Surgical Outcomes of Mediastinal Paragangliomas
Surgical Management and Long-Term Outcomes of Mediastinal Paragangliomas: Case Series and Literature Review
Forhad Ullah, MD¹, Shirmeena Begum, PharmD², Federico Steiner, MD³
- Department of Cardiac Surgery Boston Children’s Hospital, Boston, MA, USA
- Department of Oncologic Pharmacology The Mount Sinai Hospital, New York, NY, USA
- Department of Thoracic Surgery Morristown Medical Center, Morristown, NJ, USA
OPEN ACCESS
PUBLISHED: 30 November 2025
CITATION: Ullah, F., et al., 2025. Surgical Management and Long-Term Outcomes of Mediastinal Paragangliomas: Case Series and Literature Review. Medical Research Archives, [online] 13(11).
https://doi.org/10.18103/mra.v13i11.7084
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.18103/mra.v13i11.7084
ISSN 2375-1924
ABSTRACT
Background: Mediastinal paragangliomas (PGLs) are rare neuroendocrine tumors originating from extra-adrenal chromaffin tissue, accounting for less than 0.3% of mediastinal masses. They are often non-secretory but have the potential to grow and cause secondary mass effects. They also carry a potential risk of metastasis if left untreated. Advancements in imaging, genetics, and cardiopulmonary support have enhanced long-term outcomes. This study provides an updated follow-up of three surgically treated mediastinal PGLs and examines the existing literature on recurrence, metastasis, and outcomes.
Methods: A retrospective case series from a single institution involving three patients who underwent resection of middle mediastinal PGLs via cardiopulmonary bypass (CPB) was examined. Long-term follow-up data (7-12 years) were obtained from clinical records. A systematic literature review (PubMed, Embase, Scopus; from inception July 2025) identified studies reporting mediastinal PGL resection with over six months of follow-up. The extracted data encompassed surgical approach, recurrence, metastasis, and complications.
Results: All three patients were female, aged 48 to 75 years, and presented with non-secretory middle mediastinal PGLs. Using CPB through a median sternotomy, all cases achieved complete (R0) resection. One patient experienced unilateral vocal cord paralysis that required treatment; no additional significant complications arose. There were no biochemical, radiographic, or clinical recurrences during the 7-12 years of follow-up. A literature review of 169 reported resections indicated that complete resection is the most significant predictor of local control. Historical recurrence rates (approximately 55%) have diminished to less than 10% in contemporary series utilizing CPB and multidisciplinary planning. Metastasis rates (6-27%) seem to be more closely related to the status of the SDH mutation than to the anatomical location.
Conclusion: Complete surgical resection, frequently aided by planned CPB, ensures lasting local control for resectable mediastinal PGLs with minimal morbidity, though long-term monitoring remains essential due to genotype-linked metastatic risk. Our prolonged follow-up substantiates the effectiveness and safety of surgical intervention for mediastinal PGLs in contemporary practice.
Keywords: Mediastinal paraganglioma, surgery, resection, long-term outcomes, cardiopulmonary bypass, SDHB mutation, recurrence, neuroendocrine tumors
INTRODUCTION
Paragangliomas (PGLs) are neuroendocrine tumors that arise from extra-adrenal chromaffin cells; they comprise less than 3% of all PGLs and only 0.3% of mediastinal masses. These lesions can share biological secretory behavior just like their neural crest derived counterpart, pheochromocytomas. Less than 3% of PGLs exhibit secretory behavior but when present, clinical manifestations include episodic hypertension, palpitations, and other symptoms associated with increased catecholamine release.
The lack of catecholamine secretion in the majority of PGLs frequently postpones diagnosis, as these lesions are usually identified incidentally or via symptoms associated with mass effect rather than endocrine function. Non-secreting PGLs, though indolent in behavior, can exhibit local invasion and adherence to major vessels, emphasizing the importance of early diagnosis and treatment. Urine catecholamine assays combined with metaiodobenzylguanidine (MIBG) scintigraphy have become very accurate in distinguishing functional versus non-functional PGLs. When dealing with these mediastinal lesions, imaging modalities such as magnetic resonance imaging (MRI), computed tomography (CT), and positron emission tomography (PET) are necessary to precisely localize these tumors. Recent studies emphasize the importance of early multimodal imaging and genetic screening to improve resectability rates and long-term outcomes.
Over the past two decades, the genomic understanding of mediastinal PGLs has transformed clinical practice. The first susceptibility genes were found to be linked to succinate dehydrogenase (SDH) complex in the mitochondria. Mutations in subunits B and D are particularly associated with pheochromocytomas and PGLs that occur in the head/neck/chest region. The advent of next generation sequencing has greatly improved our knowledge of the genetics behind these neoplasms. Approximately 30-40% of mediastinal PGLs were found to have mutation in SDHB, SDHD, and related genes, with variable penetrance by genotype. Genetic testing has become routine but the correlation to treatment in those deemed surgically resectable is yet to be determined.
The cornerstone of management for mediastinal PGLs is surgical resection, aiming for complete (R0) resection to lower the risk of recurrence and metastasis. The anatomical proximity to vital structures in the chest (great vessels, heart, trachea, and lungs) can make these surgeries complex and invasive. The adoption of planned cardiopulmonary bypass (CPB) and, in selected cases, cardioplegic arrest has expanded the boundaries of safe resection, allowing en bloc removal of lesions. Reported perioperative mortality has fallen to under 5% in modern series, with great long-term local control when complete excision is achieved. Despite these advances, data on these mediastinal lesions remain sparse, largely limited to isolated case reports and small retrospective series with heterogeneous follow-up. Historical recurrence rates exceeding 50% have been supplanted by single-institution experiences reporting near-zero recurrence, yet the cumulative evidence has not been evaluated systematically.
Furthermore, the influence of CPB utilization, genetic status, and surveillance strategy on long-term outcomes has not been comprehensively analyzed. To address these gaps, we present a case series with extended follow-up of three patients who underwent surgical resection of non-secretory mediastinal PGLs using CPB, accompanied by a systematic literature review of all reported cases to date. This combined approach aims to (1) delineate contemporary surgical outcomes, recurrence, and metastatic patterns; (2) contextualize the role of CPB in achieving durable local control; and (3) integrate emerging genetic and imaging paradigms into the management framework of this rare entity.
MATERIALS AND METHODS
This study comprises a single institution retrospective case series of three patients with mediastinal PGLs previously reported by our group, combined with a comprehensive literature review on management and long-term outcomes. Individual patient consent was obtained for this publication. All three cases involved patients who underwent mediastinal PGL resection between 2000-2018, with follow-up extending through 2025. For the present series, attention was directed towards detailed clinical work-up, surgical management and follow-up data from the original case records. Long-term outcomes were defined as biochemical, clinical or radiographic recurrence reported during 6 months of follow-up.
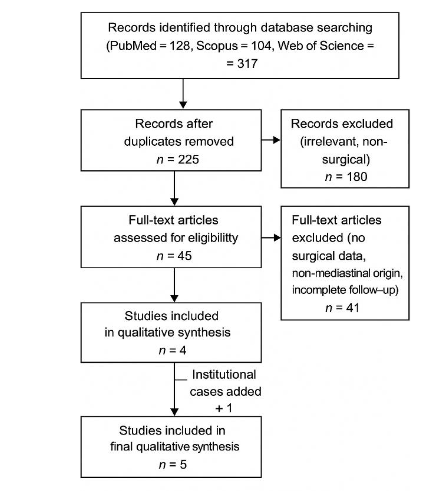
A comprehensive systematic review was conducted in accordance with PRISMA guidelines to identify all documented instances of mediastinal PGLs. We searched PubMed/Medline, Scopus, and Web of Science from the start of the databases to July 2025. The following search terms were used in combination with Boolean operators AND/OR: mediastinal paraganglioma, thoracic paraganglioma, surgery, resection, cardiopulmonary bypass, SDH, mutation, long-term outcomes, recurrence, and survival. Two authors independently reviewed the titles and abstracts, and any disagreements were settled by consensus. Studies were included if they reported: (1) histologically confirmed PGLs situated in the anterior, middle, or posterior mediastinum; (2) details of surgical resection, including operative approach or utilization of CPB; (3) confirmation of tumor status through biochemical, histologic or clinical evaluation; (4) long-term post-operative outcomes, including recurrence, metastasis, or mortality. Studies were excluded if they pertained to extra-mediastinal PGLs, lacked surgical or outcome data, or were non-English publications, reviews, or conference abstracts.
After removing duplicates, 225 records were screened from an initial 317, and 45 full-text articles were evaluated for eligibility. Four studies met the inclusion criteria, and one institutional study was added, bringing the total number of studies for final synthesis to 5. In total, 169 resections across 5 studies (including the present series) were evaluated. Data were extracted from each study regarding patient demographics, tumor location and functionality, surgical technique, utilization of cardiopulmonary bypass, completeness of resection, duration of follow-up, and outcomes. The Joanna Briggs Institute (JBI) Checklist for case reports and case series was used to rate the methodological quality of the studies. Studies that scored heterogeneity of data and limited sample sizes, results were summarized descriptively.
CASE SERIES
In this case series, we report three patients who were diagnosed with middle mediastinal PGLs. Two of the patients presented with chronic non-productive cough and one patient presented with an enlarging neck mass that was found to be a carotid body PGL with incidental finding of a mediastinal neoplasm. Initial diagnosis of these masses was made using CT imaging. All patients had laboratory work-up ruling out secretory behavior. None of the patients had any genetic work up prior to surgery. Two patients had genetic testing after surgery with one of the patients found to have a mutation in the SDHD gene. Endobronchial ultrasound-guided biopsy and video-assisted thoracoscopy were performed to obtain biopsy and evaluate characteristics of the lesion. The largest mass in this cohort measured 5.3 x 3.6 x 3.2 cm and the smallest measured 3.5 x 3.0 x 2.0 cm. All these masses were found to be in the aortopulmonary (AP) window. Given the anatomic location, all patients underwent surgical resection via median sternotomy with planned CPB.
RESULTS
In our earlier publication of these cases, all three cases achieved complete (R0) resection with no significant complications. One patient had left sided vocal cord paralysis that caused hoarseness but was later resolved with injection of the left vocal cord. Each patient recovered uneventfully without any hemodynamic instability. Surgical specimens were tested for immunoreactivity, and they were found to have proteins and serum markers consistent with PGLs. Histology showed the characteristic Zellballen pattern, which consists of small nests of chromaffin cells separated by fibrovascular stroma. Post-operative follow-up included immediate close monitoring within two weeks of surgery and as-needed basis thereafter. Surveillance included PET-CT scan at 6- and 12-month post-surgery followed by annual PET-CT scan with a full clinical exam to assess for recurrence and/or metastasis. In case 1, the patient was in good physical condition and despite her age, tolerated surgery very well. At annual follow-up, no recurrence or metastasis was seen. Functional status was intact in the years following surgery, with no secondary effects from surgery itself, including stroke, heart failure, arrhythmias, or cardiopulmonary failure. In case 2, mediastinal PGL was identified while being worked up for a carotid body mass. This patient underwent staged resection, with carotid body lesion resection first and then resection of mediastinal PGL three months later. Both surgeries were tolerated well, and no recurrence or metastasis was seen in 9 years of follow-up. In case 3, this patient was found to have a necrotic mediastinal PGL. Surgical resection in this patient was longer and more complex. The lesion was removed intact without any capsular tears. Due to central necrosis, there was concern that this mass had higher risk of recurrence and metastasis. This patient had undergone genetic testing 2 years after surgical resection and was found to have a mutation in the SDHD gene. Summarized characteristics and clinical data can be found in Table 1.
| Case | 1 | 2 | 3 |
|---|---|---|---|
| Age / Sex | 75 / F | 48 / F | 66 / F |
| Symptoms at Presentation | Non-productive cough | Incidental findings during carotid body PGL workup | Non-productive cough and fatigue |
| Secretory Status | Non-secretory | Non-secretory | Non-secretory |
| Tumor Size (cm) | 5.3 × 3.6 × 3.2 | 3.7 × 3.5 × 2.0 | 3.5 × 3.0 × 2.0 |
| Necrosis | Absent | Absent | Present (central necrosis) |
| Genetic Mutation | Testing not performed | None | SDHD |
| Histopathology | Zellballen pattern; no mitotic activity | Zellballen pattern; no mitotic activity | Zellballen pattern; no mitotic activity |
| Immunoprofile | Chromogranin A, CD56, synaptophysin, S100 | Chromogranin A, synaptophysin, S100 | Chromogranin A, synaptophysin, CD56, S100, vimentin |
| Postoperative Complications | Left vocal cord paralysis (resolved with treatment) | None | None |
| Follow-up Duration | 12 years | 9 years | 7 years |
| Follow-up Imaging | PET-CT scan | PET-CT scan | PET-CT scan |
| Recurrence | None | None | None |
| Metastasis | None | None | None |
DISCUSSION
Mediastinal PGLs are rare and surgically demanding tumors due to their proximity to major vessels and cardiac structures. A cumulative total of 169 cases were analyzed, but quantitative analysis was not feasible due to small sample size and variation in management. Historical data indicated a significant local recurrence rate (approximately 55%) and notable metastatic potential (around 27%), particularly in cases of incomplete resection. Outcomes have improved thanks to better imaging, better management of catecholamines during surgery, and the use of CPB for safe exposure. In modern cohorts, complete (R0) resection correlates with superior local control. The most extensive dedicated retrospective analysis, involving 51 patients, indicated no local tumor recurrences during a median follow-up of 8 years among those who underwent resection, despite a high incidence of peri-operative complications (66%). Metastatic disease occurred in 20% overall, but only 6% could be definitively traced to a mediastinal primary, emphasizing that certain metastases originate from extra-mediastinal disease in syndromic or multifocal contexts. The Mayo Clinic experience demonstrated the safety of sternotomy with selective CPB to mobilize tumors abutting the heart or great vessels, with excellent symptom control and survival; one intra-operative death from hemorrhage was reported. Although recurrence data were not specifically quantified, the high long-term survival rates endorse surgery as the primary treatment when feasible. Advanced intraoperative imaging and perfusion monitoring have further reduced perioperative mortality and improved CPB efficiency in mediastinal tumor resections.
Our institutional case series includes long-term follow-up: three middle mediastinal PGLs in the aortopulmonary (AP) window were removed with CPB and had R0 resection and no recurrence at 7-12 years. The only short-term problem was paralysis of one vocal cord, which resolved with vocal cord injection. For anterior/middle lesions closely associated with the great vessels, pre-planned CPB (and, if necessary, cardioplegic arrest) can mitigate the risk of bleeding and facilitate en bloc resection. Recent studies indicate that CPB is utilized in about 45% of complex cohorts with a low operative mortality rate.
Genetic testing has become common practice in patients who have pheochromocytoma/PGLs. Patients with mediastinal lesions are more likely to have SDH mutations, particularly SDHB/SDHD. These genetic mutations increase the likelihood of recurrence and metastasis. Reported metastatic rates for mediastinal primaries exhibit significant variability across studies (6-69%), attributed to referral biases, inconsistent genotyping, and the inclusion of metastases originating from non-mediastinal primaries. Thorpe et al. looked at long term outcomes in patients with metastatic PGLs and pheochromocytomas who were treated with multimodal therapy and found that median survival was 11.5 years from the time of diagnosis with 51% of patients showing progression despite treatment. When mediastinal PGLs become metastatic, outcomes are poor despite multimodal treatment which includes surgical debulking, chemotherapy, and radiotherapy. Prior to genetic testing, malignant potential was mostly based on the presence of metastatic disease. Contemporary reports demonstrate considerable variability in the use of imaging and genetic testing among mediastinal PGL cohorts. Earlier reports such as those by Lamy et al. and Brown et al. relied primarily on cross-sectional imaging (CT or MRI) for diagnosis, without incorporation of molecular or functional studies. Subsequent analyses, including Gurrieri et al., introduced PET/CT and MRI in pre-operative and follow-up protocols but did not routinely perform genotyping. Only recent large-scale series, particularly that of Kanj et al., have incorporated comprehensive SDH gene mutation testing, revealing that approximately two-thirds (66%) of tested patients harbored pathogenic variants. This finding, together with the integration of functional imaging into the diagnostic algorithm, underscores the growing significance of genotype phenotype correlations in the management and longitudinal surveillance of mediastinal paragangliomas. In the present series, selective SDH mutation testing post-operatively and routine PET-CT surveillance were performed, aligning with current best practices for thoracic paraganglioma management. Genetic testing in this patient population is paramount as it can now allow earlier diagnosis and comprehensive treatment to prevent metastasis. Large-scale genetic registries demonstrate that SDHB and SDHD mutation carriers should undergo lifelong surveillance given their cumulative metastatic risk approaching 40%. Imaging follow-up increasingly favors 68Ga-DOTATATE PET/CT for detection of multifocal or metastatic disease and for surveillance in SDH mutation carriers; MIBG sensitivity can be limited in thoracic/cardiac PGLs. Due to the genotype-linked metastatic risk, post-operative surveillance is recommended even after R0 resection. R0 surgery, often with planned CPB, provides lasting local control with low recurrence for resectable mediastinal PGLs. The risk of metastasis is influenced more by tumor biology (e.g., SDHx) than by the mediastinal location alone. Our series provides enduring, empirical validation of these trends.
CONCLUSION
Mediastinal PGLs can present with significant anatomic complexity. However, they can be safely and effectively treated with careful pre-operative evaluation and surgical planning. The evolution of peri-operative care, better imaging techniques, and the use of CPB have all led to a reduction in recurrence and perioperative mortality rates. Complete (R0) resection is still the most important part of curative therapy. Genetic profiling, especially SDH mutation screening, should be included in evaluation and follow-up protocols because it can help predict the likelihood of recurrence or metastases. Our results confirm that with the right surgical approach and careful monitoring after surgery, mediastinal PGLs can lead to long-lasting disease-free results. Long-term outcomes continue to improve due to advances in genetic counseling, functional PET imaging, and multidisciplinary collaboration.
LIMITATIONS AND NEXT STEPS
Due to the rarity of mediastinal PGLs, it is difficult to do prospective or randomized studies. Most available data derive from small retrospective series, mixed case series or experiences at a single institution. Differences in genetic testing, follow-up duration, and outcome reporting make direct comparison challenging. Furthermore, insufficient molecular characterization may lead to an underestimation of the actual prevalence of hereditary SDH-related diseases. Multicenter registries should be the focus of future research to better understand the natural history of the disease, optimize surgical techniques, and standardize post-operative surveillance protocols.
Conflict of Interest: The authors have no conflicts of interest to declare.
Table 2. Reported resections of mediastinal paraganglioma > 6 months: recurrence, metastasis, and long-term complications
| Study (Year) | Cohort / Lesions | Surgical Approach | Follow-Up Range (Years) | Local Recurrence | Metastasis (Any) | Notable Long-Term Complications | Genetic Testing Performed |
|---|---|---|---|---|---|---|---|
| Lamy et al. (1994) | 79 anterior/middle mediastinal | Mixed; CPB used selectively | 1-15 (mean 8.2) | 55.7% | 26.6% | Operative mortality 5.3% | Not reported (pre-genetic testing era) |
| Brown et al., Mayo Clinic (2008) | 14 mediastinal resections | Sternotomy (10), thoracotomy (4); CPB in 6 | 1-19.4 (median 2.3) | 0% | 0% | 1 intra-op death; persistent HTN | Limited (SDHB/SDHD selectively tested) |
| Gurrieri et al. (2018) | 22 anterior/middle mediastinal | CPB in 45.5% | 1-18 (median 8.2) | 0% | 27.2% | Operative mortality 4.5% | Partial SDH testing (SDHB, SDHD) |
| Kanj et al. (2023) | 51 mediastinal PGLs (~75% resected) | Mixed approaches | 1-25 (median 8.0) | 0% | 20% overall; 6% from mediastinal primary | Peri-op complications 66%; no deaths | Comprehensive SDHx panel performed |
| Current Series (2025) | 3 middle mediastinal | Sternotomy with CPB; R0 resection | 7-12 (mean 9.7) | 0% | 0% | None | Selectively tested post-operatively (SDHD positive in 1 patient) |
REFERENCES
- De Palma A, Lorusso M, Di Gennaro F, Quercia R, Pizzuto O, Garofalo G, et al. Pulmonary and mediastinal paragangliomas: rare endothoracic malignancies with challenging diagnosis and treatment. J Thorac Dis. 2018;10(9):5318-5327.
- Lack EE. Tumors of the adrenal gland and extra-adrenal paraganglia. AFIP Atlas of Tumor Pathology. 1997.
- Plouin PF, Gimenez-Roqueplo AP. Pheochromocytomas and secreting paragangliomas. Orphanet Journal of Rare Diseases. 2006; 1:49.
- Chen H, Sippel RS, O’Dorisio MS, et al. The clinical manifestations of PGL. World J Surg. 2002; 26(8):1023-1030.
- Kanj A, et al. Mediastinal PGLs: a 51-case retrospective analysis. Ann Thorac Surg. 2023;115(2):385-394.
- Ilias I, Pacak K, et al. MIBG scintigraphy in functional PGLs. J Clin Endocrinol Metab. 2003; 88(9):4080-4086.
- Janssen I, Chen CC, Millo CM, et al. PET/CT imaging of pheochromocytoma and paraganglioma with 68Ga-DOTATATE: a prospective single-center study. J Nucl Med. 2021;62(10):1428-1434.
- Baysal BE, et al. Mutations in SDHD cause hereditary PGL type 1. Science. 2000;287(5454):848-851.
- Niemann S, Müller U. SDHC mutations in PGL. Nat Genet. 2000;26(3):268-270.
- Burnichon N, et al. SDH mutations in PGL: genotype-phenotype correlations. J Clin Endocrinol Metab. 2010;95(2):959-968.
- Ullah F, et al. Long term follow up of 3 patients after resection of mediastinal PGL necessitating cardiopulmonary bypass: case series. J Surg Case Rep. 2024;(12):501.
- Brown ML, Zayas GE, Abel MD, et al. Mediastinal PGLs: the Mayo Clinic experience. Ann Thorac Surg. 2008;86(3):946-951.
- Gurrieri C Amico G, et al. Surgical management of mediastinal PGL: single-center experience and review. J Thorac Dis. 2018;10(5):2944-2953.
- Lamy AL, et al. Mediastinal PGLs: a clinicopathologic and immunohistochemical study of 79 cases. Cancer. 1994;73(8):2248-2258.
- Asa SL, Mete O. Endocrine Pathology: PGL. Springer; 2018.
- Li, Wilson W. L., et al. Management of Large Mediastinal Masses: Surgical and Anesthesiological Considerations. Journal of Thoracic Disease, vol. 8, no. 3, 2016, pp. E175-E184.
- Ghayee, Hans K., et al. Mediastinal Paragangliomas: Association with Mutations in the Succinate Dehydrogenase Genes and Aggressive Behavior. Endocrine-Related Cancer, vol. 16, no. 1, 2009, pp. 291-299.
- Burnichon, Nelly, et al. The Succinate Dehydrogenase Genetic Testing in a Large Prospective Series of Patients with Paragangliomas. J Clin Endocrinol Metab, vol. 94, no. 8, 2009, pp. 2817-2827.
- Thorpe MP, et al. Long-Term Outcomes of 125 Patients with Metastatic Pheochromocytoma or PGL Treated With 131-I MIBG. J Clin Endocrinol Metab. 2020;105(3): e494-501.
- Hamidi, O., et al. Malignant Pheochromocytoma and Paraganglioma: 272 Patients Over 55 Years. J Clin Endocrinol Metab, vol. 102, no. 9, 2017, pp. 3296-3305.
- Taieb D, Pacak K. Current approaches and recent developments in the management of head and neck paragangliomas and pheochromocytomas. Endocr Rev. 2021;42(3):329-377.
- Janssen I, et al. 68Ga-DOTATATE PET/CT in PGL surveillance. J Nucl Med. 2015;56(3):399-405.

