Survival Outcomes in Newly Diagnosed Multiple Myeloma
Patient Characteristics, Treatment Patterns, and Survival Outcomes in Newly Diagnosed Multiple Myeloma: A Single-Center Study from South India
Beulah Elizabeth Koshy1*, Linu Abraham Jacob1, Sabeena K Choudhary1, MC Suresh Babu1, Lokesh K N1, A H Rudresha1, Rajeev LK1, Smitha C Saldanha
- Department of Medical Oncology, Kidwai Memorial Institute of Oncology, Dr M H Marigowda Road, Bengaluru, Karnataka, India.
OPEN ACCESS
PUBLISHED: 31 May 2025
CITATION: KOSHY, Beulah Elizabeth et al. Patient Characteristics, Treatment Patterns, and Survival Outcomes in Newly Diagnosed Multiple Myeloma: A Single-Center Study from South India. Medical Research Archives, [S.l.], v. 13, n. 5, may 2025. Available at: <https://esmed.org/MRA/mra/article/view/6593>.
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.18103/mra.v13i5.6593
ISSN 2375-1924
Abstract
Background: Multiple myeloma (MM) is a malignant plasma cell disorder with significant morbidity and mortality, particularly in aging populations. This study aims to evaluate the demographic, clinical, and prognostic characteristics of MM patients at our institute, assess treatment patterns, and analyze survival outcomes to guide risk-adapted therapeutic strategies.
Methodology: This retrospective observational study was conducted on 255 newly diagnosed MM patients between January 2022 and March 2024. Baseline demographics, clinical features, and biochemical markers were analyzed. Patients were staged according to the Revised International Staging System (R-ISS), and treatment regimens, including autologous stem cell transplantation (ASCT), were documented. Survival outcomes, including progression-free survival (PFS) and overall survival (OS), were assessed. Statistical analyses were performed using SPSS software (version 24.0). For comparisons, a p value < 0.05 was considered as statistically significant.
Results: The cohort had a mean age of 55.4 years, with a male predominance (67%). The most common CRAB feature was bone disease. R-ISS stage III disease was seen in 65.1% of patients, with significantly higher relapse rates (80.7%) than stages I (0%) and II (12.2%) (p < 0.001). VRd was the most effective induction regimen, with a relapse rate of 26.7% versus 74.7% for CyBorD (p < 0.001). ASCT significantly reduced relapse rates (9% vs. 62.1%, p < 0.001). Median PFS was 15 months, and survival outcomes favored deeper responses and ASCT.
Conclusion: This study highlights the prognostic impact of R-ISS stage, induction regimens, and ASCT on MM outcomes in the setting of a developing country. VRd-based induction and ASCT improved PFS, emphasizing the need for optimal therapy selection in resource-limited settings.
Keywords
Multiple Myeloma, Treatment Patterns, Survival Outcomes, Autologous Stem Cell Transplantation, R-ISS
Introduction
Multiple myeloma (MM) is a malignant plasma cell disorder characterized by clonal proliferation, leading to overproduction of monoclonal immunoglobulins or free light chains, which cause end-organ damage such as bone lesions, renal impairment, anemia, and hypercalcemia. This genetically complex and heterogeneous disease arises from tumor cell alterations and changes in the bone marrow microenvironment. MM follows a relapsing course with progressive resistance to therapies, primarily affecting individuals aged 65 and older. Its rising prevalence in aging populations, such as India, makes it a significant public health concern.
Diagnosis of MM is based on the revised International Myeloma Working Group (IMWG) criteria, which a biopsy-proven plasmacytoma, along with at least one myeloma-defining event (MDE). MDEs include CRAB criteria (hypercalcemia, renal failure, anemia, or lytic bone lesions), bone marrow clonal MRI.
Risk stratification is critical for guiding treatment. The Revised International Staging System (R-ISS), introduced in 2015, integrates the International Staging System (ISS), serum lactate dehydrogenase (LDH) levels, and high-risk cytogenetic abnormalities detected by FISH to provide a more accurate prognostic model. High-risk features, such as del(17p), t(4;14), t(14;16), and elevated LDH, are associated with poorer outcomes. The recently developed R2-ISS further refines risk stratification by incorporating additional markers like chromosome 1q gain/amplification (1q+).
The treatment landscape for MM has evolved significantly. For transplant-eligible patients, quadruplet induction therapy combining bortezomib, lenalidomide, dexamethasone (VRd), and a CD38-targeted monoclonal antibody like daratumumab (DVRd) followed by high-dose melphalan and autologous stem cell transplantation (ASCT), is now standard. Maintenance therapy, particularly dual-agent regimens for high-risk patients, is recommended to prolong remission. For transplant-ineligible patients, quadruplet regimens such as Dara-VMP and Isa-VRd are widely used. Despite these advances, nearly all patients eventually relapse, with progressively shorter remission durations and poorer outcomes.
In India, access to novel therapies and ASCT has improved outcomes, but challenges such as financial constraints, limited infrastructure, and poor adherence persist, particularly in resource-limited settings. Real-world data from a Canadian cohort study highlight a significant efficacy-effectiveness gap, with MM patients in real-world settings experiencing 75% higher mortality rates than those in clinical trials. Given these challenges, there is a pressing need for region-specific data on disease presentation, treatment patterns, and outcomes to inform clinical practice and policy. This study aims to provide real-world insights into the demographic, clinical, and prognostic characteristics of MM patients at our institute, evaluate the impact of baseline characteristics and initial treatment on outcomes, and contribute to evidence guiding risk-adapted therapeutic strategies in diverse healthcare settings.
Patients and Methods
This retrospective observational study included 255 newly diagnosed multiple myeloma (MM) patients who presented to our institute between January 1, 2022, and March 31, 2024. Diagnosis and staging were performed in accordance with the International Myeloma Working Group (IMWG) criteria.
Clinical variables analyzed included patient demographics, major presenting complaints, comorbidities, and the presence of CRAB features (hypercalcemia, renal failure, anemia, and bone lesions). Biochemical parameters assessed were total protein levels, lactate dehydrogenase (LDH), beta-2 microglobulin, and the percentage of plasma cells on bone marrow biopsy. Additionally, involved light and heavy chains, Revised International Staging System (R-ISS) staging, and details of initial and subsequent lines of treatment, including autologous stem cell transplantation (ASCT), were recorded.
Treatment outcomes following primary therapy were documented, and patients were monitored for disease relapse. Those who relapsed were initiated on second- and third-line treatment regimens and followed up accordingly. Data collection and analysis were conducted to evaluate disease progression, treatment response, and survival outcomes.
Statistical analysis
Statistical analysis was performed using SPSS software (version 24.0). Descriptive statistics were used to summarize baseline characteristics. Continuous variables were expressed as means ± standard deviations (SD) or medians with interquartile ranges (IQR), as appropriate, while categorical variables were presented as frequencies and percentages. Given the non-normal distribution of the data, non-parametric tests were employed for group comparisons. The Kruskal-Wallis test was used to assess differences in progression-free survival (PFS) across Revised International Staging System (R-ISS) stages and treatment regimens. For pairwise comparisons, the Mann-Whitney U test was applied to assess PFS between early and late relapsers, as well as between different induction regimens. Associations between categorical variables, such as relapse rates across R-ISS stages and treatment groups, were examined using chi-square tests. Kaplan-Meier survival analysis was performed to estimate median PFS, and survival curves were compared using the log-rank test. Odds ratios (OR) with 95% confidence intervals (CI) were calculated to evaluate the risk of mortality between treatment groups. A p-value <0.05 was considered statistically significant for all analyses.
Results
Patient Characteristics and Baseline Data
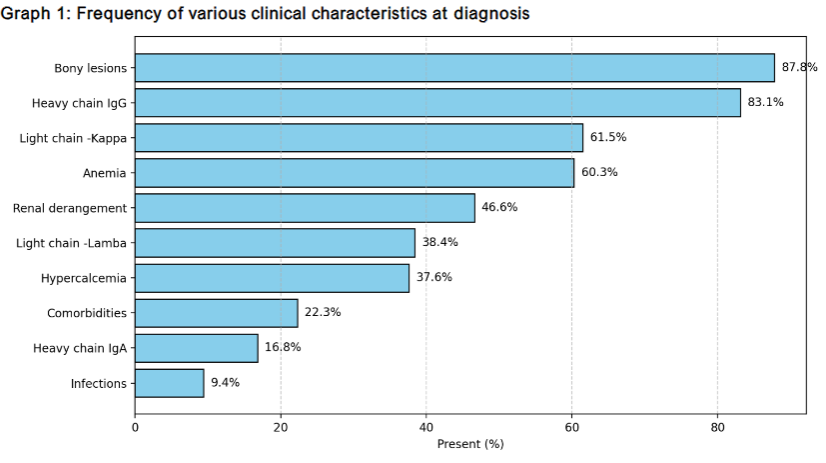
A total of 255 patients were included in this study, with a male predominance (67%) and a mean age of 55.4 years (standard deviation [SD]: 11.4). The median follow-up duration was 17 months (range: 6-23 months), and the median overall survival (OS) was not reached by the end of the study period. At the conclusion of the study, 238 patients (93.3%) were alive, while 17 patients (6.7%) had died. Among the enrolled patients, 141 (55.2%) experienced relapses, with a median progression-free survival (PFS) of 15 months (range: 2.5-18 months). Comorbidities were present in 22.3% of patients at baseline, and 9.4% required hospitalization due to infections. The most common “CRAB” features at presentation were bone disease, followed by anemia, renal dysfunction, and hypercalcemia. Regarding paraprotein analysis, the kappa light chain was more prevalent than the lambda light chain, and IgG was the most common heavy chain. Consequently, IgG kappa was identified as the most frequent paraprotein subtype.
Biochemical and Disease Staging Parameters
The median beta-2 microglobulin level was 7.3 mg/L (range: 2.2-12.46 mg/L), the median lactate dehydrogenase (LDH) level was 179 U/L (range: 130-266 U/L), and the median percentage of plasma cells in bone marrow biopsy was 20% (range: 10-60%). Based on the Revised International Staging System (R-ISS), the majority of patients (n=166, 65.1%) were classified as stage III, while 22.4% (n=57) and 12.5% (n=32) were classified as stage II and stage I, respectively.
Relapse Patterns and RISS Stage Correlation
Relapse rates varied significantly across RISS stages. None of the 32 patients in RISS stage I experienced relapse, whereas 7 of 57 patients (12.2%) in stage II and 134 of 166 patients (80.7%) in stage III relapsed (p < 0.001). Biochemical parameters also differed across RISS stages: median beta-2 microglobulin levels were 2.25 mg/L, 3.30 mg/L, and 6.60 mg/L for stages I, II, and III, respectively. Similarly, median LDH levels were 170.5 U/L, 179 U/L, and 288 U/L, and median plasma cell percentages in bone marrow were 19.5%, 19%, and 21% for stages I, II, and III, respectively.
Treatment Regimens and Outcomes
The most used upfront induction regimens were VRd (bortezomib, lenalidomide, dexamethasone), VTd (bortezomib, thalidomide, dexamethasone), Vd (bortezomib, dexamethasone), and CyBorD (cyclophosphamide, bortezomib, dexamethasone). Most of the patients (119/255) received CyBorD due to renal impairment at presentation. Relapse rates varied significantly among regimens: 74.7% (89/119) of patients on CyBorD, 74.1% (23/31) on Vd, 50% (2/4) on VTd, and 26.7% (27/101) on VRd relapsed (p < 0.001), indicating the superior efficacy of VRd. The overall response rate (ORR) to induction therapy, including complete response (CR), very good partial response (VGPR), and partial response (PR), was 85.4% (n = 218). Among 25 patients who underwent bone marrow evaluation after 8 cycles of induction, CR was observed in 4 patients (16%). Most patients achieved PR or stable disease (SD) (n = 136), while 115 patients (45.0%) attained VGPR. Relapse rates were significantly lower in patients who achieved CR or VGPR (26.8%, 32/119) compared to those with PR/SD (80.1%, 109/136) (p < 0.001), highlighting the protective effect of deeper responses.
Progression-Free Survival (PFS) Analysis
PFS, defined as the time from initial treatment to death, progression, or initiation of a new therapy, varied significantly across RISS stages (p < 0.001). Patients in RISS stage I had no relapses and a median PFS of 10.5 months (range: 7-15 months), while those in stage II and III had median PFS of 11 months (range: 4-18 months) and 16 months (range: 2.5-15 months), respectively. Relapsed patients were categorized into early (relapse ≤12 months) and late (relapse >12 months) relapsers. Early relapsers (n = 17) exhibited significantly higher mean beta-2 microglobulin levels (10.14 mg/L vs. 7.98 mg/L, p = 0.034).
Impact of Autologous Stem Cell Transplantation (ASCT)
Since 2022, 33 patients underwent ASCT at our institution, with 23 consolidated at first remission and 10 as part of subsequent lines of therapy. Only 2 of these patients relapsed (p < 0.001), and 29 remained on maintenance therapy. The median time to relapse in ASCT patients was not reached. In contrast, among the 222 patients who did not receive ASCT, 138 (62.1%) relapsed, compared to only 3 (9%) in the ASCT group (p < 0.001).
Subsequent Lines of Therapy and Mortality
Among patients who relapsed after first-line therapy, 56 received two subsequent lines of treatment, while 35 received only one. Median PFS-2 and PFS-3 were not reached. At the end of the study, 17 patients had died, 16 of whom (94.1%) had relapsed. In contrast, only 125 of 238 surviving patients (52.5%) had relapsed (p < 0.001). Among deceased patients, 6 had received VRd (37.5%) and 10 had received other regimens (62.5%) (p = 0.062). The odds ratio for death in the VRd group compared to other regimens was 0.347 (95% confidence interval: 0.114-1.058), suggesting a potential protective effect of VRd against mortality.
Survival Analysis
Kaplan-Meier survival analysis revealed a mean PFS of 22.7 months (standard error: 0.276 months). The Kruskal-Wallis test demonstrated significant differences in PFS across treatment regimens (p < 0.001), with VRd demonstrating superior outcomes compared to other regimens.
Discussion
This study provides a comprehensive analysis of patient characteristics, treatment outcomes, and relapse patterns in a cohort of 255 patients with multiple myeloma. The epidemiology and clinical outcomes of multiple myeloma in India exhibit notable differences from those observed in Western populations, primarily characterized by an earlier age of onset and resource limitations. While multiple myeloma is traditionally considered a disease of the elderly, with an average age at diagnosis ranging from 65 to 75 years in Western cohorts, studies from India consistently report a mean age approximately a decade younger. In our study, the mean age at diagnosis was 55.4 years (SD: 11.4), aligning with previously published Indian data, including findings from our own institution.
The observed sex ratio in our cohort aligns with both Indian and Western studies, showing a slight male predominance. Clinically, most patients presented with bone disease, followed by anemia, renal dysfunction, and hypercalcemia. The prevalence of anemia (60.3%) and hypercalcemia (37.6%) closely match the findings of Jacob et al. Renal dysfunction (46.6%) was less frequent than reported by Yanamandra et al. (2021) but higher than in Jacob et al. Similarly, the prevalence of bone disease (87.8%) is consistent with data from both Jacob et al. and Yanamandra et al.
Our findings indicate that the majority of patients (65.0%) presented with RISS stage III disease, a significantly higher proportion than RISS stages I (12.5%) and II (22.3%). This observation contrasts with previous Indian studies, where RISS stage II was more frequently reported than stage III. Additionally, 9.4% of newly diagnosed patients in our institution presented with infections requiring hospital admission, a lower prevalence than that reported in other studies. This discrepancy may be attributable to our restrictive inclusion criteria, which considered only severe infections necessitating hospitalization.
A substantial proportion of patients (56.8%) exhibited elevated LDH levels at diagnosis, a figure comparable to the 57% prevalence reported by Yanamandra et al. (2021). However, this rate was considerably higher than that reported in other studies (10-15%). Similarly, elevated beta-2 microglobulin levels were observed in 61.1% of patients, aligning with the findings of Hussain et al., who reported a prevalence of 67%. Both of these biomarkers were associated with higher relapse rates and lower progression-free survival (PFS), further establishing their prognostic significance in our cohort. In contrast, bone marrow plasma cell percentage did not emerge as a predictive or prognostic factor.
Our study revealed a one-year overall survival (OS) rate of 97%, with only 10 out of 255 patients succumbing within the first year. The one-year PFS rate was 88.3%, with 30 patients experiencing disease progression or relapse. These survival outcomes are comparable to recently published Indian studies, including those by Yanamandra et al. (2021). Among the 33 patients who underwent autologous stem cell transplantation (ASCT), only one death occurred, resulting in a one-year PFS and OS of 96.97%.
Limitations
This study has several limitations. The sequential enrollment of newly diagnosed multiple myeloma patients without stratified sampling may limit the generalizability of our findings. Consequently, the correlation of PFS with RISS staging, first-line chemotherapy regimens, and response at the end of induction therapy may not be fully representative of the broader disease spectrum. However, despite this limitation, our findings suggest that lower RISS stages, VRd induction therapy, and achieving complete response (CR) or very good partial response (VGPR) serve as protective factors against relapse. Additionally, this study did not assess the potential impact of extramedullary disease at presentation. Comparisons with previous Indian studies were also constrained by differences in staging criteria, as most earlier investigations utilized ISS staging rather than RISS staging. Consequently, extrapolations were necessary when comparing one-year OS data and other prognostic parameters.
Another limitation pertains to the use of RISS staging for prognostication at diagnosis instead of the currently recommended R2-ISS staging. Furthermore, individual chromosomal abnormalities were not analyzed, limiting insights into high-risk cytogenetic subsets. Treatment regimens were determined by resource availability, financial considerations, and social support rather than strict adherence to international guidelines, reflecting the challenges of managing multiple myeloma in a resource-limited setting. Lastly, the study did not document the incidence of adverse effects associated with chemotherapy, immunomodulatory agents, or monoclonal antibodies. Minimal residual disease assessment post-induction was also not performed, limiting insights into deeper response evaluation.
Conclusion
Within its limitations, this study underscores the critical importance of early diagnosis, effective induction therapy, and autologous stem cell transplantation (ASCT) consolidation in improving outcomes for patients with multiple myeloma (MM). The findings highlight the prognostic significance of baseline characteristics such as elevated lactate dehydrogenase (LDH) and beta-2 microglobulin levels, as well as the protective effects of achieving deep responses (complete response [CR] or very good partial response [VGPR]) and utilizing the VRd (bortezomib, lenalidomide, dexamethasone) regimen. The superior outcomes observed in patients undergoing ASCT further reinforce its role as a cornerstone of MM management, particularly in resource-limited settings.
Future studies should prioritize the integration of high-risk cytogenetic profiling, MRD assessment, and uniform treatment guidelines to refine risk-adapted therapeutic strategies. By addressing these gaps, researchers and clinicians can further optimize outcomes for MM patients, particularly in diverse healthcare settings where resource limitations pose significant challenges. This study contributes to the growing body of evidence guiding MM management and underscores the need for continued innovation and collaboration in the field.
Conflict of Interest:
None.
Funding Statement:
None.
Acknowledgements:
None.
References:
- Malard F, Neri P, Bahlis NJ, Terpos E, Moukalled N, Hungria VTM, et al. Multiple myeloma. Nat Rev Dis Prim. 2024; 10:45. https://doi.org/10.1038/s41572-024-00529-7
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548. https://doi.org/10.1016/S1470-2045(14)70442-5
- Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised International Staging System for Multiple Myeloma: A Report from the International Myeloma Working Group. J Clin Oncol. 2015; 33:2863-9. https://doi.org/10.1200/JCO.2015.61.2267
- JJ, Wester R, Bertsch U, Waage A, et al. Second Revision of the International Staging System (R2-ISS) for Overall Survival in Multiple Myeloma: A European Myeloma Network (EMN) Report Within the HARMONY Project. J Clin Oncol. 2022;40:3406-18. https://doi.org/10.1200/jco.21.02614
- Sonneveld P, Dimopoulos MA, Boccadoro M, et al. Daratumumab, bortezomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2024;390(4):301-313. https://doi.org/10.1056/NEJMoa2312054
- Voorhees PM, Sborov DW, Laubach J, et al. Addition of daratumumab to lenalidomide, bortezomib, and dexamethasone for transplantation-eligible patients with newly diagnosed multiple myeloma (GRIFFIN): Final analysis of an open-label, randomised, phase 2 trial. Lancet Haematol. 2023;10(10):e825-e837. https://doi.org/10.1016/S2352-3026(23)00217-X
- Moreau P, Hulin C, Perrot A, Arnulf B, Belhadj K, Benboubker L, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab and followed by daratumumab maintenance or observation in transplant-eligible newly diagnosed multiple myeloma: Long-term follow-up of the CASSIOPEIA randomised controlled phase 3 trial. Lancet Oncol. 2024. https://doi.org/10.1016/s1470-2045(24)00282-1
- Kaedbey R, Reece D, Venner CP, McCurdy A, Su J, Chu M, et al. Long-term follow-up of outcomes including progression-free survival 2 in patients with transplant-ineligible multiple myeloma in real-world practice: A multi-institutional report from the Canadian Myeloma Research Group (CMRG) database. EJHaem. 2024;5:474-84. https://doi.org/10.1002/jha2.894
- Leleu X, Hulin C, Lambert J, et al. Isatuximab, lenalidomide, dexamethasone and bortezomib in transplant-ineligible multiple myeloma: The randomized phase 3 BENEFIT trial. Nat Med. 2024. https://doi.org/10.1038/s41591-024-03050-2
- Kumar SK, Therneau TM, Gertz MA, et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc. 2004;79(7):867-874. https://doi.org/10.4065/79.7.867
- Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study [published correction appears in Leukemia. 2012 May;26(5):1153. Nari, Hareth [corrected to Nahi, Hareth]]. Leukemia. 2012;26(1):149-157. https://doi.org/10.1038/leu.2011.196
- Moreau P, Kumar SK, San Miguel J, et al. Treatment of relapsed and refractory multiple myeloma: Recommendations from the International Myeloma Working Group. Lancet Oncol. 2021;22(3):e105-e118. https://doi.org/10.1016/S1470-2045(20)30756-7
- Yanamandra U, Saini N, Chauhan P, et al. AYA-Myeloma: Real-World, Single-Center Experience Over Last 5 Years. J Adolesc Young Adult Oncol. 2018;7(1):120-124. https://doi.org/10.1089/jayao.2017.0034
- Jacob LA, Suresh Babu MC, Lakshmaiah KC, et al. Multiple myeloma: Experience of an institute in limited resource setting. Indian J Cancer. 2017;54(1):340-342. https://doi.org/10.4103/ijc.IJC_87_17
- Yanamandra U, Sharma R, Shankar S, et al. Survival outcomes of newly diagnosed multiple myeloma at a tertiary care center in North India (IMAGe: 001A Study). JCO Glob Oncol. 2021;7:704-715. https://doi.org/10.1200/go.20.00625
- Gupta R, Kaur G, Kumar L, et al. Nucleic acid based risk assessment and staging for clinical practice in multiple myeloma. Ann Hematol. 2018;97(12):2447-2454. https://doi.org/10.1007/s00277-018-3457-8
- Kastritis E, Terpos E, Roussou M, et al. Evaluation of the Revised International Staging System in an independent cohort of unselected patients with multiple myeloma. Haematologica. 2017;102(3):593-599. https://doi.org/10.3324/haematol.2016.145078
- Hussain A, Almenfi HF, Almehdewi AM, Hamza MS, Bhat MS, Vijayashankar NP. Laboratory Features of Newly Diagnosed Multiple Myeloma Patients. Cureus. 2019;11(5):e4716. Published 2019 May 22. https://doi.org/10.7759/cureus.4716

