SIDS and Vaccination Risks: A Critical Review
Sudden Infant Death Syndrome: A Review and Re-evaluation of Vaccination Risks
Paul Nathan Goldwater1, Reginald M. Gorczynski2, Edward J. Steele3
- Adelaide Medical School, Faculty of Health and Medical Sciences, Adelaide University, Adelaide, SA, Australia.
- Institute of Medical Science & Departments of Immunology and Surgery, University of Toronto, Canada.
- Melville Analytics Pty Ltd and Immunomics, Brisbane, Australia.
OPEN ACCESS
PUBLISHED: 31 March 2025 in the Medical Research Archives, Volume 13 Issue 3
CITATION: Goldwater, P., N., Gorczynski, R., M., Steele, E., J., 2025. Sudden Infant Death Syndrome: A Review and Re-evaluation of Vaccination Risks. Medical Research Archives, [online] 13(3). https://doi.org/10.18103/mra.v13i3.6349
COPYRIGHT: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI https://doi.org/10.18103/mra.v13i3.6349
REVIEW ARTICLE
ABSTRACT
Sudden infant death syndrome (SIDS) remains a leading cause of infant mortality. Medical science’s failure to elucidate its cause is opprobrious. SIDS shares many epidemiological traits with infectious diseases. Autopsy findings show very consistent findings in ~90% of cases, and immunological investigations consistently reveal underlying activation of inflammatory pathways. Surprisingly, most mainstream researchers have largely ignored these key findings. The “Triple Risk Hypothesis” remains the mainstay for mainstream researchers. The hypothesis encompasses three main ideas: 1) a vulnerable infant, 2) a critical development period and 3) an exogenous stressor which combine lethally. Stressors include prone sleeping, smoke exposure, and overheating. Infections, though often overlooked, can trigger deadly immune responses. The COVID-19 pandemic and the use of parenterally administered SARS-Cov-2 vaccines provided an example of a lethal immunopathogenicity in elderly comorbid patients. During the pandemic, we reviewed papers on the impact of injected vaccines on infant mortality. The papers analysed vaccine adverse event reporting systems (VAERS) to conclude a possible causal relationship existed. It is of particular note that approximately 50% of cases in the VAERS dataset have been diagnosed as Sudden Infant Death Syndrome (SIDS). VAERS are known to capture a small minority of vaccine associated adverse events (estimated to be ~1%). Studies indicate that vaccination is preventative, but control groups often have more SIDS risk factors. The findings from the VAERS are of significant concern to the scientific, medical, public, and governmental communities. This review provides a scientific analysis of this apparent problem and proposes a case for vaccines that provide IgA-based mucosal immunity to replace (where appropriate) the potentially harmful parenterally administered vaccines currently in use. In addition to recommending the use of new safe vaccines, we highlight the role of breastfeeding in the prevention of sudden infant death syndrome (SIDS).
Keywords: Sudden infant death syndrome, vaccination, immunisation, adjuvants, breastfeeding
Introduction and background
The causes of sudden infant death syndrome (SIDS) (as defined in Krous et al) and Sudden Unexplained Infant Death (SUID) in the first year of life still combine to account for much of the infant mortality rate (IMR) and remain a mystery in modern developed industrialised countries, despite great advances in health care systems. This IMR has largely ranged over the period 2009 to 2019 from about 1.6 to 6 deaths per 1000 live births per year and understanding this conundrum remains a very real research challenge for medicine and paediatrics. We suggest that an improved understanding may require the addition of new lateral thinking along with research approaches and insights based on both old reliable knowledge and new facts, and more recent data relevant to the problem. One of us (PNG), has spent the best part of the past 35 years on this intractable problem and has concluded that the mainstream approaches may well be flawed at an unrecognised fundamental level. It is in that spirit this critical analytical review has been written with new colleagues as suggested from the fields of mucosal immunology, as well molecular-cellular and clinical immunology (RMG, EJS). This paper now focuses on the role of infant infection episodes resulting in sudden unexpected death in the context of the immaturity and/or breakdown of normal Innate and Adaptive acquired immunity. The review used data derived from peer-reviewed papers obtained from PubMed, Google Scholar and Open Evidence. We suggest below that while regular breast feeding by a healthy mother plays a known protective role, there is evidence that in contrast, the childhood vaccination schedule is causally associated as a trigger for SIDS. Such insights have been re-enforced from lessons learned of the importance of both innate and acquired mucosal immunity during the public health response to COVID-19, particularly a detailed analysis of the effects of collapse of SARS-CoV-2 immunity in elderly co-morbid vulnerable patients. The paper expands on, and extends, the main themes and conclusions reached earlier which emphasized the clear importance of understanding SIDS epidemiology, pathology observations and laboratory data derived from autopsy (advanced in the context of the proposed ‘Infection and Epidemiology’ approach to the problem).
Short comings in mainstream SIDS research
Mainstream SIDS researchers have held to the tenets of the Triple Risk Hypothesis without due consideration of all the clinical, epidemiological and pathological information. A dogmatic assertion that SIDS has its origin within the central nervous system encompassing homeostatic disruption of heart, breathing and arousal functions has adversely confined research into the problem. Guntheroth and Spiers concluded the hypothesis added little if any improvement of our understanding of the cause of SIDS. Mainstream researchers have found few, if any consistent correlations with epidemiology or pathology. The hypothesis posits a vulnerable infant, a critical developmental period, and an exogenous stressor (such as prone sleeping position, overheating, or exposure to tobacco smoke). These factors can be applied to numerous infectious diseases or immunological challenges. A major part of the problem has been the mainstream embrace of a confusing notion that underlying pathogenetic mechanisms are heterogeneous in nature. An examination of the pathological findings tells another story: a heterogeneous mechanism would offer a panoply of pathological findings, whereas in SIDS we find intrathoracic petechial haemorrhages in ~90% of cases. Occam’s razor would suggest this would be due to a single mechanism. The remaining approximately 10% includes inborn errors of metabolism, cardiac arrhythmias, and other conditions. Because of the distraction by the Triple Risk Hypothesis and its focus on the sleeping environment, a wealth of information has been missed through under-investigation of important pathological findings including liquid unclotted heart blood, the intrathoracic petechial haemorrhages and organ weight changes. Raised fibrin degradation products was a significant finding yet only two publications have considered clotting abnormalities in SIDS. In addition, petechiae involving the thymus (a non-respiratory organ) and the epidcardium and pleura have been attributed to air-pressure changes within the chest, or processes involving asphyxiation. Animal studies have only provided confusing and inconsistent results. The possibility that petechiae result from an immunopathological process has not been considered. Additionally, there has been limited progress in explaining the organ weight changes observed in SIDS, with the thymus showing the most notable differences. The fact that sera from SIDS cases is often lethal to chick embryos or that staphylococcal toxins or staphylococcal enterotoxin genes are a frequent finding in SIDS cases has been ignored by the mainstream. Of particular concern is that key and established epidemiological risk factors have been under-investigated. Little heed has been given to the work of Ponsonby and colleagues whose 1993 Tasmanian study revealed a 10-fold increased risk of SIDS if prone sleeping babies had features of infection, while in supine babies there was no increased risk. The study was supported by the 1999 findings of the Nordic SIDS Epidemiological study of Helweg-Larsen and colleagues whose study observed a 29-fold increased risk of SIDS with infection. The nature of the epidemiology of the efficacy of induced immunity by vaccination in relation to SUID and SIDS surely qualifies for close examination as vaccination, in many ways, mimics infection and deserves detailed discussion. This issue is addressed below.
The ‘Infection and Epidemiology’ model
In our view the ‘Infection and Epidemiology’ explanation better fits the autopsy and pathology laboratory data. To allow a focus on the analyses which follow we paraphrase the main previous conclusions viz. ‘a most likely causal epidemiological factor is that SIDS is caused by a sudden dual infection with a respiratory virus and toxigenic bacteria with the evidence suggesting a respiratory viral infection, which could possibly act as a SIDS trigger, …. and in many studies, more than 75% of SIDS babies featured recent or active respiratory tract infections.’ Co-authors RMG and EJS find this conclusion compelling and logical. It fits with a wealth of new data and recent analyses of the causes of the lethal respiratory crises during the COVID-19 pandemic. It also underlines the importance of the classic respiratory-crisis sequence of uncontrolled viral replication in the lungs and respiratory tract, followed by extensive epithelial viral damage with subsequent rapid growth of opportunistic resident microflora or opportunistic exogenous toxigenic bacterial infection. When left unchecked, as in lethal cases of the acute respiratory crisis caused by SARS-CoV-2 in vulnerable patients, these events can rapidly lead to severe bronchitis and pneumonia and death.
Discussion
Lessons from COVID-19 and the importance of mucosal immunity
Local mucosal immunity both innate and adaptive in respiratory tract infections. The experience analysing relevant immunological and viral mutation genetic data during the recent COVID-19 Pandemic (involving SARS-CoV-2 infections) reminded us, and alerted us, to the key factors that led to the rapid deaths of immune-defenceless elder co-morbid patients in aged care and nursing facilities. In these facilities in Melbourne, Australia in 2020 (June-Sept) during the first COVID-19 wave to hit the State of Victoria (before the vaccine roll-out) the median age at Covid-associated death from the respiratory crisis was 84 yr. The other indicator from the pandemic was that the coronavirus caused a relatively mild ‘common cold infection’ in healthy people but spread on a pandemic global scale via asymptomatic or mildly infected healthy individuals. These ‘carriers’ shedding virus passed on a potentially lethal ‘common cold’ coronavirus infection to the vulnerable elderly. This seemed true then and again later when assessed by the Fauci group in 2023 as such events could lead to a potentially lethal outcome. However, our attention to deeper underlying causal factors narrowed in the light of the protection failure of the roll-out of the global mRNA spike protein vaccine during 2021. We use the term “vaccine failure” deliberately based on these and many other hard extensive epidemiological data and re-evaluation analyses following the pandemic including the importance of induced secretory IgA immunity. Further, in 2020 a year before the vaccine roll-out in a very large population-level observational study in Denmark it was clear that prior infection with SARS-CoV-2 afforded clear protection against re-infection. This is an expected result if normal acquired mucosal ‘natural’ immunity involving protective dimeric and highly avid secretory IgA antibodies were induced through oro-nasal infection in a prior epidemic wave in Denmark in subsequent protected individuals. It was concluded that the main feature of the failure of the COVID-19 vaccines was they did not protect from, nor prevent transmission of, SARS-CoV-2 because of a failure to pay attention to, and activate, front-line mucosal innate and adaptive immunity. In Melbourne in June -Sept 2020 when healthy asymptomatic carriers of SARS-CoV-2 spread the coronavirus into closed aged care facilities and nursing homes, the ‘immune defenceless elderly comorbid’ subgroups therein, lacking immunity from prior exposure, had a complete absence of normal Innate Immune defences allowing uncontrolled viral replication and thus a rapid respiratory crisis. The environments (fomite surfaces of all types) within these facilities were almost certainly extensively contaminated by the liberated viral-laden aerosols. All the most vulnerable died during the first wave in Melbourne. In other instances, a mortality rate of 25.6% was noted among hospitalized elderly patients with COVID-19 infection where the vaccine did not provide protection. In those over 80 years the mortality rate was 30.6%. Matsumura and colleagues reported a mortality rate of 25.8% in vaccinated nursing home residents exposed during the Omicron variant outbreak. We offer now some underlying protective mechanisms or lack thereof, relevant in such patients.
The main anti-viral nonspecific yet potent and protective innate immune enzymes
are the APOBEC (apolipoprotein B mRNA-editing, catalytic polypeptide) and ADAR (adenosine deaminase acting on RNA) deaminases, which are potent gene mutators of RNA (and DNA) viral genomes. These are but two key sets of molecules in a vast cast of up to a thousand diverse proteins and RNA enzyme/effector molecules targeting many facets of typical pathogen life cycles (viral or bacterial). These are all unleashed in the first few hours in a healthy cell when foreign pathogen entry is ‘sensed’. This is cascade-gene expression activation of the Interferon Stimulated Gene pathways involving type I and type III IFN inducible innate anti-viral immunity. These are ‘germline encoded’ immediately reactive cell defence responses. They have evolved over eons of evolutional time in all eukaryotic cells. The APOBEC and ADAR deaminase mutators can cripple the replicating virus trying to gain replicative traction by introducing many lethal mutations throughout the length of the 29,903 nucleotide (nt) SARS-CoV-2 RNA genome. The APOBEC cytosine deaminases cause cytosine (C) to uracil (U, read as thymine T) mutations and the ADAR adenosine deaminase mutators cause adenosine (A) to inosine (I) (read as guanine, G) mutations. In the infected aged-care and nursing facilities the SARS-CoV-2 can replicate freely without mutating, whereas infected normal health care visitors and health care staff these deaminases progressively mutate and cripple and thus dampen and attenuate the further virulence of the virus.
The vaccines delivered parenterally via a ‘jab in the arm’ or intramuscularly (i.m) thus would not selectively stimulate essential protective mucosal adaptive immunity, such as protective dimeric secretory IgA antibodies into mucosal secretions. Vaccine efficacy was monitored by induction of IgG, or systemic immunity, not IgA or mucosal immunity. This dichotomy is now accepted even by the Fauci led group who advised the White House on COVID-19 vaccination strategy. By one informed reasonable immunological view all they did was complicate matters by inducing inappropriate systemic immunity of inflammatory complement-fixing IgG antibodies (i.e. the wrong type of immunity for mucosal respiratory tract infections of this type). It is therefore necessary for respiratory tract infections entering via the oral-nasal portal of entry to apply stimulating vaccine antigens also via the oral-nasal route. This will reliably induce protective dimeric secretory IgA mucosal immunity which is an avid antigen binder and viral, bacterial cell adhesin and toxin neutralizing antibody. It is non-complement fixing and a blocker of the complement fixing properties of co-stimulated IgG that can concurrently seep into mucosal areas via capillaries and potentially excite unnecessary inflammatory foci. A role for this type of ‘natural mucosal immunity’ clearly must have occurred in the natural post-infection immunity cycles prior to the vaccine rollout from detailed longitudinal population studies in 2020.
Prolonged breast feeding and incidence of SIDS
It should be now clear why we consider this factor, or lack thereof, as a major risk factor in SIDS epidemiology. For about 50 years now many babies are quickly shunted off the breast onto formula milks. This is a troubling trend in both advanced industrialized and developing countries where such products are promoted. A continuous supply of highly avid viral and pathogen-adhesin specific neutralising activity by secretory IgA antibodies is very important. The mother produces and secretes these antibodies in very high concentration particularly in colostrum but also the early and later milk. These antibodies would cover the whole spectrum of pathogen antigen specificities experienced by the feeding mother in real-time. This (together with important immune cells transferred in breast milk) would seem essential to health in such babies (backed up also by an intact healthy Innate Immune system, below). This is because the acquired or Adaptive Immune system is still developing in neonates in first year of life. All the major breast feeding national support groups have recognised this for many years (e.g. Australian Breast Feeding Association, https://www.breastfeeding.asn.au) and population-based data strongly support their claims. The consensus is that breastfeeding for four to six months or even longer can be associated with up a 60 percent lower risk of SIDS. When these facts and realities are placed in the context of maternal mucosal secretory IgA immunity this is not surprising to us. The absence of real prolonged breast feeding in western industrialised countries must now be considered as a key infection suppressive and protective factor against SIDS irrespective of other putative triggers that may be identified. Immunisation during pregnancy offers a means of providing IgA to the newborn via colostrum and breastmilk.
Nonspecific innate mucosal immunity stimulated via oro-nasal infection
There is now another additional antigen non-specific immunity-based factor about mucosal immunity that has been brought into sharp relief during and following the COVID-19 pandemic and should now be discussed at length. During this period and discussed in detail by us, it was clear that the early Innate Immune response to respiratory viral infections was crucial in SARS-CoV-2 immunity, and had to be fully functional. As implied above Type I and type III interferon (IFN) inducible anti-viral immunity (that activates APOBEC, ADAR enzymes) is particularly affected in elderly COVID-19 patients allowing the SARS-CoV-2 virus to flourish. This elderly co-morbid subgroup clearly displayed these deficits in Innate Immunity and these deficits were also evident in many other studies.
Childhood vaccination and SIDS
Residual incidence of SIDS in advanced industrialized countries: clear role of the childhood vaccine schedule in the first year of life -Miller & Goldmann 2011. Why is there a residual incidence of SIDS occurring within the IMR of 2 to 6 deaths per 1000 live births per year? Should it be much lower given the great success of modern medicine in the developed world? What are the causes of this residual rate? We have discussed how lethal inadvertent loss of Innate Immunity can put a baby at increased risk of SIDS. The putative role of prior vaccination is thus a seriously important issue and needs to be confronted cooly and objectively. The issue has had an extremely contentious and destructive history dating to the 1990s and earlier. This medical and biomedical controversy emerged with great intensity in the late 1990s following the 1998 claims in London linking measles mumps rubella (MMR) vaccination to autism by British consultant gastroenterologist Dr Andrew Wakefield. The details can be found via a Google search. A similar quite savage press campaign was launched against Australian academic Dr Judy Wilyman in 2015 because of her important systematic analysis of vaccine efficacy and safety over the past 40 or so years. That controversy can also be accessed via Google search.
We summarise this issue systematically and at some depth and cover recent developments and data analyses published in peer-reviewed journals (2020-2023). These are the very important findings initiated and led by two United States statisticians and biomedical informaticians, NZ Miller and GS Goldman first in 2011 and in subsequent publications.
As defined at many accessible data bases infant mortality rate (IMR) is the number of deaths of infants under one year old per 1,000 live births in the same year in that country. In the first big study and analysis for the year 2009 the IMR data were assembled from The World Factbook. In the more recent wider and deeper analyses for year 2019 the data are from the UNICEF Data Warehouse. Childhood immunization schedules with the number of infant vaccine doses required by each nation were collected from the World Health Organization, the European Centre for Disease Prevention and Control and national governments. As indicated Miller and Goldman are statisticians and bioinformaticians they analyse objective data using standard statistical tools and approaches. Their exact analyses and conclusions can be easily checked by other similarly qualified and competent statisticians analysing the same data. For year 2009 over 30 developed countries the IMR range was 2.31 to 6.22. For year 2019 over 44 developed countries the IMR range was 1.59 to 5.42. In both years the United States had the highest IMR rates at 5.42 and highest number of vaccine doses scheduled in that year. At time of submission of our paper we are not aware that their exact analyses and precise conclusions have been refuted in any peer-reviewed publication. However, as made clear in an updated reanalysis and response to a critic (Dr. E. Bailey and her students, a professor at Brigham Young University, BYU), levelled a criticism of these studies which remains ‘unpublished’ as a preprint at the medRxiv website. While remaining unconvinced by this unfocused and non-peer-reviewed article, we instead refer readers to a more recent and systematic critical response and data analysis update.
Consequences of multiple doses of adjuvant-associated parenterally administered vaccines given to babies in the first year of life – Miller & Goldman 2011
We now address this central issue in the spirit of encouraging new directions of SIDS research and vaccine development. The Miller and Goldman 2011 study is entitled “Infant mortality rates regressed against number of vaccine doses routinely given: Is there a biochemical or synergistic toxicity?” This appeared in the peer-reviewed SAGE journal Human Experimental Toxicology. Two of us (RMG, EJS) are traditionally trained immunologists accustomed to the important value of vaccines and the very foundations (raison d’etre) of modern immunology. Our experience analysing what happened in COVID-19 and vaccine roll-out deconstructed those foundational beliefs. Over the past 5 years we have undergone a rethink of vaccine efficacy (and safety) and now examine claims of vaccine protective efficacy and safety with scepticism and seek proof through rigorous clinical trial data (Stage I, II, III, etc). Indeed, given what we now know, summarised here, and based on our experience analysing what happened in COVID-19 and vaccine roll-out challenged those foundational beliefs, and after critically examining claims of vaccine protective efficacy and safety, we suggest a re-think of all mandated and scheduled vaccinations, particularly in vulnerable developing babies and children, until we can achieve a consensus on the cost/benefits at the scientific and clinical level.
The epidemiology of childhood vaccine efficacy and safety across 30 advanced industrialised countries (irrespective of antigen specificity) of newborn babies in the first year of life strongly implies (in a regression analysis) that increased numbers of vaccine doses could cause sudden infant death syndrome. There are profound implications arising from that global study over 15 years ago. The Miller and Goldman report of 2011 is not widely known or cited and can easily be missed in literature searches.
Figures 1, 2 Taken from Miller & Goldman 2011 Open Access publication.
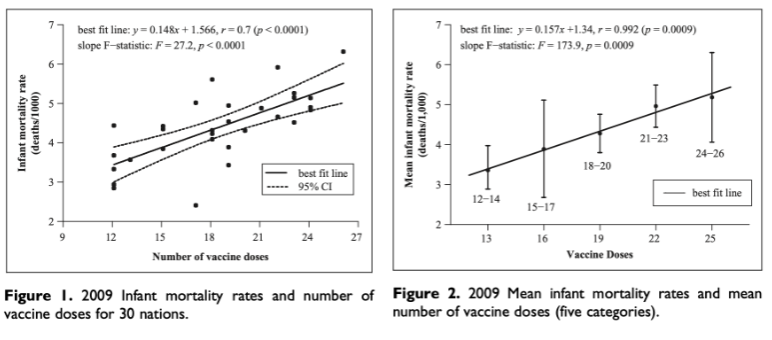
We note the following:
- There is a clear putative causal correlation between the number of vaccine shots and incidence of and infant mortality and probably SIDS. Doubling the number of shots in the first year of life doubles the number of SUID (and SIDS) deaths.
- The regression is clearly independent of antigen specificity, thus the number of adjuvant doses could be very important in a causal pathway, possibly reflecting very strong non-specific immune stimulatory cytokine effects that exacerbate the regulation of Innate Immunity on a systemic scale in newborn babies. Another consideration is the effect on Blood Brain Barrier permeability.
The US vaccination schedule provides 6, 5 and 6 vaccines at 2, 4 and 6 months respectively. In our considered opinion this number of vaccine-adjuvant shots are likely to cause systemic immunological havoc in the baby’s normal immune development and many other normal central nervous system (CNS) developmental interconnected processes that are in synchrony with a baby’s healthy growth. The latter include, but may not be limited to, direct neurological wiring and cytokine cross talk that is now recognised as a well-established phenomenon since the pioneering 1974 work of Ader and Cohen and others. The excellent 2018 book by Edward Bullmore and the classic textbook by Ader, Felten and Cohen are now essential cross-disciplinary background reading for researchers in the SIDS field. A recent sample of this field includes key cytokines involved such as Interleukin-1 beta (IL-1β) which is essential for normal CNS development and synaptic connections. It has been shown to influence axonal dynamics and growth, as well as synaptic function. Other cytokines include Interleukin-6 (IL-6) which participates in neurogenesis and glial cell development. Elevated levels of IL-6 during critical periods of brain development can disrupt these processes and lead to behavioural changes associated with neurodevelopmental disorders, and Tumor Necrosis Factor-alpha (TNF-α) which participates in synaptic formation, maturation, plasticity and neural circuit function. In addition, Interleukin-33 (IL-33) which is produced by astrocytes, stimulates microglial synapse engulfment and normalises synapse number and neural circuit function, and Interleukin-13 (IL-13) the product of group 2 innate lymphoid cells, is critical for the development of inhibitory synapses and social behaviour.
SIDS events which temporally cluster in proximity to infant vaccine doses – Miller 2021
This is another important and significant analysis by Miller (as sole author) of 2605 infant deaths (from 1990-2019) reported to the United States Adverse Event Reporting System (VAERS) database. In this careful focused analysis Miller reports that 58% of the infant deaths clustered within 3 days post-vaccination and 78.3 % clustered within 7 days post-vaccination. He reported that this excess of deaths during the early post-vaccination period was statistically significant (p <0.00001). In his paper Miller observed that in 2011 a European hexavalent vaccine manufacturer, GlaxoSmithKline (GSK), produced a confidential report on SIDS which was made publicly available by the Italian Court. Sudden deaths occurring within 20 days after hexavalent vaccination were documented and seemingly passed off as insignificant as not exceeding the background incidence or expected number of cases. The manufacturer’s conclusion that its hexavalent vaccine does not increase the risk of sudden death was erroneous as the confidential report showed that 62.7 % of these deaths clustered within 3 days post-vaccination and 89.6 % occurred within 7 days post-vaccination, and more significantly, 97 % (65 of the 67 reported infant deaths) occurred in the first 10 days post-vaccination while just 3% (2 of the 67 infant deaths) occurred in the next 10 days.
An earlier study by Silvers and colleagues examined fatalities reported to the Vaccine Adverse Event Reporting System (VAERS) from 1990 to 1997. The study found that nearly half of the documented deaths were attributed to sudden infant death syndrome (SIDS). However, because of the subsequent trend of decreasing numbers of deaths reported to VAERS since 1992-1993 appeared to parallel the decrease in overall SIDS cases in the US general population following implementation of the ‘Back to Sleep’ program the authors concluded that findings of past controlled studies showing an association between infant vaccination and SIDS is coincidental and not causal. In a separate study of Haemophilus influenzae b vaccination, Moro and colleagues noted that 51% of death reports with autopsy/death certificate records were attributed to SIDS. This finding plus that of Miller adds to our concern.
Miller’s review of the medical literature discusses the various pathogenic mechanism explanations behind these fatal events. These include the role of inflammatory cytokines as neuromodulators in the medulla of the infant brain that can precede an abnormal response to the accumulation of carbon dioxide and extreme aberrant respiratory control induced by vaccine adjuvants that cross the Blood Brain Barrier. This type of systemic-wide inflammation (multiple cytokines crossing the Blood Brain Barrier) impacting the pathology of an “inflamed” brain is now well documented as discussed already. Finally, Miller discusses the known synergistic biochemical toxicity caused by multiple vaccines administered concurrently. It is difficult not to concur with Miller’s conclusion … “While the findings in this paper are not proof of an association between infant vaccines and infant deaths, they are highly suggestive of a causal relationship.” This understatement does not escape us!
Update and reaffirmation of 2011 data: 2019 data confirms correlation between number of vaccine doses and infant mortality rate – Goldman & Miller 2023
As well as a systematic refutation of the unpublished, yet internet-posted, claims of a group of critics, Goldman and Miller reaffirm their 2011 report for year 2009 on the more recent data for 2019. On expanding the data assessment from the top 30 to the 46 nations with the best IMR scores they report a statistically significant positive correlation between the number of vaccine doses and IMR. This study replicates their original study using updated 2019 data – that is “a replication of our original study using updated 2019 data corroborated the trend found in our first paper (r = 0.45, p = .002).” If they systematically add data from more background noise of underdeveloped and third world countries, they clearly dilute the significance of the original reported regression. The sole unpublished critics wanted Miller and Goldman include all (uncontrolled) data from 185 reporting countries, a clearly spurious proposition. Upon doing that even with the presence of many confounding variables, they still show a residual (small) … “statistically significant positive correlation of r = 0.16 (p < .03) … that corroborates the positive trend in our study” which they reported in 2011.
Recent data from neonatology has provided interesting findings in relation to vaccination of premature infants and development of apnoea. The randomised trial of hospitalized preterm infants resulted in higher odds of apnoea within 48 hours after the 2-month vaccinations versus no vaccinations. The authors’ interpretation favoured current vaccination recommendations for hospitalized preterm infants; they considered apnoea a non-serious adverse event. Outside hospital, preterm immunisation could have a different outcome.
Encouragement should be given to vaccination during pregnancy; pertussis-containing vaccines given to pregnant women provides high levels of specific IgA in breast milk. Vaccination before 24 weeks, between 24 and 27+6 weeks, or between 28 and 31+6 weeks does not significantly impact levels of antigen-specific IgA in colostrum or breast milk at 14 days postpartum. Gestational immunization with the BNT162b2 mRNA COVID-19 vaccine during the second or third trimester induces a robust IgA response in human milk with particularly high levels of IgA in both colostrum and mature milk with higher levels of IgA being induced after vaccination in the second trimester compared to the third trimester.
Limitations
It is important to recognise that the present review, by its nature, has limitations. The first would be criticism of the use of IMR data as a possible surrogate for SIDS. The VAERS data used only diagnoses of sudden unexplained infant death and SIDS. Such diagnoses are subject to variation and coding issues. However, given that SIDS makes up some 43% of IMR cases and that the cases were evenly distributed within the populations studied, this would suggest the detected risk ratios are likely to be applied reasonably accurately. The sex distribution (60% male) fits with usual SIDS epidemiology as Goldman and Miller have pointed out.
Miller (2021) noted ‘VAERS is a passive surveillance system, which means that reports about adverse events are not actively solicited or automatically collected. Moreover, parents are rarely warned to look for serious adverse reactions in their vaccinated children. Underreporting is a known limitation of passive surveillance systems; VAERS only captures a fraction of actual adverse events. A recent report prepared by Harvard Pilgrim Health Care for the U.S. Department of Health and Human Services (HHS) (2010) found that “fewer than 1% of vaccine adverse events are reported.” This means that infant deaths and SIDS cases that occur post-vaccination may be underreported by a factor of 100.
Unfortunately, there are no studies relating to the 1970s and 1980s, periods of peak SIDS incidence. Had data been available additional insights into the problem may well have been acquired. The other limitation for this review may arise externally and illogically from vested interested protagonists of parenteral vaccines who would likely call us “anti-vaxxers” or more likely ignore us as part of citation amnesia or the ‘disregard syndrome’.
We declare we are not anti-vaccination but provide this review in the hope that the advances already made in the development of safe and effective vaccines that induce, where appropriate, mucosal immunity (as opposed to, again where appropriate systemic vaccines) are acknowledged by the pharmaceutical industry, governments, the public and SIDS researchers alike, to recognize the limitation that the approach “one size fits all” is simply scientifically incorrect. There will, of course, be a transition period in which we hope all the positive and negative elements and difficulties can be ironed out for the babies, children and adults of the future.
Conclusion
There is an urgent need for a serious generously funded public health research program to study all these identified issues properly in developed countries able to afford it. There is a strong sense and unease abroad in the general public that SIDS is just the lethal tip of the iceberg. Of consideration is a subset of babies that survive the first year of the often-mandated vaccine schedule but for developmental problems to arise beyond the first year. Indeed, they may in theory later display, or develop, a variety of developmental and neurological disorders – autism spectrum disorder (ASD), full blown autism itself, Asperger’s syndrome, attention-deficit/hyperactivity disorder (ADHD), the most common mental disorders affecting children. Recently Miller and his associate Hooker have also addressed this issue by analysing health outcomes in vaccinated versus unvaccinated children uncovering associations with developmental delays, asthma, ear infections and gastrointestinal disorders.
In our opinion we suspect the rise in the incidence of all these disorders over the past 30-40 years is not simply ‘greater awareness” or improved reporting. It may well be related to the Nationally mandated childhood vaccine schedules. Indeed, we can specifically ask: Is this a reason why we now need in an apparently ‘wealthy’, and also by implication, ‘healthy’ country like Australia, a Federal Taxpayer-funded National Disabilities Insurance Scheme (NDIS)? This review does not prove an association between infant vaccinations and SIDS, it reveals unusual patterns highly suggestive of a causal relationship and should elicit safety concerns. One obvious conclusion from our review of specific and focused relevant data is that a truer understanding of the ‘SIDS Enigma’ and its real epidemiological risk factors may have been staring at us in plain sight. We offer this critical review and set of scientific-based explanations in that spirit.
Conflict of Interest: The authors have no conflicts of interest to declare.
Funding Statement: None.
Acknowledgements: None.
References:
- Krous HF, Beckwith JB, Byard RW, et al.: Sudden infant death syndrome and unclassified sudden infant deaths: a definitional and diagnostic approach. Pediatrics. 2004;114:234 – 2388. DOI: 10.1542/peds.114.1.234
- Raab CP. Sudden Unexpected Infant Death (SUID) and Sudden Infant Death Syndrome (SIDS). Merck (MSD) Manual. Professional Version. Published Feb 2023. Accessed January 8, 2025 https://www.msdmanuals.com/professional/pediatrics/miscellaneous-disorders-in-infants-and-children/sudden-unexpected-infant-death-suid-and-sudden-infant-death-syndrome-sids
- Miller NZ, Goldman S.: Infant mortality rates regressed against number of vaccine doses routinely given: Is there a biochemical or synergistic toxicity? Human Experimental Toxicology, 2011:30:1420–1428. DOI: 10.1177/0960327111407644
- Goldman GS, Miller NZ.: Reaffirming a Positive Correlation Between Number of Vaccine Doses and Infant Mortality Rates: A Response to Critics. Cureus 2023;15(2), e34566. DOI 10.7759/cureus.34566.
- Goldwater PN.: The Science (or Nonscience) of Research Into Sudden Infant Death Syndrome (SIDS). Front. Pediatr. 2022;10:865051. https://doi.org/10.3389/fped.2022.865051
- Goldwater, P.N.: Current SIDS research: time to resolve conflicting research hypotheses and collaborate. Pediatr Res. 2023;12:1–5. https://doi.org/10.1038/s41390-023-02611-4
- Lindley RA, Steele EJ.: Analysis of SARS-CoV-2 haplotypes and genomic sequences during 2020 in Victoria, Australia, in the context of putative deficits in innate immune deaminase anti-viral responses. Scand J Immunol. 2021;94:e13100. https://doi.org/10.1111/sji.13100
- Guntheroth WG, Spiers PS.: The triple risk hypotheses in sudden infant death syndrome. Pediatrics. 2002;110: e64. https://doi.org/10.1542/peds.110.5.e64
- Goldwater PN, Gebien DJ.: Metabolic acidosis and sudden infant death syndrome: overlooked data provides insight into SIDS pathogenesis. World J Pediatr 2024; https://doi.org/10.1007/s12519-024-00860-9
- Goldwater PN, Williams V, Bourne AJ, Byard RW.: Sudden infant death syndrome: a possible clue to causation. Med J Austr 1990;153:59-60. DOI: 10.5694/j.1326-5377.1990.tb125473.x
- Emura I, Usuda H.: Biochemical, Cytological and Histopathological Examination of Sudden Unexpected Death in Infancy. Pathology International. 2011;61:469-474. https://doi.org/10.1111/j.1440-1827.2011.02690.x
- Hanssen TA, Jørgensen L.: Obstruction of the Lung Capillaries by Blood Platelet Aggregates and Leucocytes in Sudden Infant Death Syndrome. APMIS : Acta Pathologica, Microbiologica, Et Immunologica Scandinavica. 2010;118: 958-967. https://doi.org/10.1111/j.1600-0463.2010.02651.x
- Sayers NM, Drucker DB, Hutchinson IV, Barson AJ.: Preliminary investigation of lethally toxic sera of sudden infant death syndrome victims and neutralization by commercially available immunoglobulins and adult sera. FEMS Immunol Med Microbiol. 1999;5:193 – 198. https://doi.org/10.1111/j.1574-695X.1999.tb01343.x
- Malam JE, Carrick GF, Telford DR, Morris JA.: Staphylococcal Toxins and Sudden Infant Death Syndrome. J Clin Pathol. 1992;45:716 – 721. https://doi.org/10.1136/jcp.45.8.716
- Highet AR, Goldwater PN. :Staphylococcal enterotoxin genes are common in Staphylococcus aureus intestinal flora in sudden infant death syndrome (SIDS) and live comparison infants. FEMS Immunol Med Microbiol. 2009;57:151-155. https://doi.org/10.1111/j.1574-695X.2009.00592.x
- Ponsonby AL, Dwyer T, Gibbons LE, Cochrane JA, Wang YG.: Factors potentiating the risk of sudden infant death syndrome associated with the prone position. N Engl J Med. 1993;329:377-382. DOI: 10.1056/NEJM19930805329060
- Helweg-Larsen K, Lundemose JB, Oyen N.: et al. Interactions of infectious symptoms and modifiable risk factors in sudden infant death syndrome. The Nordic Epidemiological SIDS study. Acta Pediatrica. 1999;88: 521- 527. https://doi.org/10.1111/j.1651-2227.1999.tb00168.x
- Steele EJ, Lindley RA.: Analysis of APOBEC and ADAR Deaminase-Driven Riboswitch Haplotypes in COVID-19 RNA Strain Variants and the Implications for Vaccine Design. In Understanding the Origin and Global Spread of COVID-19 (Wickramasinghe, Gorczynski, R.M & Steele, E.J., eds) p.111 – 143 (World Scientific Publishing Co. Pty Ltd, Singapore, 2022. https://doi.org/10.1142/9789811259081_0005
- Gorczynski RM, Lindley RA, Steele EJ, Wickramasinghe NC.: Nature of Acquired Immune Responses, Epitope Specificity and Resultant Protection from SARS-CoV-2. J.Pers. Med. 2021;11(12): 1253. https://doi.org/10.3390/jpm11121253
- Fauci AS, Lane HC, Redfield RR.: COVID-19 – Navigating the Uncharted. New Engl J Med 2020;382:1268-1269. DOI: 10.1056/NEJMe2002387
- Morens DM, Taubenberger JK, Fauci AS.: Rethinking next-generation vaccines for coronaviruses, influenzaviruses, and other respiratory viruses. Cell Host & Microbe. 2023;31:146-157. DOI: 10.1016/j.chom.2022.11.016
- Subramanian SV, Kumar A.: Increases in COVID-19 are unrelated to levels of vaccination across 68 countries and 2947 counties in the United States. Eur J Epidemiol 2021;36:1237-1240. https://doi.org/10.1007/s10654-021-00808-7
- Klein NP.: Added Benefit of COVID-19 Vaccination after Previous Infection N Engl J Med. 2022;386:1278-1279. DOI: 10.1056/NEJMe2201380
- Muttappallymyalil J, Nair SC, Changerath R, et al.: Vaccination Rate and Incidence of COVID-19 and Case Fatality Rate (CFR): A Correlational Study Using Data From 2019 to 2021. Cureus. 2022;14: e28210. DOI: 10.7759/cureus.28210
- Pisanic N, Antar AAAR, Hetrich MK, et al.: Early, robust mucosal secretory IgA but not IgG response to SARS-CoV-2 spike in oral fluid is associated with faster viral clearance and COVID-19 symptom resolution. J Infect Dis. 2024; jiae447. doi: https://doi.org/10.1101/2024.02.21.24303146
- Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S.: Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: A population-level observational study. Lancet 2021;397:1204-1212. DOI: 10.1016/S0140-6736(21)00575-4
- Díaz-Menéndez M, de la Calle-Prieto F, Montejano R. et al.: Clinical Characteristics and Outcome of Hospitalized Elderly Patients With COVID- 19 After Vaccine Failure. Vaccine. 2022;40:4307-4311. https://doi.org/10.1016/j.vaccine.2022.06.003
- Matsumura Y, Yamamoto M, Shinohara K, et al. et al. High Mortality and Morbidity Among Vaccinated Residents Infected With the SARS-CoV-2 Omicron Variant During an Outbreak in a Nursing Home in Kyoto City, Japan. Am J Infect Control 2023;51:800-806. https://doi.org/10.1016/j.ajic.2022.09.007
- Schoggins JW, Rice CM.: Interferon-stimulated genes and their antiviral effector functions. Curr Opin Virol. 2011;1:519-525. https://doi.org/10.1016/j.coviro.2011.10.008
- Schneider WM, Chevillotte MD, Rice CM.: Interferon-stimulated genes: A complex web of host defenses. Annu Rev Immunol. 2014;232:513-545. https://doi.org/10.1146/annurev-immunol-032713-120231
- Tomasi TB, Bienenstock J.: Secretory immunoglobulins. Adv Immunol . 1968;9:1-96. https://doi.org/10.1016/S0065-2776(08)60441-1
- Russell MW, Mestecky J.: Mucosal immunity: The missing link in comprehending SARS-CoV-2 infection and transmission. Front. Immunol. 2022;13:957107. https://doi.org/10.3389/fimmu.2022.957107
- Steele EJ, Chaicumpa W, Rowley D.: Isolation and biological properties of three classes of rabbit antibody to Vibrio Cholerae. J Infect Dis. 1974;130:93-103. https://doi.org/10.1093/infdis/130.2.93
- Steele EJ.: Efficiency of antibody classes in cholera immunity. PhD diss. University of Adelaide, 1975.
- Bleier BS, Ramanathan M, Lane AP.: COVID-19 vaccines may not prevent nasal SARS-CoV-2 infection and asymptomatic transmission. Otolaryngol Head Neck Surg. 2021;164:305-307. https://doi.org/10.1177/0194599820982633
- Schiavone M, Gasperetti A, Mitacchione G, Viecca M, Forleo GB.: Response to: COVID-19 reinfection vaccinated individuals as a potential source of transmission. Eur J Clin Invest. 2021;51:e13544. https://doi.org/10.1111/eci.13544
- Wilyman J.: A critical analysis of the Australian government’s rationale for its vaccination policy. Doctor of Philosophy thesis, University of Wollongong, 2015.
- Landa-Rivera JL, Pérez-Pérez, J, González-Núñez M. et al.: Population-Based Survey Showing That Breastfed Babies Have a Lower Frequency of Risk Factors for Sudden Infant Death Syndrome Than Nonbreastfed Babies. Breastfeed Med. 2022;17:182–188. https://doi.org/10.1089/bfm.2021.0113
- Lucas C, Wong P, Klein J, et al.: Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463-69. https://doi.org/10.1038/s41586-020-2588-y
- Acharya D, Liu G-Q, Gack MU.: Dysregulation of type I interferon responses in COVID-19 Nat. Rev Immunol. 2020;20:397- 398. https://doi.org/10.1038/s41577-020-0346-x
- Blanco-Melo D, Nilsson-Payant BE, Liu W-C, et al.: Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell. 2020;181:1036-1045. DOI: 10.1016/j.cell.2020.04.026
- Hadjadj J, Yatim N, Barnabei L, et al.: Impaired type I interferon activity and exacerbated inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718-724. DOI: 10.1126/science.abc6027
- Netea MG, Giamarellos-Bourboulis EJ, Domı́nguez- Andrés J, al.: Trained Immunity: A tool for reducing susceptibility to and the severity of SARS-CoV-2 infection. Cell. 2020;181:969-977. DOI: 10.1016/j.cell.2020.04.042
- Zhang Q, Bastard P, Liu Z, et al.: Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370:eabd4570. DOI: 10.1126/science.abd4570
- Moderbacher CR, Ramirez SI, Dan, JM, et al.: Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183:996-1012. DOI: 10.1016/j.cell.2020.09.038
- Sette A, Crotty S.: Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184, 1-20. DOI: 10.1016/j.cell.2021.01.007
- Xiao Y, Lidsky PV, Shirogane Y, et al.: A defective viral genome strategy elicits broad protective immunity against respiratory viruses. Cell 2021;184:6037-6051. DOI: 10.1016/j.cell.2021.11.023
- Oh JE, Song E, Moriyama M, et al.: Intranasal priming induces local lung-resident B cell populations that secrete protective mucosal antiviral IgA. Science Immunology 2021;6:eabj5129. DOI: 10.1126/sciimmunol.abj5129
- Afkhami S, D’Agostino MR, Zhang A. et al.: Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell 2022;185:896–915. DOI: 10.1016/j.cell.2022.02.005
- Lobaina Y, Chen R, Suzarte E, et al.: The Nucleocapsid Protein of SARS-CoV-2, Combined with ODN-39M, Is a Potential Component for an Intranasal Bivalent Vaccine with Broader Functionality. Viruses. 2024;16:418. https://doi.org/10.3390/v16030418
- Hooker BS, Miller NZ.: Analysis of health outcomes in vaccinated and unvaccinated children: Developmental delays, asthma, ear infections and gastrointestinal disorders. SAGE Open Med. 2020;8:1-11. doi: 10.1177/2050312120925344
- Miller NZ.: Vaccines and sudden infant death: An analysis of the VAERS database 1990-2019 and review of the medical literature. Toxicol Rep. 2021;8:1324-1335. https://doi.org/10.1016/j.toxrep.2021.06.020
- Nysetvold E, Mika T, Elison W, et al.: Infant vaccination does not predict increased infant mortality rate: correcting past misinformation [PREPRINT]. medRxiv. 2022;09:1-25. doi: https://doi.org/10.1101/2021.09.03.21263082
- Bullmore, E. The Inflamed Mind: A radical new approach to depression. Simon & Schuster, London, New York, 2018.
- Ader R, Cohen N.: Behaviourally conditioned immunosuppression. Psychosom Med 1974;37:333-342. https://www.researchgate.net/profile/Nicholas-Cohen-4/publication/21993670_Behaviorally_Conditioned_Immunosuppression/links/626c54b8b277c02187d60ee3/Behaviorally-Conditioned-Immunosuppression.pdf
- Gorczynski LV, Gorczynski CP, Terzioglu T, Gorczynski RM.: Pre- and Postnatal Influences of Neurohormonal Triggering and Behaviour on the Immune System of Offspring Adv Neuroimmune Biol. 2011;1:39-51. DOI: 10.3233/NIB-2011-004
- Ader R, Felten DL, Cohen N.: Psychoneuroimmunology, 4th edition, 2 volumes, Academic Press/Elsevier, 2006. https://shop.elsevier.com/books/psychoneuroimmunology/ader/978-0-12-088576-3
- Solek CM, Farooqi NAI, Brake N, et al.: Early Inflammation Dysregulates Neuronal Circuit Formation In Vivo via Upregulation of IL-1β. J Neuroscience. 2021;14:6353-6366. https://doi.org/10.1523/JNEUROSCI.2159-20.2021
- Velloso FJ, Wadhwa A, Kumari E, et al.: Modestly Increasing Systemic Interleukin-6 Perinatally Disturbs Secondary Germinal Zone Neurogenesis and Gliogenesis and Produces Sociability Deficits. Brain, Behavior, and Immunity. 2022;101: 23-36. https://doi.org/10.1016/j.bbi.2021.12.015
- Sager REH, Walker AK, Middleton FA, et al.: Changes in Cytokine and Cytokine Receptor Levels During Postnatal Development of the Human Dorsolateral Prefrontal Cortex. Brain, Behavior, and Immunity. 2023;111:186-201. https://doi.org/10.1016/j.bbi.2023.03.015
- Boulanger LM.: Immune Proteins in Brain Development and Synaptic Plasticity. Neuron. 2009;64: 93-109. DOI: 10.1016/j.neuron.2009.09.001
- Vainchtein ID, Chin G, Cho FS, et al.: Astrocyte-Derived Interleukin-33 Promotes Microglial Synapse Engulfment and Neural Circuit Development. Science. 2018;359: 1269-1273. DOI: 10.1126/science.aal3589
- Barron JJ, Mroz NM, Taloma SE, et al.: Group 2 innate lymphoid cells promote inhibitory synapse development and social behavior. Science. 2024;386:eadi1025. DOI: 10.1126/science.adi1025
- Krause RM.: The Search for Antibodies with Molecular Uniformity. Adv. Immunol. 1970;12:1-56. https://doi.org/10.1016/S0065-2776(08)60167-4
- Silvers LE, Ellenberg SS, Wise R, et al.: The epidemiology of fatalities reported to the vaccine adverse event reporting system 1990-1997. Pharmacoepidemiol. Drug Saf 2001;10:279-285. https://doi.org/10.1002/pds.619
- Moro PL, Jankosky C, Menschik D, et al.: Adverse events following Haemophilus influenzae Type B Vaccines in the Vaccine Adverse Event Reporting System, 1990-2013. J Pediatr 2015;166:992–997. https://doi.org/10.1016/j.jpeds.2014.12.014
- Greenberg RG, Rountree W, Staat MA, et al.: Apnea After 2-Month Vaccinations in Hospitalized Preterm Infants: A Randomized Clinical Trial. JAMA pediatrics. 2025; doi:10.1001/jamapediatrics.2024.5311
- Daniel O, Loughnan M, Quenby M, et al. Antibody in Breastmilk Following Pertussis Vaccination in Three-Time Windows in Pregnancy. Pediatr Infect Dis J. 2025;44(2S):S66-S69. doi:10.1097/INF.0000000000004696.
- Kigel A, Vanetik S, Mangel L, et al. Maternal Immunization During the Second Trimester With BNT162b2 mRNA Vaccine Induces a Robust IgA Response in Human Milk: A Prospective Cohort Study. Am J Clin Nutrition. 2023;118(3):572-578. doi:10.1016/j.ajcnut.2023.07.013.
- Bairoliya N, Fink G.: Causes of Death and Infant Mortality Rates Among Full-Term Births in the United States Between 2010 and 2012: An Observational Study. PLoS Medicine. 2018;15:e1002531. https://doi.org/10.1371/journal.pmed.1002531
- Garfield E.: Bibliographic negligence: a serious transgression. The scientist. 1991;5(23):14. https://www.the-scientist.com/bibliographic-negligence-a-serious-transgression-60359
- Ginsburg I.: “The Disregard Syndrome: A Menace to Honest Science?” The Scientist 2001 December. https://www.the-scientist.com/the-disregard-syndrome-a-menace-to-honest-science-53924

