Transformative Small Mobile Stem Cells in Regenerative Medicine
Small Mobile Stem Cells: A Transformative Paradigm for Regenerative Medicine
Abdulkader Rahmo, PhD1
- President & CSO SMSbiotech, San Marcos, CA 92078, United States
smsbiotech.com
[email protected]
OPEN ACCESS
PUBLISHED :31 October 2025
CITATION: Rahmo, A., 2025. Small Mobile Stem Cells: A Transformative Paradigm for Regenerative Medicine. Medical Research Archives, [online] 13(10). https://doi.org/10.18103/mra.v13i10.6984
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.18103/mra.v13i10.6984
ISSN 2375-1924
ABSTRACT
Small Mobile Stem cells represent a transformative advance in regenerative medicine. Acting as circulating “smart messaging” cells, they possess the unique ability to sense and respond to tissue-specific environments, orchestrating repair processes dynamically. Through organ-tailored modulation of gene expression programs, SMS cells introduce a novel paradigm of adaptive regeneration, one that transcends traditional models of stem cell differentiation. This approach addresses critical gaps left by conventional stem cell therapies and advanced therapies, offering a more responsive and integrative solution to tissue repair and restoration.
Keywords
Small Mobile Stem Cells, Regenerative Medicine, Tissue Repair, Gene Expression, Adaptive Regeneration
Introduction
Regenerative medicine has long pursued the goal of repairing damaged tissues using engineered cells, biomaterials, and gene therapies. Yet this approach often overlooks a fundamental biological mechanism underlying natural repair. Emerging research has uncovered the extraordinary properties of Small Mobile Stem (SMS) cells, which are robust, highly motile stem cells that circulate throughout the body and function as “intelligent messengers.” These cells possess the ability to sense their microenvironment, withstand hostile conditions, engage in targeted intercellular communication, and fine-tune regenerative signaling to meet the specific needs of each tissue.
Unlike conventional stem cells, which follow linear differentiation pathways, SMS cells demonstrate a higher-order biological adaptability. They appear to adaptively interpret local cues, coordinate context-specific responses, and activate organ-targeted repair programs. Rather than representing an incremental improvement in stem cell therapy, SMS cells embody a fundamental shift in the paradigm of regenerative medicine, one that emphasizes responsiveness, precision, and systemic integration. The present commentary aims to (1) frame the emerging concept of regenerative intelligence, (2) articulate the biological and therapeutic rationale for Small Mobile Stem cells, and (3) outline study designs and translational opportunities that can advance this field into clinical application.
Missed Potential in Regeneration Science
Regenerative medicine has traditionally centered on replacing or engineering damaged tissues using biomaterials, engineered cells, or gene therapies. While these approaches have yielded significant progress, they often overlook the body’s intrinsic cellular communication networks that naturally coordinate repair. A useful parallel can be found in immunology: circulating T and B lymphocytes detect and respond to threats, but their actions frequently overshoot, resulting in chronic inflammation or tissue damage.
In contrast, Small Mobile Stem (SMS) cells embody a regulated form of regenerative intelligence. These motile stem cells circulate systemically, sense local microenvironments, and respond with precision, modulating their activity to exert tissue-specific effects without triggering maladaptive outcomes. Their ability to promote repair without collateral damage suggests that SMS cells represent a previously unrecognized dimension in classical regenerative frameworks.
SMS Cells “Catalyze” Tissue Regeneration
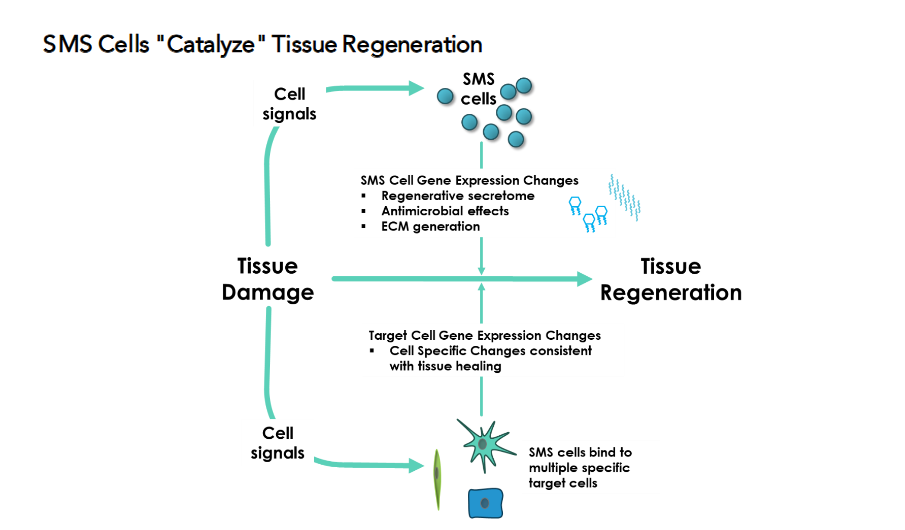
Small Mobile Stem cells engage in direct, tissue-tailored interactions with target cells, enabling context-specific regenerative responses:
- Cartilage: In the hypoxic, avascular environment of cartilage, SMS cells bind to chondrocytes and deliver pro-repair signals that stimulate extracellular matrix synthesis—activating healing pathways in a niche typically resistant to regeneration.
- Lung: SMS cells selectively interact with alveolar type II epithelial progenitors, promoting their proliferation and inducing angiogenesis, key processes for restoring gas exchange and reversing damage in conditions such as emphysema.
Moreover, SMS cells are able to reshape the gene expression of target cells in response to different tissue environments, a versatility that distinguishes them from conventional stem cells and positions them as universal mediators of tissue-specific regeneration.
The Framework of Regenerative Intelligence
Small Mobile Stem (SMS) cells function as “intelligent messengers” within a distributed regenerative network. They appear to detect microenvironmental stressors including hypoxia, inflammation, and cellular damage, and recalibrate target cells gene expression and secretory profiles to restore tissue homeostasis. This role transcends traditional notions of “stemness.” SMS cells operate as dynamic regulators, bridging immune modulation, antimicrobial defense, and regenerative signaling. While conventional therapies often attempt to impose repair externally, SMS biology reveals an innate, self-directed system designed to restore equilibrium from within.
Why This Concept Is Revolutionary
Scientific breakthroughs often begin as overlooked ideas. Just as germ theory and the complexity of the immune system were once underestimated, regenerative medicine may have missed its most profound discovery: naturally circulating, intelligent cells capable of orchestrating multi-organ repair with precision and adaptability.
SMS cells are not a mere refinement of existing therapies; they represent a new class of biology. These cells integrate core dimensions of tissue regeneration, including inflammation, fibrosis, apoptosis, angiogenesis, and extracellular matrix remodeling. Their ability to modulate these processes in a coordinated, context-specific manner positions SMS cells as a unified platform for disease modification, bridging the gap between immune regulation and structural repair in ways previously thought impossible.
Implications of SMS Cell Biology
| Implication | Description |
|---|---|
| Multi-Tissue Versatility | Active across diverse conditions, from osteoarthritis to COPD. |
| Immunologic Precision | Modulate inflammation adaptively, avoiding off-target effects of systemic drugs. |
| Structural Regeneration | Promote repair of epithelial, endothelial, and stromal compartments. |
| Exacerbation Control | Effect secretion of antimicrobial and immunomodulatory proteins to reduce flare-ups. |
| Broad Applicability | Address anti-microbial and anti-inflammatory phenotypes beyond narrow biologic targets. |
| Targeted Delivery, and directed migration | Nebulized SMS cells reach alveolar niches, bypassing limitations of injectables. Systemically injected SMS cells use homing mechanisms to reach affected tissues. |
| Age-Related Benefit | Restore regenerative capacity diminished by aging. |
| Biomarker Potential | Transcriptomic/proteomic signatures enable personalized interventions. |
| Complementary Integration | Enhance existing therapies like bronchodilators and biologics. |
| First-in-Class Modification | Combine structural repair, immune balance, and functional restoration. |
Future Directions
Small Mobile Stem (SMS) cells represent a bold leap forward in regenerative medicine. With multi-tissue versatility, structural repair capabilities, targeted immune modulation, and the potential to modify disease at its root, SMS cells transcend the limitations of current therapies that target narrow inflammatory subtypes. These cells operate as a platform for regenerative care. The challenge now lies with the scientific community: to recognize this paradigm shift, rigorously investigate its mechanisms, and lead the translation of SMS biology into broad, intelligent functionality, restoring damaged architecture, reviving adaptive healing lost with age, and reducing exacerbations through innate antimicrobial defense.
Their biomarker-driven adaptability and seamless compatibility with existing therapeutic modalities position SMS cells as the first truly integrative transformative therapies that redefine the boundaries of medicine.
Conclusion
The emergence of Small Mobile Stem cells marks the dawn of a new era in regenerative science. These cells embody a form of regenerative intelligence which are capable of sensing, signaling, and orchestrating adaptive tissue repair across diverse biological landscapes.
Just as the immune system revolutionized our understanding of defense, SMS cells may fundamentally reshape our understanding of healing. The responsibility now falls to researchers, clinicians, and innovators to explore this opportunity with rigor and imagination in order to move beyond symptom management and toward therapies that achieve true structural and functional restoration.
Acknowledgement
I would like to express my deepest gratitude to my colleagues for their invaluable contributions to advancing our understanding of SMS cell biology and its applications. I wish to recognize Professor Peter Wagner, Dr. Roger Schechter, and Dr. Jason Kirkness. In fact, the dedication of the entire scientific and medical team has been instrumental in exploring the intricacies of regenerative medicine.
Conflict of Interest Statement:
Author reports the following relationships/activities/interests: discoverer of SMS cells; current President of SMSbiotech Inc. These affiliations could be perceived as potential conflicts of interest. No other conflicts are declared.
Funding Statement:
This research was funded by SMSbiotech Inc., a privately held biotechnology company.
References:
- Rahimi Darehbagh R, Seyedoshohadaei SA, Ramezani R, Rezaei N. Stem cell therapies for neurological disorders: current progress, challenges, and future perspectives. Eur J Med Res. 2024; 29(1):386.
- Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10(9):200223.
- Poliwoda S, Noor N, Downs E, Schaaf A, Cantwell A, Ganti L, Kaye AD, Mosel LI, Carroll CB, Viswanath O, Urits I. Stem cells: a comprehensive review of origins and emerging clinical roles in medical practice. Orthop Rev (Pavia). 2022; 14(3):37498.
- A. Rahmo, R. Schechter. Small Mobile Stem Cells, Unique Regenerative Effects on Lung of Emphysema Animal Model, Currently Undergoing Phase 1 Clinical Trial. Am J Respir Crit Care Med. 2024;209:A6431.
- Lee MS, Stebbins MJ, Jiao H, Huang HC, Leiferman EM, Walzack BE, et al. Comparative evaluation of isogenic mesodermal and ectomesodermal chondrocytes from human iPSCs for cartilage regeneration. Sci Adv. 2021; 7(21):eabf0907.
- Rahmo A, Kirkness J, Schechter R. A study to investigate small mobile stem cells (SMS cells) in participants aged 39 to 69 years with chronic obstructive pulmonary disease. ACTRN12624001140549. ANZCTR. 2024 https://anzctr.org.au/Trial/Registration/TrialReview.aspx?id=386994.
- Lee MS, Stebbins MJ, Jiao H, Huang HC, Leiferman EM, Walzack BE, et al. Comparative evaluation of isogenic mesodermal and ectomesodermal chondrocytes from human iPSCs for cartilage regeneration. Sci Adv. 2021; 7(21):eabf0907.
- Banh L, Cheung KK, Chan MWY, Young EWK, Viswanathan S. Advances in organ-on-a-chip systems for modelling joint tissue and osteoarthritic diseases. Osteoarthritis Cartilage. 2022;30(7):1050–61.
- Yang Y, Lin H, Shen H, Wang B, Lei G, Tuan RS. Mesenchymal stem cell-derived extracellular matrix enhances chondrogenic phenotype of and cartilage formation by encapsulated chondrocytes in vitro and in vivo. Acta Biomater. 2018;69:71–82.
- Paggi CA, Teixeira LM, Le Gac S, Karperien M. Joint-on-chip platforms: entering a new era of in vitro models for arthritis. Nat Rev Rheumatol. 2022;18(4):217–31.
- Thorp H, Kim K, Kondo M, Grainger DW, Okano T. Fabrication of hyaline-like cartilage constructs using mesenchymal stem cell sheets. Sci Rep. 2020;10(1):20869.
- Sekiya I, Katano H, Mizuno M, Koga H, Masumoto J, Tomita M, et al. Alterations in cartilage quantification before and after injections of mesenchymal stem cells into osteoarthritic knees. Sci Rep. 2021;11:13832.
- Lin W, Wang M, Xu L, Tortorella M, Li G. Cartilage organoids for cartilage development and cartilage-associated disease modeling. Front Cell Dev Biol. 2023;11:1125405.
- Basil MC, Katzen J, Engler AE, Guo M, Herriges MJ, Kathiriya JJ, et al. The cellular and physiological basis for lung repair and regeneration: Past, present, and future. Cell Stem Cell. 2020;27(4):482–502.
- Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A. 2011;108(52):E1475–83.
- Zacharias WJ, Frank DB, Zepp JA, Morley MP, Alkhaleel FA, Kong J, et al. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature. 2018;555(7695):251–5.
- Somoza RA, Welter JF, Correa D, Caplan AI. Chondrogenic differentiation of mesenchymal stem cells: challenges and unfulfilled expectations. Tissue Eng Part B Rev. 2014;20(6):596–608.

