Zinc Oxide Nanomaterials: Synthesis and Biomedical Uses
Effect of Synthetic Routes on Physicochemical Properties and Biomedical Applications of Zinc Oxide Nanomaterials and Quaternary Ammonium Compounds
Dr. Satyawati Sudhir Joshi
- Professor (Retired), Department of Chemistry, Savitribai Phule Pune University, Pune, Maharashtra, India
OPEN ACCESS
PUBLISHED: 31 March 2025
CITATION: Joshi, SS., 2025. Effect of Synthetic Routes on Physicochemical Properties and Biomedical Applications of Zinc Oxide Nanomaterials and Quaternary Ammonium Compounds. Medical Research Archives, [online] 13(3). https://doi.org/10.18103/mra.v13i3.6439
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI https://doi.org/10.18103/mra.v13i3.6439
ISSN 2375-1924
Abstract
ZnO has acquired a large amount of attention due to its immense potential for fundamental studies regarding effects of morphology, dimensionality, and size dependent physical and chemical properties as well as their variety of applications. In the studies of nanomaterials, it has been observed that the size and shape of a nanomaterial depends on the nature of the stabilizer, i.e. surfactant, ligand, polymer to salt ratio, reaction temperature and time. The synthetic method applied also plays a role. The synthetic methods discussed in this review explain how the properties, morphology changes, affect the size, shape, and activity of ZnO nanomaterial. Especially, the electrochemical method has not been used so far. Its advantages are described. Recent advances in research on biomedical applications using ZnO indicate its potentiality as an anticancer, antidiabetic drug. Biocides, primarily those containing quaternary ammonium compounds, are heavily used in hospital environments, and various industries (food, water, cosmetic, etc). Quaternary ammonium compounds ultimately reach the environment via waste water and may remain there for a long time, due to their poor biodegradability. The potential implications of use in the emergence of antibiotic resistance have been paid little attention. This review also discusses the significance of quaternary ammonium compounds in biomedical applications. We have used tetra alkyl quaternary ammonium salt as capping agent and as a supporting electrolyte in the synthesis of ZnO NPs by electrochemical method. This method produces fine ZnO particles in the pure form. Combination of ZnO, quaternary ammonium compounds and use of electrochemical synthesis method may help in developing a new strategy towards sensing and drug delivery applications. The versatile use of quaternary ammonium compounds has been reported, their risk factors must be taken into account while choosing the compounds. Although ZnO NPs are proven to be a future potential, their toxicity is the main concern and more in-depth understanding should be developed. Understanding of cellular and molecular pathways and clinical trials will be required in future.
Keywords
Zinc oxide nanoparticles, quaternary ammonium compounds, electrochemical synthesis, biomedical applications, drug delivery, toxicity.
1. Introduction
WHY ZnO?
ZnO nanoparticles (ZnO NPs) in the form of various structures are excellent candidates for numerous applications viz. as sensors, variastors, as antifungicides in paints and pigments, in cosmetics and in flat panel displays. Current interest lies in their biomedical applications including drug delivery, bioimaging, biosensing, wound healing, to name a few. Also used in tissue engineering, a luminescent semiconductor used to visualize tissue cells. Nanoparticle fluorescence imaging has been used in gene detection, protein analysis, enzyme activity evaluation, element tracing, cell tracking, early-stage disease diagnosis, tumour related research. In addition to drug delivery applications, ZnO NPs have been widely explored for various other biomedical applications, including anticancer, antidiabetic, antibacterial, antifungal, and anti-inflammatory activities.
PROPERTIES OF ZnO
Zinc oxide exists in nature as a mineral in the crust of the Earth. It has been recognized as GRAS (Generally Recognized as Safe) by FDA. ZnO is less toxic, inexpensive, does not react with most of the pharmaceutical active ingredients studied so far. ZnO is one of the most extensively studied functional oxides with a direct wide band gap (3.37 eV) and a large excitation binding energy (60 meV), which makes it as one of the most important functional material with unique properties of near-UV emission, optical transparency, electric conductivity, and piezoelectricity. ZnO has attracted a large amount of attention due to its immense potential for fundamental studies regarding effects of morphology, dimensionality, and size dependent physical and chemical properties as well as their applications in photocatalysis, gas sensors and optoelectronic devices.
The semiconductor properties of ZnO affect their ability to generate reactive oxygen species, (ROS) and cause apoptosis. The electrons (e-) in semiconductors contain energies within certain bands and the band gap, a void region extending from top of the filled valence band to the bottom of vacant conduction band, measures approximately 3.3 eV for crystalline ZnO. UV rays contain sufficient energy to promote e- to the conduction band, leaving behind holes (h+). e- and h+ migrate to the surface of the NPs and react with oxygen and hydroxyl ions, respectively. This leads to the formation of superoxide and hydroxyl radicals. Zn2+ is an indispensable trace element for adults (~10 mg of Zn(2+) per day is recommended) and it is involved in various aspects of metabolism. Chemically, the surface of ZnO is rich in -OH groups, which can be readily functionalized by various surface decorating molecules.
Synthesis of ZnO Nanoparticles (NPs), nanostructures with controlled size and shape is very important in controlling their physical, chemical, optical properties. These properties of ZnO depend on its dimension, morphology, crystallinity and nature of synthetic route as well. ZnO NPs less than 100 nm are considered relatively biocompatible. ZnO is an FDA approved food additive and is generally recognised as safe and non-toxic in low concentrations.
Our aim is to understand the effect of synthetic route, the variety of reagents used like polymers, ligands on the formation of nanomaterial, and their activity as a catalyst, as a nanomedicine. It is very interesting to know how the morphology changes, how the activity of nanomaterial changes with respect to the synthetic conditions. Also, various experimental parameters such as reaction time, temperature, concentration of precursors and capping molecule as well as choice of solvent affect the shape and size of the NPs. The habit modification and morphological changes of some inorganic materials in microsize and nanosize are discussed in another review.
In most of these studies, polymers play multiple roles as a fuel in combustion synthesis, encapsulating agent and as a habit modifier in other synthesis method applied. We have observed that the size, shape, morphology of the synthesized material depends on various factors like nature of polymer, its degree of polymerization, molecular weight, reaction time, synthetic method applied and also on heat of reaction. In the methods applied at high temperature, rapid nucleation time gives rise to short burst of nuclei which might react with intermediate species and the reactions are more kinetically controlled.
Nanoparticles are small and thermodynamically unstable. After the primary nucleation, the particles grow via molecular addition. Particles can grow by aggregation with other particles called secondary growth. Their growth rates may be arrested during the reaction either by adding surface protecting agents. Nanocrystal dispersions are stable if interaction between the capping groups and solvent is favourable providing an energetic barrier to counter act van der Waals’ forces. When the synthesis was carried out at low temperatures, nucleation process is slow and thermodynamically driven process. With aging, growth process becomes more favourable. Final morphology of the material depends on equilibrium conditions related to minimum surface energy, rate of nucleation and growth. Using Polyvinyl alcohol (PVA), Polyvinyl pyrrolidone (PVP), surfactants we have prepared Cu, Pd, Pt, Ag, and Au metals in nanosize. Nanomaterials of oxides such as ZnO, Fe2O3, TiO2, Co3O4 to name a few and composites of metal-oxide have been synthesized in our group.
For the utilization of ZnO for various practical applications one has to consider the following points such as poor chemical stability and intrinsic defects of ZnO nanomaterials. Through surface functionalization the surface properties can be controlled. In the context of surface modification, we have used quaternary ammonium compounds (QAC) as capping agent and as a supporting electrolyte in our work. The present review is aimed at how this combination of ZnO and QAC can be utilized for biomedical applications in current scenario. The purpose of this review is also to analyse the prevalent traditional methods of preparing ZnO NPs as well as studying harmful side effects. Further the synthesis methods used so far by our group have been discussed along with their advantages. Electrochemical method of synthesis is discussed in detail, not reported much but very useful for preparing nanomaterial in the purest form with easy isolation of the product. Quaternary ammonium compounds (QACs) are widely utilized in medical field, but impact of QACs on the emergence of antibiotic resistance in patients and the environment is not attended so far. The versatile uses and their demerits are discussed in this review.
2. Synthesis Methods used
2.1 RADIATION-INDUCED SYNTHESIS:
Gamma radiolysis of aqueous solution is an effective method to reduce transition metal ions to zero metallic state. The advantage of the radiolytic synthesis lies in the fact that the main reducing agent is the solvated electron which has a very negative redox potential. This enables the reduction of metal ion to zero valent state. The advantages of this method are particles are pure and highly stable. In our group, various nanomaterials viz. metal nanoparticles such as gold, silver, Cu, Pd, were synthesized by radiation chemical route. Capped copper nanoclusters were successfully synthesized by gamma radiolysis method by optimizing various conditions like metal ion concentration, polymer or surfactant concentration and pH. The increasing amount of capping agent was responsible for decrease in size as small as 17 nm of the metal clusters. The radiolytic method provides copper nanoparticles in fully reduced and highly pure state compared to other synthetic routes. Formation of copper nanoclusters (FCC) was confirmed by X-ray diffraction technique. UV-vis spectrophotometry was employed to examine changes in plasmon resonance absorption peaks of copper metal. The purity of copper particles was further confirmed by electron spin resonance studies. Transmission electron microscopy results revealed the particle size distribution from 17 to 80 nm. Electron diffraction pattern confirms FCC copper phase.
Solvated electron e-aq and H. are powerful reducing agents, so that they easily reduce metal ions like Cu+ and Ag+ to zerovalent state. In this synthesis process, alcohols like ethanol, isopropanol are used as OH. radical scavenger. to form R.HOH radical. Polymers like PVA, PVP are used as stabilizers or capping agents.
2.2 GAMMA RADIOLYSIS METHOD: EFFECT OF PARTICLE SIZE ON SHAPE
Gamma radiolysis method was used to prepare polyvinyl alcohol (PVA) capped silver nanoparticles by optimizing various conditions like metal ion concentration and polymer (PVA) of different molecular weights. The role of different scavengers was also studied. The decrease in particle size was observed with increase in the molecular weight of capping agent. γ-radiolytic method provides silver nanoparticles in fully reduced and highly pure state. XRD (X-ray diffraction) technique confirmed the zero valent state of silver. Optical studies were done using UV-visible spectrophotometer to see the variation of electronic structure of the metal sol. Transmission Electron Microscopic (TEM) studies reveal the fcc geometry. The TEM show clearly split Debye-Scherrer rings. The d values calculated from the diffraction ring pattern are in perfect agreement with the ASTM data. Ag particles less than 10 nm are spherical in shape, whereas the particles above 30 nm have structure of pentagonal biprisms or decahedra, referred to as multiply twinned particles. ZnO NPs can be prepared by this method using oxidizing radical via oxidation. Here one has to use N2O as oxidizing radical to scavenge reducing radicals i.e. hydrated electrons.
2.3 POLYMER COMBUSTION METHOD:
a. Earlier, spherical- and cuboid shaped ZnO, Cu-ZnO and Co3O4 NPs were synthesized using aqueous thermolysis method and studied the role of polymer interactions during aqueous themolysis. Aqueous polymer thermolysis or combustion (burning in presence of oxygen) is an attractive technique for the production of different oxides. The method involves gelling and subsequent exothermic redox reaction between oxidizer (e.g. metal nitrates) and a fuel (e.g. urea, citric acid, hydrazine) to yield a desired product. The large amount of gases produced during the combustion, promotes the decomposition of precursor gel and produces the nano-oxide particles. Zinc oxide nanoparticles were successfully prepared using PVA and PVP. The influence of oxidation temperature on structural properties of ZnO nanoparticles using different polymers of varied molecular weights was studied systematically, understanding the polymer interactions. The polymer is adsorbed and acting as a bridge between particles. The linear chains of PVA can be cross-linked in aqueous medium, i.e. water. The cross-linking between the chains may provide small cages wherein the ‘sol’ of the reactant mixture gets trapped. During thermolysis, the ‘sol’ trapped in the cages may get converted to ultrafine particles of zinc oxide. Thus, the cages formed by the cross-linking may offer resistance to the agglomeration of the particles and the particle growth.
b. Polymeric materials have been found to be ideal candidates for the synthesis of organic–inorganic nanomaterials. We have obtained Co3O4-decorated graphene oxide (GO) nanocomposites by a simple polymer combustion method. Polyvinyl alcohol (PVA) of two different molecular weights, 14,000 and 125,000, was used for the synthesis. The pristine sample was annealed at 300, 500, and 800°C. PVA has played an important role in the formation of GO and Co3O4 nanoparticles. Synthesized Co3O4–GO nanocomposites were characterized by X-ray diffraction, Fourier transform infrared, Raman, electron paramagnetic resonance, transmission electron microscopy, and vibrating sample magnetometry. Reflection peaks at 12° and 37° in an X-ray study confirm the formation of Co3O4–GO. Raman study validates the presence of GO in nanocomposites of Co3O4–GO. Room temperature ferromagnetism was observed in all annealed samples. The highest coercivity of 462 G was observed for 300°C annealed samples as compared with bulk Co3O4. These studies show the potential application of Co3O4 NPs as nano medicine-based hyperthermia in the cancer treatment.
c. Comparison of chemical and gamma radiolytic method; Effect of shape on catalytic activity. Stable nanocluster catalysts prepared by chemical and γ-radiolytic reduction methods were found to give very high turn-over frequency numbers in hydrogenation of styrene oxide and 2-butyne-1,4-diol (B3D) as compared to the conventional catalysts. A systematic study was carried out on the effects of different transition metals, their reduction methods, types of polymer used as a capping agent, and the concentration and composition of solvent used during catalyst preparation on the size and shape of nanoparticles. The reduction method of metal precursor directly influenced the morphology of the nanoparticles, affecting the catalyst activity considerably. The cubic-shaped nanoparticles (5–7 nm) were obtained in chemical reduction, while radiolytic reduction method gave spherical nanoparticles (1–7 nm).
d. The formation of silver nanoparticles (Ag NPs) in an aqueous solution using gamma-radiolysis in the presence of poly (N-vinylpyrrolidone) (PVP) is followed at various doses. At lower dose spherical Ag NPs are formed, however, with increase in dose an increasing fraction of nano decahedron and truncated triangles are developed. With dose spherical, decahedron and/or truncated triangles grew independently indicating separate nucleation pathways. To gain quantitative information of plasmonic properties we have compared optical spectra of Ag NPs obtained under different conditions. The effects of the concentrations of silver ions and stabilizer, counter anion, ionic strength, solvent and pH of the reaction medium on the dipolar plasmon frequency are investigated. Synthesized Ag nanoplates and NPs are characterized using various techniques; X-ray powder diffraction (XRD), transmission electron microscopy (TEM) and ultraviolet–visible absorption spectroscopy (UV–vis). A high yield of nanoplates is obtained under optimized conditions, 3.0 × 10−2 M Ag+ ions and 0.01% (w/v) PVP. Change in the concentrations of Ag+ ions and PVP resulted in samples with fewer nanoplates compared to nanospheres indicating inhibition of growth of nanoplates. Use of alcohols instead of water resulted in samples with only nanospheres. Neither the effect of counter anion nor the change in pH (5–9) has any influence on the yield of nanoplates. Ag nanoplates are found to be highly stable under deaerated condition. However, presence of oxygen plays a role in transforming nanoplates to nanospheres. A plausible mechanism for the effect of O2, aging and solvent is discussed.
2.4 GREEN SYNTHESIS METHOD
Silver nanoparticles were synthesized by green route using a traditional medicinal plant Adhatoda vasica having high therapeutic potential. This protocol gives synthesis of stable silver nanoparticles of size 16.8 nm. FTIR study suggested that various biomolecules including vasicine and vasicinone may be responsible for capping and stabilizing of silver nanoparticles. The advantages of this method are synthesis at room temperature very rapid, cost-effective, eco-friendly, non-toxic and no requirement of any accelerant or capping agent.
Bacteria-mediated synthesis of Ag NPs was carried out. HR -LCMS and Blast analysis revealed a detailed bioreduction mechanism of Ag NPs. Size and shape of Ag NPs are controlled by bacteria and media components. Bacteria-mediated synthesized Ag NPs showed enhancement in the antibacterial activity of ampicillin antibiotic.
2.5 CATALYTIC APPLICATIONS:
2.5.1 Effect of morphology on photocatalytic behaviour:
a. Optical and photocatalytic properties of single crystalline ZnO NPs were studied. Crystalline flowerlike ZnO NPS were synthesized by an aminolytic reaction at the air- liquid interface in an aqueous media at an alkaline pH. A thin visible film was formed at the air-liquid interface by self-assembly of flowerlike ZnO. Fourier transform infrared (FTIR) spectroscopy was used to study the chemical composition of the sample, in order to derive a plausible growth mechanism. The molecules are held together by the intermolecular and intramolecular hydrogen bonding. This fact is confirmed from the FTIR spectra. Figure 1 shows comparative FTIR spectra of as-prepared ZnO (ZM4), ZnO at the air-liquid interface and in the sediments.
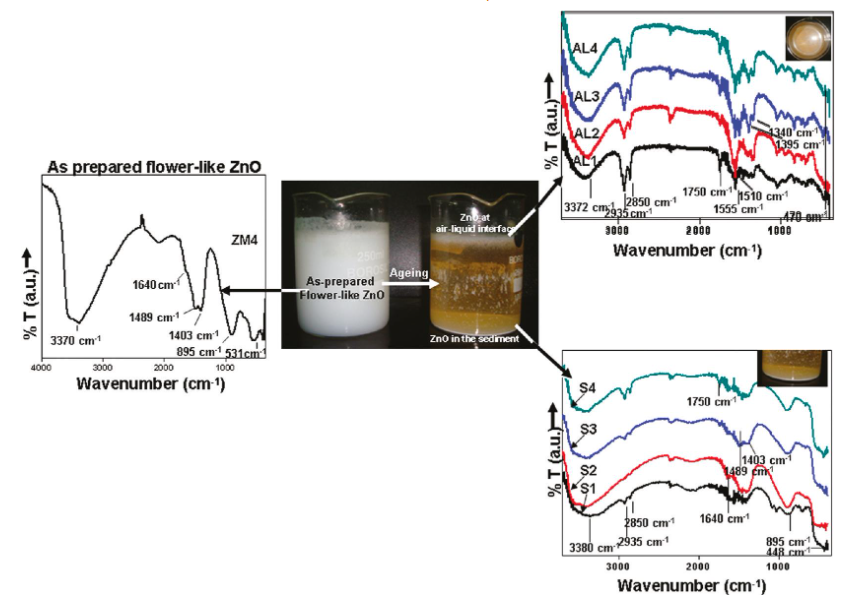
The photodegradation of methylene blue over the flowerlike ZnO catalyst formed at the air-liquid interface and in the sediments shows enhanced photocatalytic activity. The sub-bands formed due to surface defects facilitate separation of charge carriers increasing their lifetime, leading to enhanced photocatalytic activity of flowerlike ZnO.
2.5.2 Use as Burn rate catalyst:
a. The catalytic effect of two different sizes of alpha-Fe2O3 nanoparticles synthesised using an electrochemical method was investigated on the thermal decomposition of ammonium perchlorate (AP) using differential scanning calorimetry as a function of catalyst concentration. The nanosized ferric oxide particles exhibited more of a catalytic effect on the thermal decomposition of AP than commercial Fe2O3 particles. A lowering of the high-temperature decomposition of AP by 59°C was observed after mixing with 2 wt. per cent of alpha-Fe2O3 particles with the very fine size of 3.5 nm. The mixture produced a high heat release of 4.574 kJ/g compared to 0.834 kJ/g of pure AP. The kinetic parameters were evaluated using Kissinger method. The decrease in the activation energy and increase in rate constant confirmed the catalytic activity of these nanoparticles.
b. The catalytic effect of p-type nano CuO and CuCr2O4 synthesized using electrochemical method has been investigated on the thermal decomposition behavior of ammonium perchlorate (AP) using differential scanning calorimetry as a function of catalyst concentrations. The nano copper chromite showed best catalytic effect as compared to nano cupric oxide in lowering the high temperature decomposition by 118°C at 2 wt.%. The high heat release of 5.430 and 3.921 kJ/g were observed in presence of nano CuO and CuCr2O4 respectively. The kinetic parameters were evaluated using Kissinger method. The decrease in the activation energy and increase in rate constant in both the oxides confirmed the enhancement in catalytic activity of ammonium perchlorate. A mechanism based on electron transfer process has also been proposed for AP in presence of nano metal oxides.
2.6 EFFECT OF QUATERNARY AMMONIUM SALTS ON MAGNETIC BEHAVIOUR:
The electrochemical synthesis of alpha Fe2O3 nanoparticles was performed using quaternary ammonium salts viz. TPAB, TBAB and TOAB in an organic medium by optimizing current density and molar concentration of the ligand. The role of ligands in the formation of a phase, structure and magnetic properties was investigated in details. The effect of increasing chain length on the particle size confirmed that as the chain length increases from propyl to octyl, the particle size decreases. X-ray diffraction spectra of as prepared samples and TEM analysis confirmed the amorphous nature of iron oxide. TEM showed beads of iron oxide joined together with a size distribution in the range of 6–30 nm. Iron oxide capped with TOAB indicated superparamagnetic nature at room temperature. The resultant internal magnetic field of 506 mm/s due to hyperfine splitting clearly established the formation of α-Fe2O3. The infrared spectroscopy and pH measurements revealed the binding of tetra alkyl ligand with iron oxide.
3. Electrochemical Synthesis: Features
An electrochemical synthesis is achieved by passing an electric current between two or more electrodes separated by an electrolyte, and the synthesis takes place at the electrode-electrolyte interface. Several features which distinguish electrosynthesis from other synthetic methods are as follows:
- Electrochemical synthesis takes place close to the electrode within the electric double layer, which has a very high potential gradient of 105 Vcm-1. Under these conditions, the reactions often lead to products which cannot be obtained in a chemical synthesis.
- The product is deposited on the electrode in the form of a thin film or a coating. Further, a solid-liquid interface facilitates the growth of conformal coatings on substrates of any shape, especially if a suitably shaped counter electrode is employed to provide uniform polarization.
- Electrochemical synthesis is a low-temperature technique limited by the boiling point of the electrolyte. This can be raised by using molten salt electrolytes.
- Kinetic control can be exercised by controlling the current passed through the cell, while thermodynamic control can be exercised by choosing the applied cell potential.
- An electrochemical synthesis is an oxidation or a reduction reaction. By fine-tuning the applied cell potential, the oxidizing or reducing power can be continuously varied and suitably selected.
- The film composition can be controlled by varying the bath composition.
- The experiments are simple to perform and the instruments are inexpensive and readily available.
3.1 DESIGNING AN ELECTROCHEMICAL SYNTHESIS:
The success of an electrosynthetic reaction depends on the proper choice of a number of reaction parameters:
- Choice of electrodes inert or reactive
- Choice of an electrolyte
- Choice of temperature, pH, concentration, and composition of the electrolyte solution
- Choice of the cells divided or undivided. In a typical electrosynthesis, the reactant dissolved in the electrolyte is deposited as a solid product. Consequently, the activity of the reactant decreases as the reaction proceeds. The two important parameters that determine the course of the reaction are (i) the deposition current and (ii) the cell potential. Of the two, any one can be controlled as a function of time during the reaction.
3.2 CHOICE OF SOLVENT AS AN ELECTROLYTE:
Choice of solvent depends on their properties such as polarity, dielectric constant, surface tension, etc.; play an important role in controlling the morphology and size of NPs.
Explanation of the terms involved:
- Nonpolar solvents: have zero or small dipole moments and low dielectric constant (<5) and are typically used for dissolving non-polar substances.e.g. benzene, hexane, pentane, toluene, chloroform and diethylether. Some nonpolar solvents with slightly large dipole moments and dielectric constant such as dichloro methane tetrahydrofuran (THF), and ethyl acetate are useful as solvents for large number of reactions.
- Polar Aprotic solvents: have large dipole moments and higher dielectric constant (>10) along with some water solubility. They lack O-H or N-H bond (aprotic) and therefore do not participate in Hydrogen bonding, e.g. acetone, acetonitrile and dimethyl formamide (DMF).
- Polar Protic solvents: have large dipole moments and high dielectric constant (polar) and contain O-H or N-H bonds (protic). Therefore, they can form Hydrogen bonds. e.g. Water, ethanol, acetic acid, t-butanol, ammonia.
The structural properties can be tailored by using mixed solvent systems changing their mixture ratio and nature of cosolvent. Cosolvent is miscible with water and increases the solubility of poorly soluble substance. Ethanol and glycerol are used in food science and pharmaceutical science. Ethanol is used in drug delivery formulations. The mixture of solvents provides extra degrees of freedom in tailoring the properties of solution. Each molecule of water in liquid state can form four hydrogen bonds with the solute molecules leading to the formation of three-dimensional network structures. During this rearrangement, large number of water molecules move collectively and dynamically, accompanied by large energy fluctuations. Ethanol is used as a polar co-solvent which is often used to study release kinetics, solubility and formulation of drug. Furthermore, it also acts as a dehydrating agent and gives directly ZnO NPs instead of zinc hydroxide without any heat treatment. Alcohol molecules can form two hydrogen bonds giving rise to one dimensional structure consisting of chains of molecules. In case of alcohol-water mixture the structure and properties result from the balance between hydrophilic and hydrophobic part of alcohol molecule. Due to polar mixed solvent system they offer faster synthesis due to fast electrolysis rate.
It is well known that capping agent is needed during the synthesis of ZnO due to its poor crystallization at low temperature. In present study, TBAB is used as a capping agent in electrochemical synthesis of ZnO. It also works as supporting electrolyte, which changes the electrical atmosphere in the reaction medium. It possesses 4 alkyl chains with a symmetrical structure and has a large hydrophobic volume. Quaternary ammonium salts act as electrolytes and are reported to show unusual properties such as high viscosities with large temperature coefficient, long dielectric relaxation time, and high partial molar heat capacities. TBAB is a cationic capping agent in which TBA+ ions form polyhedral clatharate hydrates with many molecules of water of crystallization. Patel and coworkers have studied behaviour of quaternary ammonium salts and their effect on the micellar properties of surfactants. According to them, tetra butyl ammonium ions show tendency to be rejected from aqueous phase due to strong cohesive force between water-water bonding and get adsorbed at the interface in contact with their aqueous solution. They reduce the surface tension of water and become more surface active, facilitating elongated growth by reducing the effective head area due to screening effect.
We have reported synthesis of cadmium sulphide nanoparticles using simple approach by using various alcohols and their mixture with water as a reaction medium. We observed that the solvent plays effective role not only in controlling the particle size but also in the structure. We obtained cubic phase in pure alcohol while hexagonal phase was obtained in mixture of alcohol and water. The possible mechanism for the structural phase transition is discussed on the basis of change in surface energy and solvent-solvent interaction.
Though there are reports on preparation of ZnO nanomaterials by polymer combustion, sol-gel, polyol, sonochemical, coprecipitation, hydrothermal, low temperature aqueous method and so on, synthesis by electrochemical method by use of mixture of simple alcohol and water has not been reported so far. Being nontoxic and biocompatible, using ethanol as cosolvent, water as main medium and their mixture in various proportions we have prepared ZnO NPs by electrochemical method using quaternary ammonium salts as supporting electrolyte. ZnO NPs were synthesized by electrochemical method in an undivided two electrode cell, consisting of pure Zn metal foil (sacrificial anode) and Pt foil (cathode).
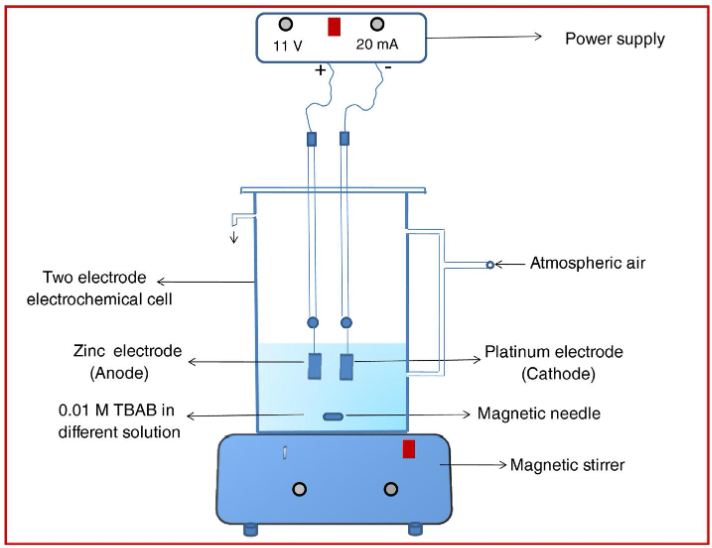
During all experiments, electrodes of 0.5 mm thickness and area 1 cm × 1 cm were used. Also, a constant distance of 1 cm was maintained between two electrodes. Before the experiment, Pt electrode was cleaned by dipping it in concentrated nitric acid and Zn electrode was cleaned by sonication in absolute ethanol for few minutes. Both electrodes were then washed thoroughly with deionized water. Electrolytic medium was prepared by dissolving 20 mg TBAB (0.01 M) in 65 mL of the desired solvent, such as pure ethanol, water-ethanol (7:3) mixture or pure water. Here TBAB served as both stabilizing agent as well as supporting electrolyte. Continuous magnetic stirring and constant current density of 20mA was applied for 2 h. In this work, tetrabutyl ammonium bromide (TBAB) has been used as supporting electrolyte and as capping agent in different polar protic solvents viz. water, ethanol and their mixture. Lowest rate of electrolysis observed was due to less polarity of ethanol; favours formation of mesoporous NPs with small particle size, higher band gap and exclusively high surface area as compared with those synthesized in water and water-ethanol mixture. Synthesis in ethanol yielded ZnO nanospheres while involvement of water as an electrolyte gives ZnO nanospindles. Catalytic activity of sample was evaluated by degradation of Methylene blue (MB) dye under UV light irradiation. ZnO nanospheres obtained in ethanol showed highest catalytic activity. The mechanism proposed for this synthetic route may be useful for the selective synthesis of other metal hydroxide and metal oxide nanoparticles with controlled shape and size. ZnO NPs synthesized in present study may be used for different catalytic reactions due to its smaller particle size, high surface area and mesoporous nature.
3.3: QUATERNARY AMMONIUM COMPOUNDS (QAC):
Quaternary ammonium compounds (QAC) have a general chemical structure N+ R1R2R3R4 X−, are a group of ammonium salts in which organic radicals are substituted for all the four hydrogen of the original ammonium cation. They have a central nitrogen atom, which is joined to four organic radicals(R) and one acid radical(X) such as chloride or bromide ion. Among four alkyl groups, one is a long alkyl chain group containing more than eight hydrocarbons and also serves as the hydrophobic group. They are prepared by treatment of an amine with an alkylating agent. They show variety of physical, chemical and biological properties and most compounds are soluble in water and act as a strong electrolyte.
Earlier in 1900s, the antimicrobial activity of QAC was first studied. QACs are used for numerous industrial purposes, including cleaning and disinfecting farm buildings, water and waste water treatment, and antifungal treatment in horticulture. In addition, QACs are used in pharmaceutical and consumer products. QACs have been used in the following products: eyewash/artificial tears; nose decongestant lotions; facial cleansers; acne treatment; sun protection creams and lotions; baby lotions; moisturisers; pain relief poultices or creams; hair conditioners; hair colour and styling products; make-up and make-up removal products; and hand sanitizers.
Quaternary ammonium compounds (QACs) are widely distributed in hospitals, industry and cosmetics. Little attention has been focused on the potential impact of QACs on the emergence of antibiotic resistance in patients and the environment. Significant studies have been made to address the primary concerns about the health issues. Some of the reviews mentioned here report valuable information about the antimicrobial and antibacterial resistance. The literature reviews on QAC report chemical structure, fields of application, mechanism of action, susceptibility testing, prevalence, and co- or cross-resistance to antibiotics. QACs have different impacts on the minimum inhibitory concentrations of antibacterials depending on the antibacterial compound investigated, the resistance genes involved, the measuring methodology and the interpretative criteria.
Quaternary ammonium compounds (QACs) are widely used as biocides that possess antimicrobial effect against a broad range of microorganisms. These compounds are used for numerous industrial purposes, water treatment, antifungal treatment in horticulture, as well as in pharmaceutical and everyday consumer products as preserving agents, foam boosters, and detergents. Resistance toward QACs is widespread among a diverse range of microorganisms and is facilitated by several mechanisms such as modifications in the membrane composition, expression of stress response and repair systems, or expression of efflux pump genes. Development of resistance in both pathogenic and non-pathogenic bacteria has been related to application in human medicine and the food industry. QACs in cosmetic products will inevitably come into intimate contact with the skin or mucosal linings in the mouth and thus are likely to add to the selection pressure toward more QAC-resistant microorganisms among the skin or mouth flora. There is increasing evidence of coresistance and cross-resistance between QACs and a range of other clinically important antibiotics and disinfectants.
Synthesis and evaluation of the antimicrobial activity of 52 novel QACs bearing 1-3 quaternary ammonium centres was performed. Striking differences in antimicrobial activity against bacteria bearing QAC resistance genes were observed, with up to a 125-fold increase in minimum inhibitory concentration (MIC) for select structures against bacteria known to bear efflux pumps. Increased use of QACs during the COVID-19 pandemic has raised concerns about their toxicity and potentially harmful effects on humans and several aquatic organisms. Besides the toxicity of QACs, there is a growing concern that QACs can lead to increased Antimicrobial Resistance (AMR) in bacteria. Thus, this comprehensive review addresses various aspects of QACs, focusing on the distribution and fate of several QACs in environmental matrices and during wastewater treatment. In general, this paper further provides a comprehensive discussion on the classification and sources of QACs, and the resulting toxicity and development of AMR.
The bioaccumulation studies of QAC have been done during COVID-19 pandemic. Researchers determined bioaccumulation potentials of 18 QACs with alkyl chain lengths of C8-C18 in the in vitro-in vivo extrapolation (IVIVE) model using the results of human hepatic metabolism and serum protein binding experiments. The slowest in vivo clearance rates were estimated for C12-QACs, suggesting that these compounds may preferentially build up in blood. The bioaccumulation of QACs was further confirmed preferentially build up in blood. The bioaccumulation of QACs was further confirmed by the analysis of human blood (sera) samples (n = 222). Fifteen out of the 18 targeted QACs were detected in blood with the ΣQAC concentrations reaching up to 68.6 ng/mL. The blood samples were collected during two distinct time periods: before the outbreak of the COVID-19 pandemic (2019; n = 111) and during the pandemic (2020, n = 111). The ΣQAC concentrations were significantly higher in samples collected during the pandemic (median 6.04 ng/mL) than in those collected before (median 3.41 ng/mL).
In another review also, concerns about the potential emergence of Enterococcus spp. populations exhibiting resistance to both biocides or QACs and antibiotics is reported. Such concerns arise from their frequent exposure to subinhibitory concentrations of CBs in clinical, food chain and diverse environmental settings. This comprehensive narrative review aimed to explore the complexity of the Enterococcus’ response to CBs and of their possible evolution toward resistance. To that end, CBs’ activity against diverse Enterococcus spp. collections, the prevalence and roles of genes associated with decreased susceptibility to CBs, and the potential for co- and cross-resistance between CBs and antibiotics are reviewed. Significant methodological and knowledge gaps are identified, highlighting areas that future studies should address to enhance our comprehension of the impact of exposure to CBs on Enterococcus spp. populations’ epidemiology. This knowledge is essential for developing effective One Health strategies that ensure the continued efficacy of these critical agents in safeguarding Public Health.
Quaternary ammonium compounds (QACs) are among the most commonly used disinfectants. There has been concern that their widespread use will lead to the development of resistant organisms, and it has been suggested that limits should be placed on their use. While increases in tolerance to QACs have been observed, there is no clear evidence to support the development of resistance to QACs. Since efflux pumps are believed to account for at least some of the increased tolerance found in bacteria, there has been concern that this will enhance the resistance of bacteria to certain antibiotics. QACs are membrane-active agents interacting with the cytoplasmic membrane of bacteria and lipids of viruses.
Researches performed a prospective cohort study in 153 patients with Escherichia coli bacteraemia from February to September 2008 at University Hospital in Rennes. The minimum inhibitory concentrations (MICs) of antibiotics and QACs alkyldimethylbenzylammonium chloride (ADBAC) and didecyldimethylammonium chloride (DDAC) were determined by the agar dilution method and the capacity of biofilm production was assayed using the Crystal Violet method and mutation frequencies by measuring the capacity of strains to generate resistance to rifampicin. Logistic regression analysis showed that one of the significant factors related to low MICs for ADBAC (≤16 mg/L) and DDAC (≤8 mg/L), was cotrimoxazole susceptibility (odds ratio: 3.72; 95% confidence interval: 1.22-11.24; P=0.02 and OR: 3.61; 95% CI: 1.56-7.56; P<0.01, respectively). Antibiotic susceptibility to cotrimoxazole was strongly associated with susceptibility to amoxicillin and nalidixic acid (P<0.01). Community-acquired or healthcare-associated bacteraemia, severity of bacteraemia, and patient outcome were independent of the MICs of ADBAC and DDAC. The findings demonstrate an epidemiological relationship between higher MIC values of QACs in clinical E. coli isolates and antibiotic resistance.
Bacterial resistance toward commonly used biocides is a widespread yet underappreciated problem, one which needs not only a deeper understanding of the mechanisms by which resistance proliferates, but also means for mitigation. To advance understanding of this issue, scientists recognized a polyaromatic structural core analogous to activators of QacR, a negative transcriptional regulator of the efflux pump QacA, and envisioned a series of quaternary ammonium compounds (QACs) based on this motif. Using commercially available dye scaffolds, they synthesized and evaluated the antimicrobial activity of 52 novel QACs bearing 1-3 quaternary ammonium centres. Striking differences in antimicrobial activity against bacteria bearing QAC resistance genes have been observed, with up to a 125-fold increase in minimum inhibitory concentration (MIC) for select structures against bacteria known to bear efflux pumps. Based on these findings, general trends in structure-resistance relationships have been identified, laying the groundwork for future mechanistic studies.
Another usage of (QACs) represents their role as effective classes of disinfectant agents in dental materials and resin nanocomposites. QACs have been used as monomer and micro/nanofiller in restorative dentistry. They possess one or more methacrylate functional groups to participate in polymerization reactions. QACs with multiple methacrylate groups can also be used as crosslinking agents. Furthermore, QACs with chain length from ∼12 to 16 have higher antimicrobial activity in cured dental resins. In general, increasing the chain length leads to a threshold value (critical point) and then it causes decrease in the antimicrobial activity.
4. Biomedical Applications of ZnO Nanomaterials:
4.1 ANTICANCER ACTIVITY:
Despite of advancement in medicinal field cancer remains one of the fatal diseases. Traditional treatments include chemotherapies. However there are concerns about stability and poor solubility. Destruction of healthy cells, hair loss, and drug resistance are some of the side effects. In this aspect, nanoscience and nanotechnology has opened up new alternatives. ZnO NPs are one of the most valuable metal oxide NPs owing to their high biocompatibility and low toxicity. ZnO exhibit different types of surface charge behaviour due to their electrostatic properties; utilized for anticancer activity. ZnO NPs demonstrate their anticancer activity by inducing ROS generation and also by inducing apoptosis. Electrostatic interactions between positively charged nanomaterials and target cells are believed to play an important part in cellular adhesion and uptake. ZnO NPs exhibit a different type of surface charge behaviour, because of the neutral hydroxyl groups chemisorbed on to their surface. Protons (H+) move out from the particle surface in aqueous medium at high pH, leaving a negatively charged surface with partially bonded oxygen atoms (ZnO_). At lower pH, protons from the environment are transferred to the particle surface, resulting in a positively charged surface (ZnOH2+). The isoelectric point of 9–10 indicates that ZnO nanoparticles will have a strong positive surface charge under physiological conditions. Given that cancer cells frequently contain a high concentration of anionic phospholipids on their outer membrane and large membrane potentials interactions with positively charged ZnO nanoparticles are expected to be driven by electrostatic interactions, thereby promoting cellular uptake, phagocytosis and ultimate cytotoxicity. ZnO nanoparticles have also been shown to exhibit strong protein adsorption properties, which can be used to modulate cytotoxicity, metabolism or other cellular responses.
In a recent study, researchers have developed a screen-printed des-carboxy-prothrombin (DCP) immunosensor using ZnO NPs for accurate DCP assessment in the detection of liver cancer. DCP is a novel biomarker for detecting liver cancer that has a sensitivity of roughly 70% and a specificity of approximately 100%. As a result, the DCP immunosensor developed is simple, cheap, and reliable, with the potential to be used at home to screen for early-stage liver.
4.2 ANTIBACTERIAL ACTIVITY:
The antibacterial activity of ZnO NPs also lies in their ability to induce oxidative stress. Zn+ ions, released by ZnO, interact with the thiol group of respiratory enzymes, inhibiting their action. It has been demonstrated that ZnO NPs affect the cell membrane and lead to ROS formation. Thus, when bacterial cells come into contact with ZnO NPs, they absorb Zn+, which then inhibits the action of respiratory enzymes, generates ROS, and produces free radicals, causing oxidative stress. ROS irreversibly damage bacterial membranes, DNA, and mitochondria, resulting in the death of cells.
Synthesized ZnO-NPs, which have natural antibacterial effects and are photocatalytic in the ultraviolet (UV-B) light range, can create potent hydroxyl (-OH) free radicals to kill dangerous pathogens and germs at wound sites. This observation led to the development of a 3D printed customized wound-healing template made of ZnO-NPs that were uniformly scattered within an alginate template, which can be easily created and contour-printed to the exact size and depth of a wound. 3D printing consists of the adding of material layer by layer, allowing for the fabrication of unique shapes and customizability, which are crucial in biomedical areas such as tissue engineering and pharmaceuticals. ZnO-NPs medical medication action mechanism is still a mystery. Hydrogen peroxide emission may be the essential factor in the action of therapeutic drugs. It is also possible that the mechanism is due to the binding of particles on the bacterial surface, owing to static tensions.
According to the results, the antibacterial activity of ZnO-NPs seems to be stronger than that of tiny particles. Particle dosage, treatment duration, and the NP production process influence NPs’ efficacy. Furthermore, the surface area and the size of particle variation, which are noteworthy in green-synthesized ZnO-NPs, are responsible for enhanced antibacterial activity. Future medical difficulties might benefit from green-synthesized ZnO-NPs applications in food safety and agriculture that have not yet been confirmed. Reported toxicities associated with the liver, lung, nervous system, and immune system are dose and exposure dependent. Accumulation risk is high in long-term high dose intake. Antibacterial activity of ZnO nanoparticles requires higher doses than that causing toxicity; therefore, disinfection with ZnO may be a risk for human use.
Conclusion:
In this report, an attempt is made to review the use of synthesis methods which might help in preparing ZnO NPs of various size and morphology. The versatile use of QACs has been reported, their risk factors must be taken into account while choosing the compounds. Although ZnO NPs are proven to be future potential, their toxicity is the main concern and more in-depth understanding should be developed. Understanding of cellular and molecular pathways and clinical trials will be required in future.
Conflicts of Interest Statement:
The author has no conflict of interest to declare.
Acknowledgements:
I hereby acknowledge my PhD students who have contributed their thesis work done and their contributions are cited wherever necessary.
References:
- Patil PR, Joshi SS. Polymerized organic–inorganic synthesis of nanocrystalline zinc oxide. Mater Chem Phys. 2007;105(2):354-361. doi:https://doi.org/10.1016/j.matchemphys.2007.04.072
- Ushio Y, Miyayama M, Yanagida H. Effects of interface states on gas-sensing properties of a CuO/ZnO thin-film heterojunction. Sens Actuators B Chem. 1994;17(3):221-226. doi:https://doi.org/10.1016/0925-4005(93)00878-3
- Troy CT. New phosphor view promises display advances. Photonics Spectra. 1997;31(1):34.
- Fan Z, Lu JG. Zinc Oxide Nanostructures: Synthesis and Properties. J Nanosci Nanotechnol. 2005;5(10):1561-1573. doi:10.1166/jnn.2005.182
- Pan H, Zhu Y, Ni Z, et al. Optical and Field Emission Properties of Zinc Oxide Nanostructures. J Nanosci Nanotechnol. 2005;5(10):1683-1687. doi:10.1166/jnn.2005.183
- Subramanian V, Wolf EE, Kamat P V. Green Emission to Probe Photoinduced Charging Events in ZnO−Au Nanoparticles. Charge Distribution and Fermi-Level Equilibration. J Phys Chem B. 2003;107(30):7479-7485. doi:10.1021/jp0275037
- Weissenberger D, Gerthsen D, Reiser A, et al. Influence of the measurement procedure on the field-effect dependent conductivity of ZnO nanorods. Appl Phys Lett. 2009;94(4):042107. doi:10.1063/1.3075849
- Wang ZL, Kong XY, Ding Y, et al. Semiconducting and Piezoelectric Oxide Nanostructures Induced by Polar Surfaces. Adv Funct Mater. 2004;14(10):943-956. doi:https://doi.org/10.1002/adfm.200400180
- Li Y, Zhou X, Hu X, Zhao X, Fang P. Formation of Surface Complex Leading to Efficient Visible Photocatalytic Activity and Improvement of Photostabilty of ZnO. The Journal of Physical Chemistry C. 2009;113(36):16188-16192. doi:10.1021/jp9056863
- Xiang Q, Meng GF, Zhao HB, et al. Au Nanoparticle Modified WO3 Nanorods with Their Enhanced Properties for Photocatalysis and Gas Sensing. The Journal of Physical Chemistry C. 2010;114(5):2049-2055. doi:10.1021/jp909742d
- Özgür Ü, Alivov YaI, Liu C, et al. A comprehensive review of ZnO materials and devices. J Appl Phys. 2005;98(4):041301. doi:10.1063/1.1992666
- Kind H, Yan H, Messer B, Law M, Yang P. Nanowire Ultraviolet Photodetectors and Optical Switches. Advanced Materials. 2002;14(2):158-160. doi:https://doi.org/10.1002/1521-4095(20020116)14:2<158::AID-ADMA158>3.0.CO;2-W
- Mishra PK, Mishra H, Ekielski A, Talegaonkar S, Vaidya B. Zinc oxide nanoparticles: a promising nanomaterial for biomedical applications. Drug Discov Today. 2017;22(12):1825-1834. doi:https://doi.org/10.1016/j.drudis.2017.08.006
- Rasmussen JW, Martinez E, Louka P, Wingett DG. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin Drug Deliv. 2010;7(9):1063-1077. doi:10.1517/17425247.2010.502560
- Sharma H, Mishra PK, Talegaonkar S, Vaidya B. Metal nanoparticles: a theranostic nanotool against cancer. Drug Discov Today. 2015;20(9):1143-1151. doi:https://doi.org/10.1016/j.drudis.2015.05.009
- Zhang Y, Nayak T, Hong H, Cai W. Biomedical applications of zinc oxide nanomaterials. Curr Mol Med. 2013;13(10):1633-1645. doi:10.2174/1566524013666131111130058
- Joshi SS. Crystal Habit Modification Using Habit Modifiers. In: Kolesnikov N, Borisenko E, eds. Modern Aspects of Bulk Crystal and Thin Film Preparation. IntechOpen; 2012. doi:10.5772/28451
- Joshi SS, Patil SF, Iyer V, Mahumuni S. Radiation induced synthesis and characterization of copper nanoparticles. Nanostructured Materials. 1998;10(7):1135-1144. doi:https://doi.org/10.1016/S0965-9773(98)00153-6
- Temgire MK, Joshi SS. Optical and structural studies of silver nanoparticles. Radiation Physics and Chemistry. 2004;71(5):1039-1044. doi:https://doi.org/10.1016/j.radphyschem.2003.10.016
- Rishikeshi SN, Joshi S. Cu–ZnO nanocrystallites by aqueous thermolysis method. J Therm Anal Calorim. 2012;109(3):1473-1479. doi:10.1007/s10973-012-2212-y
- Jogdand SS, Das A, Dhayagude A, Kapoor S, Joshi SS. Role of PVA in synthesis of nano Co3O4-decorated graphene oxide. Polym Adv Technol. 2015;26(9):1114-1122. doi:https://doi.org/10.1002/pat.3543
- Telkar MM, Rode C V, Chaudhari R V, Joshi SS, Nalawade AM. Shape-controlled preparation and catalytic activity of metal nanoparticles for hydrogenation of 2-butyne-1,4-diol and styrene oxide. Appl Catal A Gen. 2004;273(1):11-19. doi:https://doi.org/10.1016/j.apcata.2004.05.056
- Dhayagude AC, Das A, Joshi SS, Kapoor S. γ-Radiation induced synthesis of silver nanoparticles in aqueous poly (N-vinylpyrrolidone) solution. Colloids Surf A Physicochem Eng Asp. 2018;556:148-156. doi:https://doi.org/10.1016/j.colsurfa.2018.08.028
- Suvidya R, Pravin L, Shubhangi B, Satyawati J. Rapid Green Synthesis and Biological Activity of Silver Nanoparticles Using Adhatoda vasica Leaf Extract. Adv Sci Eng Med. 2012;5(4):319-324. doi:10.1166/ASEM.2013.1258
- Sable SV, Kawade S, Ranade S, Joshi S. Bioreduction mechanism of silver nanoparticles. Materials Science and Engineering: C. 2020;107:110299. doi:https://doi.org/10.1016/j.msec.2019.110299
- Vaishampayan M V, Mulla IS, Joshi SS. Optical and Photocatalytic Properties of Single Crystalline ZnO at the Air–Liquid Interface by an Aminolytic Reaction. Langmuir. 2011;27(20):12751-12759. doi:10.1021/la203006n
- Vaishampayan M V, Mulla IS, Joshi SS. Low temperature pH dependent synthesis of flower-like ZnO nanostructures with enhanced photocatalytic activity. Mater Res Bull. 2011;46(5):771-778. doi:https://doi.org/10.1016/j.materresbull.2010.11.007
- Joshi S, Patil P, Krishnamurthy V. Thermal Decomposition of Ammonium Perchlorate in the Presence of Nanosized Ferric Oxide. Def Sci J. 2008;58. doi:10.14429/dsj.58.1699
- Patil PR, Krishnamurthy VN, Joshi SS. Effect of Nano-Copper Oxide and Copper Chromite on the Thermal Decomposition of Ammonium Perchlorate. Propellants, Explosives, Pyrotechnics. 2008;33(4):266-270. doi:https://doi.org/10.1002/prep.200700242
- Joshi SS, Patil PR, Nimase MS, Bakare PP. Role of ligands in the formation, phase stabilization, structural and magnetic properties of α-Fe2O3 nanoparticles. Journal of Nanoparticle Research. 2006;8(5):635-643. doi:10.1007/s11051-005-9033-x
- Therese GHA, Kamath PV. Electrochemical Synthesis of Metal Oxides and Hydroxides. Chemistry of Materials. 2000;12(5):1195-1204. doi:10.1021/cm990447a
- Yin J, Gao F, Wei C, Lu Q. Water Amount Dependence on Morphologies and Properties of ZnO nanostructures in Double-solvent System. Sci Rep. 2014;4(1):3736. doi:10.1038/srep03736
- Pang H, Gao F, Lu Q. Glycine-assisted double-solvothermal approach for various cuprous oxide structures with good catalytic activities. CrystEngComm. 2010;12(2):406-412. doi:10.1039/B904705K
- Khan Z, AL-Thabaiti SA, Obaid AY, Khan ZA, Al-Youbi AO. Effects of solvents on the stability and morphology of CTAB-stabilized silver nanoparticles. Colloids Surf A Physicochem Eng Asp. 2011;390(1):120-125. doi:https://doi.org/10.1016/j.colsurfa.2011.09.015
- Thomas J, Evans DF. Transport processes in hydrogen-bonding solvents. IV. Conductance of electrolytes in formamide at 25 and 10.deg. J Phys Chem. 1970;74(21):3812-3819. doi:10.1021/j100715a016
- Martin A, Swarbrick J, Cammarata A. Physical Pharmacy: Physical Chemical Principles in the Pharmaceutical Sciences. 3rd ed. Lea & Febiger; 1983.
- Ohmine Iwao, Tanaka Hideki. Fluctuation, relaxations, and hydration in liquid water. Hydrogen-bond rearrangement dynamics. Chem Rev. 1993;93(7):2545-2566. doi:10.1021/cr00023a011
- Patel J, Varade D, Bahadur P. Effect of tetraalkylammonium bromides on the micellar behaviour of ionic and non-ionic surfactants. Indian Journal of Chemistry – Section A Inorganic, Physical, Theoretical and Analytical Chemistry. 2004;43:715-721.
- Thakur P, Joshi SS. Effect of alcohol and alcohol/water mixtures on crystalline structure of CdS nanoparticles. J Exp Nanosci. 2012;7(5):547-558. doi:10.1080/17458080.2010.543990
- Dhayagude AC, Nikam S V, Kapoor S, Joshi SS. Effect of electrolytic media on the photophysical properties and photocatalytic activity of zinc oxide nanoparticles synthesized by simple electrochemical method. J Mol Liq. 2017;232:290-303. doi:https://doi.org/10.1016/j.molliq.2017.02.074
- Merianos JJ. Quaternary Ammonium Antimicrobial Compounds. In: Block SS, ed. Disinfection, Sterilization, and Preservation. 4th ed. Lea & Febiger; 1991:225-255.
- Walker EB. Quaternary Ammonium Compounds. In: Paulson DS, ed. Handbook of Topical Antimicrobials: Industrial Applications in Consumer Products and Pharmaceuticals. Marcel Dekker, Inc.; 2003:99-116.
- Gilbert P, Moore LE. Cationic antiseptics: diversity of action under a common epithet. J Appl Microbiol. 2005;99(4):703-715. doi:10.1111/j.1365-2672.2005.02664.x
- Buffet-Bataillon S, Tattevin P, Bonnaure-Mallet M, Jolivet-Gougeon A. Emergence of resistance to antibacterial agents: the role of quaternary ammonium compounds—a critical review. Int J Antimicrob Agents. 2012;39(5):381-389. doi:https://doi.org/10.1016/j.ijantimicag.2012.01.011
- Hegstad K, Langsrud S, Lunestad BT, Scheie AA, Sunde M, Yazdankhah SP. Does the Wide Use of Quaternary Ammonium Compounds Enhance the Selection and Spread of Antimicrobial Resistance and Thus Threaten Our Health? Microbial Drug Resistance. 2010;16(2):91-104. doi:10.1089/mdr.2009.0120
- Forman ME, Fletcher MH, Jennings MC, Duggan SM, Minbiole KPC, Wuest WM. Structure–Resistance Relationships: Interrogating Antiseptic Resistance in Bacteria with Multicationic Quaternary Ammonium Dyes. ChemMedChem. 2016;11(9):958-962. doi:https://doi.org/10.1002/cmdc.201600095
- Mohapatra S, Yutao L, Goh SG, et al. Quaternary ammonium compounds of emerging concern: Classification, occurrence, fate, toxicity and antimicrobial resistance. J Hazard Mater. 2023;445:130393. doi:https://doi.org/10.1016/j.jhazmat.2022.130393
- Zheng G, Webster TF, Salamova A. Quaternary Ammonium Compounds: Bioaccumulation Potentials in Humans and Levels in Blood before and during the Covid-19 Pandemic. Environ Sci Technol. 2021;55(21):14689-14698. doi:10.1021/acs.est.1c01654
- Pereira AP, Antunes P, Peixe L, Freitas AR, Novais C. Current insights into the effects of cationic biocides exposure on Enterococcus spp. Front Microbiol. 2024;15:1392018. doi:10.3389/fmicb.2024.1392018
- Gerba CP. Quaternary Ammonium Biocides: Efficacy in Application. Appl Environ Microbiol. 2015;81(2):464-469. doi:10.1128/AEM.02633-14
- Buffet-Bataillon S, Branger B, Cormier M, Bonnaure-Mallet M, Jolivet-Gougeon A. Effect of higher minimum inhibitory concentrations of quaternary ammonium compounds in clinical E. coli isolates on antibiotic susceptibilities and clinical outcomes. Journal of Hospital Infection. 2011;79(2):141-146. doi:https://doi.org/10.1016/j.jhin.2011.06.008
- Makvandi P, Jamaledin R, Jabbari M, Nikfarjam N, Borzacchiello A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dental Materials. 2018;34(6):851-867. doi:https://doi.org/10.1016/j.dental.2018.03.014
- Bilensoy E, Varan C. Is there a niche for zinc oxide nanoparticles in future drug discovery? Expert Opin Drug Discov. 2023;18(9):943-945. doi:10.1080/17460441.2023.2230152
- Mita Y, Aoyagi Y, Yanagi M, Suda T, Suzuki Y, Asakura H. The usefulness of determining des-γ-carboxy prothrombin by sensitive enzyme immunoassay in the early diagnosis of patients with hepatocellular carcinoma. Cancer. 1998;82(9):1643-1648. doi:https://doi.org/10.1002/(SICI)1097-0142(19980501)82:9<1643::AID-CNCR8>3.0.CO;2-B
- Anjum S, Hashim M, Malik SA, et al. Recent Advances in Zinc Oxide Nanoparticles (ZnO NPs) for Cancer Diagnosis, Target Drug Delivery, and Treatment. Cancers (Basel). 2021;13(18). doi:10.3390/cancers13184570
- Dwivedi S, Wahab R, Khan F, Mishra YK, Musarrat J, Al-Khedhairy AA. Reactive Oxygen Species Mediated Bacterial Biofilm Inhibition via Zinc Oxide Nanoparticles and Their Statistical Determination. PLoS One. 2014;9(11):e111289-. https://doi.org/10.1371/journal.pone.0111289
- Islam F, Shohag S, Uddin MdJ, et al. Exploring the Journey of Zinc Oxide Nanoparticles (ZnO-NPs) toward Biomedical Applications. Materials. 2022;15(6). doi:10.3390/ma15062160

