Racial Disparities in Crohn’s Disease Manifestations
Racial Disparities in Clinical Manifestation of Crohn’s disease
Raguraj Chandradevan 1, Porsha Okiye 2, Chakravarthy Nulu 3, Bobak Moazzami 4, Humberto Sifuentes 1*
- Department of Gastroenterology and hepatology, Medical College of Georgia, Augusta University, Augusta, Georgia, USA.
- Department of Internal Medicine, Medical College of Georgia, Augusta University, Augusta, Georgia, USA.
- Medical College of Georgia, Augusta University, Augusta Georgia, USA.
- Northside Hospital Gwinnett Georgia, USA
OPEN ACCESS
PUBLISHED: 31 March 2025
CITATION: Chandradevan, R., Porsha, O., et al., 2025. Racial Disparities in Clinical Manifestation of Crohn’s disease. Medical Research Archives, [online] 13(3). https://doi.org/10.18103/mra.v13i3.6375
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI https://doi.org/10.18103/mra.v13i3.6375
ISSN 2375-1924
ABSTRACT
Introduction: This study aims to evaluate and compare the clinical manifestations and disease progression of CD in African Americans (AA) and Caucasians (CA).
Methods: We conducted a single-center cross-sectional study of all patients with CD, both hospitalized and seen as outpatient, treated from 2019 to 2024. Chart reviews were used to identify disease location, and perianal disease, strictures, penetrating natures, endoscopy and surgical history, extra intestinal manifestation, demographics, treatment and medication use. Patients with ulcerative colitis or indeterminant colitis were excluded.
Results: A total of 167 patients were identified, comprising 43% AA and 56% CA. Mean age of diagnosis was 43±14.56 for AA and 47±15.71 for CA. The average measured BMI was higher in AA (28.55±8.07) than CA (26.01±5.93) (p<0.01; Table 1). Although there was no significant gender difference in the overall diagnosis of CD, a higher prevalence was observed among females in AA (F/M ratio: 43/27 in AAs vs. 50/45 in CA). CDs related surgeries occurred in 71% of AA and 56% of CA. Time from diagnosis to surgery was 17 years for AA and 15 years for CA with a wide range of standard deviation. No significance differences noted in extraintestinal manifestations, undergoing surgeries, treatment modalities, insurances and perianal disease. AA were more likely to have penetrating phenotype (B3) and less likely to have inflammatory (B1) and stricturing phenotypes (B2) as compared to Caucasians (p<0.005; Table 1). Multivariate logistic regression analysis revealed that the odds of having a B3 phenotype compared to a B1 phenotype were approximately 2.43 times higher for AA compared to CA (CI: 1.26-4.69) after adjusting for age, sex and BMI. In a subgroup analysis of those with the B3 phenotype, AA were less likely to use steroids compared to CA (32% Vs 53%, p < 0.05).
Discussion: This study showed that AA were more likely to develop complicated CD over time compared to CA. Higher BMI was noted among AA, however not correlated with complicated CD. AA were diagnosed younger, had a female predominance, longer time from diagnosis to surgery and underwent more surgeries. These findings suggest that CD may manifest differently in AA, though socioeconomic status, healthcare access, and awareness of the disease process may also play a role.
Keywords
Crohn’s disease, racial disparities, African Americans, Caucasians, clinical manifestations
Introduction:
Most of the clinical, epidemiology, disease characteristics, and treatment of Inflammatory bowel disease (IBD) is in Caucasian (CA) patients. Historically IBD has been viewed as a condition predominantly affects Caucasian patients in Western Europe and North America, however more recent studies indicated rising incidence of Crohn’s disease (CD) and ulcerative colitis (UC) in previously low incidence populations, including African-Americans (AA)1,2. Some studies from AA patients reveal prevalence of CD approaches that of the non-Hispanic Western population and others, with data suggesting that AA, Asian, and Hispanic patients may have higher incidence of more severe disease.3-6. These data lend credence to the suggestion that the nature of CD may be different in AA patients compared with CA patients.
Emerging literature suggests the genetic determinants6 differ by race as well as contributing to phenotypic differences in disease expression. A study conducted by Huang et al. characterized genetic loci in AAs with IBD and identified several distinct genetic loci in AAs with IBD.7 The growing literature examining variably phenotypic expression has also furthered inquisition on differences of extraintestinal manifestations between AA and CA. In Crohn’s disease increasing rate of perianal disease8, upper GI tract disease and penetrating behaviors9 of the disease were reported among AA patients.
Racial disparities have also been observed to affect clinical course and outcomes of Crohn’s disease (CD) among marginalized minorities compared to Caucasians. Many factors including inequities in social determinants of health, physical and mental barriers, and socioeconomic status (SES) have played a role in the disparities. Several studies have shown that social barriers disproportionately affect African Americans and Hispanics with CD. Barriers including transportation10, financial strains, food insecurity, medical care delays, low educational attainment, receipt of fewer therapeutic interventions are most frequently implicated10. Several studies show that socioeconomic status subscribes to gaps and serves as a predictor of poor outcomes. Walker et al conducted a retrospective study that determined race and SES are both associated with the clinical course of CD10. Findings were notable for decreased hospitalization rate with increasing income level for a CD related event, however, AA comprised most of the low-income groups affirming that disparities in disease severity are linked to social and economic inequities, especially for the uninsured. Being uninsured adds another barrier to accessing healthcare due to requirements for frequent follow up for monitoring, procedures, or treatment10.
Racial disparities have also been observed in access to healthcare utilization and higher need for healthcare visits to the emergency department (ED)11-13. Poor outcomes observed in AA patients, including longer lengths of hospitalization, higher rates of readmissions following hospitalizations and surgery, and lower health-related quality of life (HRQOL)12. Differences in utilization of IBD-related specialist services, ED visits, and infliximab that are independent of income and education.14 Access to health care and biologic agents for treating IBD has been shown to vary by socio demographics, potentially contributing to poorer outcomes and more severe disease in underserved populations.15 There are compelling studies examining AA patient barriers to health care and outcomes in real-world clinical practice in diverse communities, however strategies to overcome them need to be further explored.
Despite the increased recognition of IBD prevalence in AA patients, this has not resulted in more research into targeted treatment, effectiveness, and outcomes in this population. African American individuals currently comprise approximately 12% of the United States population but comprise as low as 3% of people included in IBD-related randomized controlled trials and 1% of people included in real-world and outcome-based studies.16,17 Therefore, we conducted a cross-sectional study to evaluate demographics, extraintestinal manifestations, surgical outcome, medication use, medical insurance, perianal disease, disease location, and behaviors to better understand CD progression in AA patients.
WellStar MCG health is a largest health system in Augusta operating with Augusta University. Our patients receive care at tertiary IBD center and reflects diverse community with approximately 56% of AA.
Methods:
This study was approved by the Institutional Review Board at the Augusta University Medical Center under the study protocol 2089342-4. We performed a cross-sectional review of the IBD registry at our tertiary center, treated from 2019 – 2024. Study participants consisted of patients diagnosed with Crohn’s disease (CD), who are being evaluated at our clinic. The data for this study was collected from the chart review of each patient. A comprehensive database was created with various data points. The patients were classified as CD by physician documentation. Patients diagnosed with ulcerative colitis and indeterminate colitis were excluded from the study. Data regarding diagnosis, year of diagnosis, date of birth, self-identified race, disease location, extent, perianal disease, stricturing and penetrating disease, endoscopy, surgical history, medication history, and extraintestinal manifestations were collected during the initial clinic visit during the study period. The age of diagnosis was determined by subtracting the year of birth from the year of diagnosis.
Similarly, the duration of the disease was determined by subtracting the year of diagnosis from the year of the most recent clinic visit. For this study, ileal involvement in CD was defined as evidence of ileal disease at any point in the disease course. Extraintestinal manifestations were determined from physician documentation and included arthralgias related to underlying IBD activity, ankylosing spondylitis/sacroiliitis, erythema nodosum, pyoderma gangrenosum, oral aphthous ulcers, ocular inflammation, osteoporosis, primary sclerosing cholangitis, or other liver disease. Data regarding current medications and endoscopic, surgical history were collected during the initial clinic visit and updated during any subsequent follow-up visits to the clinic. Surgeries were limited to those for complications or treatment of CD. For CD, this included intestinal resections, stoma creation, stricturoplasty, incision and drainage, abdominal abscess drainage, perianal fistulotomy, seton placement, stoma revision, advancement flap, or an ileal pouch-anal anastomosis (IPAA). The resulting data were subsequently abstracted from the database and divided into groups based on their self-identified race. Patients who self-identified as Caucasians (CA) or African Americans (AA) were divided into two arms for the analysis. Mixed race was not included because the ambiguity of the category would limit the conclusions. Multiple data points were analyzed between two arms to evaluate any underlying significance or differences among two groups of patients.
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). Continuous variables such as age, BMI, disease duration, and age of diagnosis were compared between CA and AA using Student’s t-test. Categorical variables, including sex, extraintestinal manifestations, surgical history, medications and treatments, insurance status, perianal disease, and Montreal disease classification, were analyzed using Pearson’s chi-square test. Logistic regression models were employed to calculate odds ratios for the likelihood of surgery, adjusting for disease location and extent within each group. A predetermined significance level of p<0.05 was used for all analyses.
Results:
DEMOGRAPHIC CHARACTERISTICS
We included 70 AA patients (42.4%) and 95 CA patients (57.5%) in our study (Table 1). The mean age at diagnosis was 43.26±14.56 for AA and 47.41±15.71 years for CA patients. The average BMI for AA patients was 28.55±8.07, which was significantly higher than that of CA patients (26.01±5.93) (p<0.01). There was no significant difference in the time from diagnosis to the most recent clinic visit between the two groups. However, AA patients had a longer average time to establish care of 17.06 ± 9.03 months compared to CA patients, who had an average follow-up of 15.96 ± 9.82 months. Although there was no significant gender difference in the overall diagnosis of CD, a higher prevalence was observed among females in AA and CA. (F/M ratio: 43/27 in AAs vs. 50/45 in CC).
| Variables | African Americans (n= 70) | Caucasians (n= 95) | P-Value |
|---|---|---|---|
| Age (mean, SD) | 43.26±14.56 | 47.41±15.71 | 0.51 |
| BMI | 28.55±8.07 | 26.01±5.93 | 0.01 |
| Duration of dx | 17.06±9.03 | 15.96±9.82 | 0.71 |
| Sex | 0.36 | ||
| Male, % | 27 (38.5) | 45 (47.3) | |
| Female, % | 43 (61.4) | 50 (52.6) | |
| Extraintestinal manifestation | 0.46 | ||
| Arthritis | 21 (30) | 31 (32.6) | |
| SLE | 0 (0) | 2 (2.1) | |
| Pyoderma gangrenosum | 3 (4.1) | 2 (2.1) | |
| Psoriasis | 1 (1.4) | 1 (1) | |
| Primary sclerosing cholangitis | 1 (1.3) | 1 (1) | |
| Surgery | 0.12 | ||
| Yes | 50 (71.4) | 56 (59) | |
| No | 20 (28.5) | 39 (41) | |
| Treatment | |||
| Biologics | 54 (75) | 80 (84.2) | 0.13 |
| Immunomodulators | 13 (18) | 19 (20) | 0.75 |
| Steroids | 26 (36.1) | 44 (46.3) | 0.18 |
| 5-ASA | 23 (31.9) | 36 (37.8) | 0.42 |
| Antibiotics | 1 (1.3) | 2 (2.1) | 0.43 |
| Combination | 22 (30.5) | 39 (41) | 0.16 |
| Insurance | 0.08 | ||
| Private | 27 (37.5) | 45 (47.3) | |
| Medicare | 17 (23.6) | 12 (12.6) | |
| Medicaid | 14 (19.4) | 15 (15.7) | |
| Veteran | 2 (2.7) | 6 (6.3) | |
| No insurance | 11 (15.2) | 17 (17.8) | |
| Peri-anal fistula | 21 (29.1) | 17 (17.8) | 0.95 |
| Peri-anal abscess | 7 (9.7) | 10 (10.5) | 0.22 |
| Montreal Crohn disease | |||
| Ileal (L1) | 8 (11.3) | 15 (15.7) | |
| Colonic (L2) | 11 (15.7) | 18 (18.9) | 0.17 |
| Ileocolonic (L3) | 49 (70) | 61 (64.2) | |
| Upper compromise (L4) | 3 (4.1) | 1 (1) | |
| B1 (inflammatory) | 12 (16.6) | 30 (31.5) | |
| B2 (stricturing) | 12 (16.6) | 24 (25.2) | 0.004 |
| B3 (penetrating) | 46 (63.8) | 41 (43.1) |
EXTRAINTESTINAL MANIFESTATIONS
Arthralgia was commonly reported in both groups; however, it was not found to be statistically significant. Thirty percentage of AA and 32% of CA patients complained of arthralgia with active CD. Less commonly reported manifestations included oral ulcers, pyoderma gangrenosum, oral ulcers, sacroiliitis and primary sclerosing cholangitis.
SURGICAL HISTORY
Although no significant difference among AA and CA patients in the need for surgeries (emergent and elective) for the CD complication or treatment, 71% of AA patients underwent surgery during the 5-year study period, compared to 59% of CA patients.
MEDICATIONS USE IN CROHN’S DISEASE
No significant difference was observed between the use of biologics, immunomodulators, steroids, 5-ASA, antibiotics and combination of medications between the two groups. In total, 84% of CA patients and 75% of AA patients received biologic treatments. Steroids were used 46% of CA patients and 36% of AA patients. A subgroup analysis of patients with the B3 phenotype of CD showed that AA patients were less likely to use steroids compared to CA (32% Vs 53%, p < 0.05).
MEDICAL INSURANCE
Our patients had variety of medical insurance plans utilized for the healthcare at our institution. Private insurance was used by 47% of CA patients and 37% of AA patients. Additionally, Medicare, Medicaid and veteran patients were seen through community care referrals. There was no significant difference between the two racial groups in terms of insurance utilization or health care access. Financial assisted programs supported 17% of CA patients and 15% of AA patients for the treatment of CD.
PERIANAL DISEASE
Perianal fistulas were observed in 29% of AA patients and 17% of CA patients. On the other hand, perianal abscess was noted 10.5% in CA patients and 9.7% of AA patients. No significance difference was found between the two racial groups in regards of perianal manifestations of CD.
DISEASE LOCATION AND BEHAVIOR
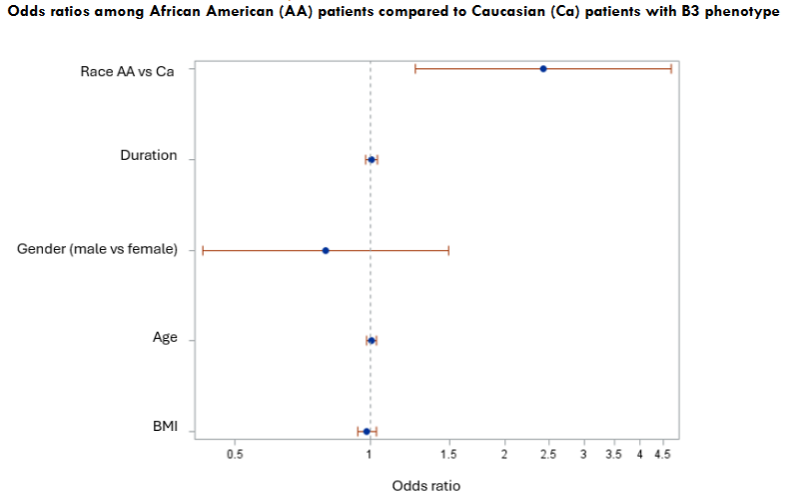
Ileocolonic disease was noted among 70% of AA patients and 64% of CA patients. No significance difference noted among two racial groups in regards of disease location of ileal, colonic or ileocolonic. However, AA patients were more likely to have a penetrating phenotype (B3) and less likely to exhibit the inflammatory (B1) or stricturing phenotypes (B2) compared to CA patients. Penetrating disease behavior noted in 63% of AA patients and 43% of CA patients. In contrast, CA patients were more likely to have stricturing disease (25%) as compared to AA patients (16.6%). After adjusting for age, sex, and BMI, AA patients had significantly higher odds of exhibiting a B3 phenotype compared to CA patients, with an OR of 2.43 (95% CI: 1.26–4.69).
Discussion:
In our analysis, we represented CD patients from a tertiary center with a diverse community with an equal distribution of AA and CA patients. This is one of the strengths of our study. Significant differences in higher BMI, high need for surgical interventions, higher reported perianal fistula, and penetrating phenotype among AA describe a more aggressive course of the disease. Patients with Crohn’s disease with delay in diagnosis or misdiagnosis result in untreated IBD can lead to persistent inflammation, development of systemic symptoms, and potential complications from aggressive disease. In our study, we noticed AA patients are slightly delayed in reaching health care/establishing care. This finding is crucial as disease severity can impact the quality of life of patients. Furthermore, several studies mentioned that disparities in disease severity were a result of social and economic inequalities (e.g. affording healthcare, delaying appointments due to financial concerns, and difficulties traveling to the provider’s office) rather than genetic differences.18 This is somewhat described in our study by higher private insurances were utilized by CA patients and AA patients were less advantaged to be involved in financial assistance needs as well as with delay in establishing care at our IBD clinic.
Surgical disparities were noted in previous studies.19 Indications for surgery in CD include perforation, enteric fistula, bleeding, malnutrition and obstruction. Typically, management of these conditions can be controlled through either outpatient or inpatient medical management, which often then allows for a planned elective procedure. However, emergency indications can occur. Examples of planned interventions that can increase the likelihood of good surgical outcomes include preoperative sepsis management with radiologically guided drain placement[s] and antibiotics, adequate time interval off immunosuppressant medications prior to surgery, and a period of nutritional support, when indicated, using total parenteral nutrition [TPN]. Existing literature indicates that care for Crohn’s disease in African American patients may have been limited and of lower quality. These disparities increase the risk of unplanned and emergency procedures, increasing the risk of major complications postoperatively. However, other risk factors for complications after intestinal surgery, such as comorbidities may also be more prevalent in AA patients undergoing surgery. In our study these findings underscore with AA undergo surgeries in 71% as compared to 56% in CA patients.
Previous studies examining racial differences in CD phenotypes have shown conflicting results. A previous meta-analysis of 47 studies found no major differences in disease location, behavior, upper gastrointestinal tract involvement, perianal involvement, or extraintestinal manifestations among racial groups.16 Other studies with small sample size populations have demonstrated that African American patients with IBD are more likely to present with severe complications, particularly in the perianal region.2,5 Our findings, based on data representative of the U.S. population with a significant African American population, suggest that they face an increased risk of complications, independent of other sociodemographic factors and comorbidities. Emerging evidence implicates other genetic factors unique to African American populations. Variants in the STAT3 and STAT5 genes, which regulate inflammatory responses, have been associated with more severe disease phenotypes, including fistulizing complications.20 Additionally, HLA-DRB1, a gene involved in immune modulation, has been linked to aggressive IBD manifestations in African American patients.6 These genetic differences not only influence the disease presentation in African American patients but also the aggressive nature of the disease, even after adjusting for age, gender, disease duration, and BMI. Collectively, these findings emphasize the need for further research into population-specific genetic and environmental influences to improve the understanding and management of CD in African American patients.
The limitations of this study include a single-center survey of a small number of patients. Due to the retrospective nature of the study, most of the surgical history/endoscopy report and physician notes were reviewed from our center only. Additionally, the use of ICD-10 codes to identify complications may result in misclassification, though this is a common limitation in studies using administrative data. Lastly, race and ethnicity in the medical records are self-reported, which could introduce inaccuracies in data collection.
Conclusion:
In summary, our study highlights notable racial disparities in CD-related complications, with African American patients exhibiting higher rates of severe outcomes, such as penetrating disease, compared to Caucasian patients. These differences likely stem from a combination of genetic factors, delayed access to medical care, and variations in healthcare coverage. Tackling these inequities is crucial to enhancing outcomes for minority populations affected by IBD. Future research should prioritize uncovering the mechanisms behind these disparities and designing targeted strategies to mitigate the burden of IBD complications in underserved communities.
Funding:
This study is supported by Fall FY24 Graduate Medical Education (GME) Research Fund, Augusta University.
References:
- Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. Jan 2012;142(1):46-54 e42; quiz e30. doi:10.1053/j.gastro.2011.10.001
- Malaty HM, Hou JK, Thirumurthi S. Epidemiology of inflammatory bowel disease among an indigent multi-ethnic population in the United States. Clin Exp Gastroenterol. 2010;3:165-70. doi:10.2147/CEG.S14586
- Nguyen GC, Torres EA, Regueiro M, et al. Inflammatory bowel disease characteristics among African Americans, Hispanics, and non-Hispanic Whites: characterization of a large North American cohort. Am J Gastroenterol. May 2006;101(5):1012-23. doi:10.1111/j.1572-0241.2006.00504.x
- Jackson JF, 3rd, Dhere T, Repaka A, Shaukat A, Sitaraman S. Crohn’s disease in an African-American population. Am J Med Sci. Nov 2008;336(5):389-92. doi:10.1097/MAJ.0b013e31816a5c06
- Alli-Akintade L, Pruthvi P, Hadi N, Sachar D. Race and fistulizing perianal Crohn’s disease. J Clin Gastroenterol. Mar 2015;49(3):e21-3. doi:10.1097/MCG.0000000000000117
- Brant SR, Okou DT, Simpson CL, et al. Genome-Wide Association Study Identifies African-Specific Susceptibility Loci in African Americans With Inflammatory Bowel Disease. Gastroenterology. Jan 2017;152(1):206-217 e2. doi:10.1053/j.gastro.2016.09.032
- Huang C, Haritunians T, Okou DT, et al. Characterization of genetic loci that affect susceptibility to inflammatory bowel diseases in African Americans. Gastroenterology. Nov 2015;149(6):1575-1586. doi:10.1053/j.gastro.2015.07.065
- Barnes EL, Kochar B, Long MD, et al. Lack of Difference in Treatment Patterns and Clinical Outcomes Between Black and White Patients With Inflammatory Bowel Disease. Inflamm Bowel Dis. Nov 29 2018;24(12):2634-2640. doi:10.1093/ibd/izy179
- Kugathasan S, Denson LA, Walters TD, et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: a multicentre inception cohort study. Lancet. Apr 29 2017;389(10080):1710-1718. doi:10.1016/S0140-6736(17)30317-3
- Walker C, Allamneni C, Orr J, et al. Socioeconomic Status and Race are both Independently associated with Increased Hospitalization Rate among Crohn’s Disease Patients. Sci Rep. Mar 5 2018;8(1):4028. doi:10.1038/s41598-018-22429-z
- Flasar MH, Johnson T, Roghmann MC, Cross RK. Disparities in the use of immunomodulators and biologics for the treatment of inflammatory bowel disease: a retrospective cohort study. Inflamm Bowel Dis. Jan 2008;14(1):13-9. doi:10.1002/ibd.20298
- Straus WL, Eisen GM, Sandler RS, Murray SC, Sessions JT. Crohn’s disease: does race matter? The Mid-Atlantic Crohn’s Disease Study Group. Am J Gastroenterol. Feb 2000;95(2):479-83. doi:10.1111/j.1572-0241.2000.t01-1-01531.x
- Azevedo RSS, de Sousa JR, Araujo MTF, et al. In situ immune response and mechanisms of cell damage in central nervous system of fatal cases microcephaly by Zika virus. Sci Rep. Jan 8 2018;8(1):1. doi:10.1038/s41598-017-17765-5
- Nguyen GC, LaVeist TA, Harris ML, Wang MH, Datta LW, Brant SR. Racial disparities in utilization of specialist care and medications in inflammatory bowel disease. Am J Gastroenterol. Oct 2010;105(10):2202-8. doi:10.1038/ajg.2010.202
- Vust S. [The child facing a serious disease]. Rev Med Suisse Romande. Oct 1994;114(10):915-7. L’enfant face a la maladie grave.
- Afzali A, Cross RK. Racial and Ethnic Minorities with Inflammatory Bowel Disease in the United States: A Systematic Review of Disease Characteristics and Differences. Inflamm Bowel Dis. Aug 2016;22(8):2023-40. doi:10.1097/MIB.0000000000000835
- Shi HY, Levy AN, Trivedi HD, Chan FKL, Ng SC, Ananthakrishnan AN. Ethnicity Influences Phenotype and Outcomes in Inflammatory Bowel Disease: A Systematic Review and Meta-analysis of Population-based Studies. Clin Gastroenterol Hepatol. Feb 2018;16(2):190-197 e11. doi:10.1016/j.cgh.2017.05.047
- Walker CH, Arora SS, Colantonio LD, et al. Rates of hospitalization among African American and Caucasian American patients with Crohn’s disease seen at a tertiary care center. Gastroenterology Report. 2016;5(4):288-292. doi:10.1093/gastro/gow036
- Montgomery SR, Jr., Butler PD, Wirtalla CJ, et al. Racial disparities in surgical outcomes of patients with Inflammatory Bowel Disease. Am J Surg. Jun 2018;215(6):1046-1050. doi:10.1016/j.amjsurg.2018.05.011
- Liu JJ, Abraham BP, Adamson P, et al. The Current State of Care for Black and Hispanic Inflammatory Bowel Disease Patients. Inflamm Bowel Dis. Feb 1 2023;29(2):297-307. doi:10.1093/ibd/izac124

