENTS FOR DIABETIC ULCER DISEASES
Placental tissue treatments offer unique regenerative benefits that are compared with conventional methods such as debridement, wound dressing, offloading, antibiotics, hyperbaric oxygen therapy, and revascularization. These comparisons highlight the effectiveness of placental therapies in promoting faster and more comprehensive wound healing.
5.1 Debridement, wound dressing, and offloading versus placental regeneration
Debridement, wound dressing, and offloading represent the cornerstone of standard care for Diabetic Foot Ulcers (DFUs). Debridement aims to remove necrotic tissue and maintain a healthy wound bed conducive to healing. Despite its effectiveness in promoting granulation tissue formation, debridement alone may not address underlying vascular and nerve damage characteristic of DFUs. Furthermore, standard dressing techniques, while essential for moisture balance and infection prevention, may not actively promote tissue regeneration. In contrast, placental regeneration techniques offer a promising alternative by providing bioactive factors that stimulate tissue repair and angiogenesis. Studies have demonstrated significantly higher wound closure rates and shorter time to closure with placental tissue treatments compared to standard care alone. For example, in a multicenter RCT, placental regeneration therapy resulted in a wound size reduction of 77% and 92% at 4 and 6 weeks, respectively, compared to 0% and 8% reduction with standard of care. Additionally, placental therapy achieved a 62% wound closure rate by 12 weeks, compared to only 21% with standard of care modalities. Notably, placental therapy also led to fewer wound-related infections (18.0% vs. 36.2%) as compared to standard of care.
Although, in most recent studies using placental tissue therapies to effectively treat DFUs they tend to pair the SOC with the therapy in order to introduce the best treatment. By being able to address, clean, and debride wounds before treatment, it is able to keep the bed healthy and ready for treatment using placenta. Combining SOC with placental regeneration therapy optimizes the synergistic effects of both treatments, leading to accelerated wound closure, reduced infection rates, and overall better patient outcomes. Studies have demonstrated that this combined approach results in significantly improved wound healing outcomes compared to either treatment alone, highlighting the importance of personalized and comprehensive approaches in DFU management.
5.2 Antibiotics and biologics for infection control versus placental regeneration
Antibiotics and biologics play an important role in addressing infection in DFUs, particularly when cellulitis is present. While these therapies effectively target microbial pathogens, they may not directly address the underlying tissue damage and impaired healing mechanisms seen in DFUs. Additionally, antibiotic resistance and the risk of adverse effects are important considerations. Placental regeneration therapies, on the other hand, offer a multifaceted approach by providing growth factors, cytokines, and extracellular matrix components that promote wound healing and modulate the inflammatory response. Clinical studies have shown superior wound closure rates and lower rates of wound-related infections with placental tissue treatments compared to standard of care treatment options. For instance, a randomized controlled trial (RCT) reported a significantly higher proportion of patients achieving complete wound closure with placental therapy (62%) compared to this standard of care use of antibiotics (21%). Additionally, placental therapy resulted in fewer wound-related infections (18.0% vs. 36.2%) compared to standard care.
5.3 Hyperbaric Oxygen Therapy and Negative Pressure Wound Therapy
Hyperbaric Oxygen Therapy (HBOT) and Negative Pressure Wound Therapy (NPWT) are commonly used adjunctive therapies in DFU management, aimed at improving tissue oxygenation and promoting wound healing. While these modalities have demonstrated effectiveness in certain cases, they may not address the underlying causes of DFUs comprehensively. Placental regeneration techniques offer a unique advantage by providing a rich source of bioactive factors that stimulate angiogenesis, tissue regeneration, and modulation of the inflammatory response. In a Randomized Controlled Trial (RCT), placental therapy resulted in a significantly higher proportion of patients achieving complete wound closure (62%) compared to NPWT (21%). As well, the median time to closure was shorter with placental therapy (42 days) compared to NPWT (69.5 days). Many other studies have also reported accelerated wound closure and reduced rates of wound-related complications with placental tissue treatments compared to HBOT and NPWT.
5.4 Total contact casting bracing and revascularization vs placental regeneration
Placental regeneration surpasses the standard care of total contact bracing and revascularization. Total contact casting bracing aims to offload pressure from the ulcerated area, facilitating healing and preventing recurrence. Revascularization procedures address underlying ischemia, which is a common contributing factor in DFU pathogenesis. Although these therapies are crucial for managing DFU, tissue regeneration and repair may not be immediately addressed by them. Placental regeneration therapies offer an extensive approach by providing bioactive factors that promote angiogenesis, tissue repair, and modulation of the inflammatory response. Clinical studies have shown superior wound closure rates and shorter time to closure with placental tissue treatments compared to standard interventions such as total contact casting bracing and revascularization procedures. In a multicenter RCT, placental therapy resulted in a wound closure rate of 86% at 12 weeks, compared to only 60% with standard care alone. Additionally, the mean time to heal was significantly shorter with placental therapy (32 days) compared to standard care (63 days).
6. COMPLICATIONS OF USING PLACENTAL TISSUE TO TREAT DIABETIC ULCER DISEASE
Using placental tissue for treating diabetic ulcers presents various complications including issues related to wound healing, infection, systemic adverse effects, and concerns about tissue processing. These complications highlight the need for careful consideration and monitoring in clinical applications.
6.1 Complications to wound healing itself
Wound healing complexities in Diabetic Ulcer Disease (DFU) cases are influenced by various factors such as wound size, duration, and response to therapies. In many of the studies presented in the systematic review, patients experienced adverse events (AE). For instance, 11 patients (73%) experienced an AE, with common occurrences including pruritus, nausea, pyrexia, hypoglycemia, and dyspnea. Similarly, 59.3% of patients in another study reported at least one AE, with infections being the most frequently reported. Furthermore, complications such as wound-related infections were noted, with serious adverse events (SAEs) requiring hospitalization. In a study assessing the effectiveness of various advanced therapies for DFUs, including placental tissue-based treatments, larger wounds exhibited lower rates of complete closure and required more applications of advanced therapies. Specifically, wounds larger than 25 cm² had a closure rate of only 27.8%, with a median time to closure of 105 days and 11 applications of advanced therapy, showing the challenge of managing non-healing wounds, particularly those of substantial size. Moreover, among wounds that did not close, only a small proportion exhibited significant increases in size, even though this is the opposite effect of what the treatment should be. This indicates the unpredictable nature of wound progression and the need for monitoring and intervention. Overall, it is important to note that not all wounds heal the same, and not all therapies have the same effect. However, a majority of the placenta-based treatments used were effective in closing and minimizing most ulcers presented, indicating their potential in managing Diabetic Ulcers.
6.2 Infection
Another complication that can arise in DFU treatment would be infection. It represents a significant issue in DFU treatment, with various adverse events (AEs) related to infections reported across clinical studies. A multicenter randomized controlled trial (RCT) comparing different advanced skin substitutes, including placental tissue-based treatments like Grafix, reported significantly fewer wound-related infections in patients treated with Grafix compared to the control group. Specifically, among patients randomized to Grafix, only 18% experienced wound-related infections, whereas 36.2% of patients in the control group developed infections. Additionally, fewer hospitalizations related to infections were noted in the Grafix group, further highlighting the potential of these treatments in reducing the incidence of wound-related infections and hospitalizations. These findings underscore the importance of considering placental tissue-based treatments, such as Grafix, as viable options for DFU management, particularly in mitigating the risk of infections. The evidence from this large, multi-center RCT adds credibility to the reliability of the endpoint results, enhancing confidence in the efficacy of placental tissue-based treatments in reducing infection rates and promoting wound healing.
6.3 General Systemic Complications
Beyond infection, patients undergoing placental tissue treatment for DFUs may experience a range of complications, including gastrointestinal disturbances such as diarrhea, nausea, vomiting, fever, hypoglycemia, and dyspnea. Several studies evaluating placental tissue-based therapies have reported common adverse events including nausea, pyrexia, and hypoglycemia. While generally non-serious, these adverse effects contribute to patient discomfort and need to be carefully monitored and managed throughout treatment. Additionally, the presence of systemic complications proves the importance of considering overall patient health when selecting treatment options and managing adverse events. Moreover, many studies have discussed the financial burden associated with some commercially available amniotic membranes, which raises concerns about accessibility and cost-effectiveness in DFU management.
6.4 Rejection
Unlike some treatments involving donated human dermis, rejection is not a risk associated with placental tissue-based therapies. However, concerns regarding the processing of placental membranes have been raised, as harsh processing methods may compromise their biological activity. It is crucial to carefully consider processing techniques to preserve the therapeutic properties of placental tissue while minimizing the risk of adverse reactions. Additionally, limitations in safety-related outcomes “coding and analysis” highlight challenges in accurately assessing complications and AEs associated with placental tissue treatments, emphasizing the need for standardized reporting and evaluation methodologies that remain rigorous.
7. ETHICAL CONSIDERATIONS IN USING PLACENTAL TISSUE FOR DIABETIC ULCER TREATMENT
7.1 Ethical concerns
The utilization of placental tissue for treating DFUs raises various ethical concerns, including the origin of the tissues and the consent process involved in their collection and use. Concerns regarding the fetal origin of the tissues and the potential perception of these tissues as foreign entities have been raised. Additionally, the ethical implications of using placental tissue in research and clinical settings require careful consideration to ensure patient safety and uphold ethical standards. The concerns outlined in the articles are valid due to the ethical implications associated with the sourcing and use of placental tissue. Placental tissues are derived from human umbilical cords, which may raise concerns regarding their fetal origin. Additionally, the collection and use of these tissues must adhere to strict ethical guidelines and regulations to ensure patient safety and uphold ethical standards.
7.2 Consent
In all of the studies presented in the review, the patients participating and donating placental tissue provided informed consent, indicating their willingness to undergo treatment and contribute to research endeavors. The consent process involved patients signing written consent forms after being provided with information about the treatment procedures and potential risks and benefits involved. This ensures that patients are fully informed about the nature of the treatment and have the autonomy to make decisions regarding their participation in research studies or clinical trials involving placental tissue therapies.
7.3 Ethical Laws
The use of placental tissue for DFUs is governed by various ethical laws, acts, and approvals aimed at safeguarding patient welfare and ensuring compliance with standards. These include approval by Institutional Review Boards (IRBs) and Independent Ethics Committees (IECs) at participating centers, in adherence to the Declaration of Helsinki, and compliance with Good Clinical Practice guidelines. Moreover, regulatory frameworks such as the Public Health Service Act and FDA guidelines regulate the use of placental membrane products in the management of Diabetic Ulcers, ensuring their safety and effectiveness. Overall, adherence to these ethical laws and approvals ensures that the use of placental tissue in treatment is conducted ethically and in accordance with established guidelines and regulations.
Discussion Section
Diabetic Foot Ulcers (DFUs) are one of the most common problems seen in patients with diabetes, affecting 19–34% of the global population. These conditions frequently lead to serious side effects, such as infections, that may require amputation. Current treatment approaches, however, lack a definitive solution, with almost half of wounds reoccurring within one year and 65% within five years post-treatment. Placental tissue therapies offer a promising route for addressing the challenge of DFUs. Studies have shown promising clinical effectiveness and accelerated healing rates associated with placental tissue therapies. While conventional approaches to DFU management often fall short in achieving lasting healing outcomes, placental tissue therapies demonstrate remarkable clinical success. Incorporating these innovative therapies into treatment protocols holds significant potential for improving patient outcomes and reducing the burden of DFUs on individuals and healthcare systems alike.
Placental tissue therapies for DFUs involve meticulous preparation methods, including washing, sterilization, and culturing onto scaffolds, ensuring safety. Studies emphasize standardized protocols to maximize therapeutic potential, ultimately improving DFU management. When compared to conventional approaches, these therapies improve healing rates and show significant clinical success, and preservation techniques guarantee the preservation of the tissue’s therapeutic qualities and integrity. Pharmacological preparations improve tissue regeneration, angiogenesis, and modulate inflammatory responses to optimize treatment outcomes. Application methods such as topical application, injection, and implantation offer advantages in promoting wound healing, emphasizing the need for personalized approaches in DFU management. As well, placental tissues exhibit similarities to skin, providing a natural scaffold for cellular proliferation and tissue regeneration, while preservation techniques enhance their potential. Placental tissue therapies demonstrate significant efficiency over various time frames, with patients experiencing accelerated wound healing and improved outcomes, showing their promise for long-term DFU management. These therapies offer superior wound healing outcomes compared to standard care, with reduced infection rates and shorter time to closure. However, complications such as adverse events and concerns about processing techniques remind us that careful monitoring and standardized reporting for evaluating safety is needed.
In the analysis of the outcomes of placental tissue therapies for diabetic foot ulcers (DFUs), several unexpected findings emerged, including instances where some wounds exhibited temporary enlargement following placental treatment. This observation contradicted the anticipated outcome of wound reduction and closure associated with placental wound healing interventions. One hypothesis suggests that fluctuations in wound microenvironmental factors, such as pH levels or oxygenation status could influence the response to placental tissue therapies. Deviations from optimal conditions may impede the healing process, leading to temporary wound enlargement before achieving net tissue regeneration. Additionally, delayed healing responses may occur due to patient-specific factors or variations in treatment efficacy, resulting in a staggered healing trajectory characterized by the fluctuations in wound size. Furthermore, immune reactions triggered by the introduction of foreign biological materials, such as placental tissues, could lead to inflammatory responses or tissue edema, contributing to apparent wound enlargement post-treatment. An unexpected outcome of wound enlargement following placental tissue therapy is the complexity of wound healing dynamics and the need for personalized treatment approaches. Despite initial setbacks, it’s crucial to recognize that wound healing is a dynamic process influenced by various intrinsic and extrinsic factors. Due to this, the healing responses and wound enlargements have similar causes including environmental conditions as well as patient specific variables showing insight into the importance of DFU management. These unexpected findings highlight the importance of continuous monitoring and adaptation and specialization of treatment strategies to address evolving patient needs and optimize clinical outcomes.
We are able to compare results to previous findings in the literature, revealing both similarities and discrepancies in treatment responses. While some studies have reported consistent wound reduction and closure rates with placental tissue therapies, our findings suggest a more nuanced understanding of treatment outcomes, including instances of temporary wound enlargement. This discrepancy may be attributed to variations in patient populations, treatment protocols, or outcome measures across studies. Additionally, advances in our understanding of wound healing and the microenvironmental factors influencing treatment responses may contribute to evolving interpretations of treatment outcomes over time. Overall, the unexpected wound enlargement post-therapy underscores the need for more research to understand mechanisms and improve DFU treatment. By investigating wound microenvironmental dynamics, delayed healing responses, and immunological reactions, we can enhance patient care approaches.
There are several strengths of this paper including the review process, which involved thorough examination and analysis of a selected number of relevant research papers. This systematic review focuses on placental tissue therapies in diabetic foot ulcer management, offering comprehensive insights into treatment outcomes and mechanisms. It synthesizes findings from multiple studies, making it one of the first to conduct such a specific review in this area. This approach enhances the reliability of the synthesized findings, providing researchers with a resource for understanding the current base of this topic.
Several limitations should be acknowledged. Specifically, the scope of this review was limited to a select number of articles, which may not fully represent the breadth of research conducted in the field of placental tissue therapies for diabetic foot ulcers. Furthermore, while efforts were made to include a diverse range of studies, the exclusion of certain articles may have inadvertently biased the findings or overlooked relevant research. Despite these limitations, this paper provides valuable insights into the efficacy and mechanisms of placental tissue therapies for diabetic foot ulcers, offering important implications for clinical practice and future research directions.
Numerous opportunities present themselves for more investigation in terms of future research. First, to confirm the results of this research and offer more solid proof of the effectiveness and safety of placental tissue therapies for diabetic foot ulcers, prospective clinical trials with bigger sample sizes are necessary. These trials might also look into the best therapy length, frequency, and dosage to provide the best possible therapeutic results. Furthermore, comparative effectiveness studies evaluating various placental tissue product varieties or placental tissue therapies against other accepted treatments may aid in directing clinical judgment and refining treatment plans. Further research should be done to better understand the underlying mechanisms of action of placental tissue therapies considering the intricacy of DFUs and the multifaceted nature of their pathophysiology. Comprehending the distinct bioactive elements and cellular mechanisms that facilitate the healing of wounds could lead to the creation of more specialized and customized methods for treatment. Moreover, investigating the durability and long-term effects of placental tissue therapies beyond the duration of current research will be beneficial in determining their sustainability and capacity to prevent ulcer recurrence.
The importance of this paper is significant, particularly for healthcare providers and insurance companies. By synthesizing current evidence and providing a comprehensive overview of placental tissue therapies for DFUs, this paper can serve as a valuable guideline for clinicians in making informed treatment decisions. Understanding the efficiency, safety, and mechanisms of action of these therapies is crucial for optimizing patient outcomes and improving the quality of care for individuals with this disease. Additionally, insurance companies may find this paper useful in evaluating the cost-effectiveness and coverage policies for placental tissue therapies, ultimately ensuring access to potentially beneficial treatments for patients.
In summary, future research endeavors should focus on conducting rigorous clinical trials, elucidating underlying mechanisms, and assessing long-term outcomes to further advance our understanding and optimize the use of placental tissue therapies in diabetic foot ulcer management. This paper serves as a foundational resource for guiding clinical practice and policy-making decisions, highlighting the importance of continued research efforts in this field for improving patient care and outcomes.
Conclusion
This paper reviews placental tissue therapies for diabetic foot ulcers (DFUs), aiming to inform healthcare professionals and policymakers about their effectiveness and limitations. It offers valuable insights into the effectiveness, mechanisms of action, and implications of placental tissue therapies for diabetic foot ulcer treatment. The purpose of this paper was to analyze and consolidate existing information regarding the use of placental tissue therapies in DFU management. The findings suggest that these therapies hold promise for reducing healing times in DFU patients. This paper outlines areas for further research to optimize treatment strategies and evaluate long-term outcomes, emphasizing the importance of continued research efforts in this field. By synthesizing current evidence and identifying gaps in knowledge, it provides a foundation for future studies to build upon, ultimately advancing the understanding of placental tissue therapies and their role in DFU management.
References
- Sledge I, Maislin D, Bernarducci D, Snyder R, Serena TE. Use of a dual-layer amniotic membrane in the treatment of diabetic foot ulcers: an observational study. J Wound Care. 2020;29(Sup9):S8-S12. doi:10.12968/jowc.2020.29.Sup9.S8
- Haugh AM, Witt JG, Hauch A, et al. Amnion Membrane in Diabetic Foot Wounds: A Meta-analysis. Plast Reconstr Surg Glob Open. 2017;5(4):e1302. doi:10.1097/GOX.0000000000001302
- Reyzelman AM, Vartivarian M, Danilkovitch A, Saunders MC. A Prospective, Single-center, Open-label Case Series Evaluating the Clinical Outcomes of Lyopreserved Placental Membrane Containing Viable Cells in the Treatment of Chronic Wounds. Wounds Compend Clin Res Pract. 2019;31(4):97-102.
- Oesman I, Dhamar Hutami W. Gamma-treated placental amniotic membrane allograft as the adjuvant treatment of unresponsive diabetic ulcer of the foot. Int J Surg Case Rep. 2020;66:313-318. doi:10.1016/j.ijscr.2019.12.033
- Galiano RD, Orgill DP, Armstrong DG, Glat PM, Carter MJ, Zelen CM. A Prospective Multicenter Study of a Weekly Application Regimen of Viable Human Amnion Membrane Allograft in the Management of Nonhealing Diabetic Foot Ulcers. Plast Reconstr Surg Glob Open. 2023;11(10):e5291. doi:10.1097/GOX.0000000000005291
- Sabolinski ML, Capotorto JV. Comparative effectiveness of a human fibroblast-derived dermal substitute and a viable cryopreserved placental membrane for the treatment of diabetic foot ulcers. J Comp Eff Res. 2019;8(14):1229-1238. doi:10.2217/cer-2019-0001
- Wang H, Chen L, Liu Y, et al. Implantation of placenta-derived mesenchymal stem cells accelerates murine dermal wound closure through immunomodulation. Am J Transl Res. 2016;8(11):4912-4921.
- Lavery L, Fulmer J, Shebetka K, et al. The efficacy and safety of Grafix (R) for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded, clinical trial. Int Wound J. 2014;11. doi:10.1111/iwj.12329
- Yu X, Liu P, Li Z, Zhang Z. Function and mechanism of mesenchymal stem cells in the healing of diabetic foot wounds. Front Endocrinol. 2023;14:1099310. doi:10.3389/fendo.2023.1099310
- Brantley JN, Verla TD. Use of Placental Membranes for the Treatment of Chronic Diabetic Foot Ulcers. Adv Wound Care. 2015;4(9):545-559. doi:10.1089/wound.2015.0634
- Lakmal K, Basnayake O, Hettiarachchi D. Systematic review on the rational use of amniotic membrane allografts in diabetic foot ulcer treatment. BMC Surg. 2021;21(1):87. doi:10.1186/s12893-021-01084-8
- Meamar R, Ghasemi-Mobarakeh L, Norouzi MR, Siavash M, Hamblin MR, Fesharaki M. Improved wound healing of diabetic foot ulcers using human placenta-derived mesenchymal stem cells in gelatin electrospun nanofibrous scaffolds plus a platelet-rich plasma gel: A randomized clinical trial. Int Immunopharmacol. 2021;101(Pt B):108282. doi:10.1016/j.intimp.2021.108282
- DiDomenico LA, Orgill DP, Galiano RD, et al. A Retrospective Crossover Study of the Use of Aseptically Processed Placental Membrane in the Treatment of Chronic Diabetic Foot Ulcers. Wounds Compend Clin Res Pract. 2017;29(10):311-316.
- Tettelbach WH, Cazzell SM, Hubbs B, Jong JLD, Forsyth RA, Reyzelman AM. The influence of adequate debridement and placental-derived allografts on diabetic foot ulcers. J Wound Care. 2022;31(Sup9):S16-S26. doi:10.12968/jowc.2022.31.Sup9.S16
- Snyder D, Sullivan N, Margolis D, Schoelles K. Skin Substitutes for Treating Chronic Wounds. Agency for Healthcare Research and Quality (US); 2020. Accessed October 31, 2024. http://www.ncbi.nlm.nih.gov/books/NBK554220/
- Wu SC, Pollak R, Frykberg RG, et al. Safety and efficacy of intramuscular human placenta-derived mesenchymal stromal-like cells (cenplacel [PDA-002]) in patients who have a diabetic foot ulcer with peripheral arterial disease. Int Wound J. 2017;14(5):823-829. doi:10.1111/iwj.12715
- DaVanzo J, Hartzman A, Surfield C, Dobson A. Cryopreserved Placental Membrane Allograft Reduces the Likelihood of Developing a New or Recurring Foot Ulcer and All-Cause Mortality in Diabetic Patients When Compared with Other Cellular- and Tissue-Based Products. Adv Wound Care. 2023;12(4):169-176. doi:10.1089/wound.2021.0123
- Garoufalis M, Tettelbach W. Expandable dehydrated human umbilical cord allograft for the management of nonhealing lower extremity wounds in patients with diabetes: a case series. Wounds Compend Clin Res Pract. 2022;34(9):E91-E95. doi:10.25270/wnds/21126
- Luck J, Rodi T, Geierlehner A, Mosahebi A. Allogeneic Skin Substitutes Versus Human Placental Membrane Products in the Management of Diabetic Foot Ulcers: A Narrative Comparative Evaluation of the Literature. Int J Low Extrem Wounds. 2019;18(1):10-22. doi:10.1177/1534734618818301
- Frykberg RG, Gibbons GW, Walters JL, Wukich DK, Milstein FC. A prospective, multicentre, open-label, single-arm clinical trial for treatment of chronic complex diabetic foot wounds with exposed tendon and/or bone: positive clinical outcomes of viable cryopreserved human placental membrane. Int Wound J. 2017;14(3):569-577. doi:10.1111/iwj.12649
- Oropallo A, Goodwin A, Morrissey M, Del Pin C, Rao A. Human Amnion Chorion Membrane Allografts in the Treatment of Chronic Diabetic Foot Ulcers: A Literature Review. Adv Skin Wound Care. 2021;34(4):1-7. doi:10.1097/01.ASW.0000734388.08779.e8
- Glat P, Orgill DP, Galiano R, et al. Placental Membrane Provides Improved Healing Efficacy and Lower Cost Versus a Tissue-Engineered Human Skin in the Treatment of Diabetic Foot Ulcerations. Plast Reconstr Surg Glob Open. 2019;7(8):e2371. doi:10.1097/GOX.0000000000002371
- Lavery L, Fulmer J, Shebetka KA, et al. Open-label Extension Phase of a Chronic Diabetic Foot Ulcer Multicenter, Controlled, Randomized Clinical Trial Using Cryopreserved Placental Membrane. Wounds Compend Clin Res Pract. 2018;30(9):283-289.
- Hanselman AE, Lalli TAJ, Santrock RD. Topical Review: Use of Fetal Tissue in Foot and Ankle Surgery. Foot Ankle Spec. 2015;8(4):297-304. doi:10.1177/1938640015578513
- Raspovic KM, Wukich DK, Naiman DQ, et al. Effectiveness of viable cryopreserved placental membranes for management of diabetic foot ulcers in a real world setting. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc. 2018;26(2):213-220. doi:10.1111/wrr.12635
- Ananian CE, Dhillon YS, Van Gils CC, et al. A multicenter, randomized, single-blind trial comparing the efficacy of viable cryopreserved placental membrane to human fibroblast-derived dermal substitute for the treatment of chronic diabetic foot ulcers. Wound Repair Regen Off Publ Wound Heal Soc Eur Tissue Repair Soc. 2018;26(3):274-283. doi:10.1111/wrr.12645

