Cannabinolic Acids: New Anticancer Drug Insights
Cannabinolic acids derivatives as a new anticancer drugs
Alexander Aizikovich, PhD
- AL&AM Pharmachem Ltd., Rehovot, Israel.
[email protected]
OPEN ACCESS
PUBLISHED: 30 June 2025
CITATION: Aizikovich, A., 2025. Cannabinolic acids derivatives as a new anticancer drugs. Medical Research Archives, [online] 13(6). https://doi.org/10.18103/mra.v13i6.6575
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI https://doi.org/10.18103/mra.v13i6.6575
ISSN 2375-1924
ABSTRACT
Intensive research of the main products of the cannabis plant – tetrahydrocannabinolic (THCA) and cannabidiolic (CBDA) acids in recent years provided supportive evidence for their anti-inflammatory and anticancer activity, as well as the absence of any psychoactive properties. The presence of a carboxyl group in the cannabinoid acid molecules opens up a truly “Klondike” opportunity to obtain different derivatives and study their biological activity. This article is dedicated to description of some nitrogen containing THCA and CBDA derivatives and in vitro and in vivo anticancer activity of these compounds toward various cancer cell lines. A study of the cannabinoid influence on the hormesis effect in a HT-29 colon cancer cell line showed its direct dependence on the structure of both the cannabinoid moiety and the side chain substituents.
Keywords: THCA, CBDA, in vitro, in vivo, anticancer drugs.
1. Introduction
Marijuana is known as a natural drug useful in the treatment of inflammation, pain, psychoses, migraine and other disorders of the nervous system. Owing to their various activity, natural cannabinoids can often be used for the development of new potential drugs especially as starting materials for organic synthesis.
Cannabinoids are active ingredients of the Cannabis Sativa plant, which mimic the effects of the endogenous cannabinoid system (endocannabinoids), and impact human body by activating cannabinoid receptors. Cannabinoid receptors include the cannabinoid type 1 (CB1) receptor, predominantly expressed in the brain, and the cannabinoid type 2 (CB2) receptor, primarily found in the immune system cells. Cannabinoids receptors as well as the entire endocannabinoid system are commonly treated as putative targets for the treatment of various diseases, including neurodegenerative diseases, multiple sclerosis and numerous inflammatory diseases such as asthma, allergic, Rheumatoid arthritis, and colitis.
Cannabinoids can be classified into endogenous cannabinoids, phytocannabinoids and synthetic cannabinoids. Endocannabinoids are produced in the human body and mostly act as neuromodulators. They play an important role in inflammation, insulin sensitivity and metabolism, and have an important role in regulating the mood, appetite, pain sensation, inflammation response and memory.
There are about 100 cannabinoids in the cannabis plant. Cannabinolic acids THCA, CBDA and their derivatives THC and CBD obtained after decarboxylation are the most widespread natural cannabinoids. While THC is the primary psychoactive component of the plant and has been used to treat a wide range of medical conditions, the most abundant non-psychoactive phytocannabinoid CBD is known to exert many positive pharmacological effects including anti-inflammatory, anti-anxiety, anti-diabetic, and anti-cancer effects. In addition, CBD is proposed to reverse some of the central side effects of THC, emphasizing the therapeutic value of the THC-CBD formulations.
Cannabis extract obtained immediately from plant material contains up to about 70-75% cannabinolic acids together with terpenoids and other materials like lignin, gums, pigments and lecithin. Crude extracts from cannabis can be used immediately for patients suffering from many diseases, but they are not suitable for pharmacological purposes. For this reason, modern research aim is the development of the new strategies enabling isolation of most important cannabinolic acids THCA and CBDA (Figure 1) which can be dosed and standardized, enabling pharmaceutical activity without psychotropic side effects.
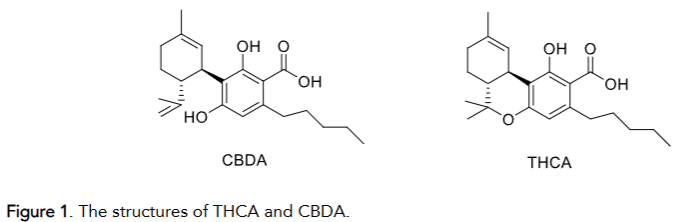
There are many publications that disclose the isolation and purification of THCA and CBDA using liquid chromatography as well as methods for isolating of cannabinoid acids on an semi-industrial scale. Currently, the most advanced process for isolation of natural cannabinolic acids from a crude cannabis plant (e.g., Cannabis saliva) is the ion exchange resin extraction resulting in THCA and CBDA with purity 95-99% and suitable for industrial production.
The acids obtained in this way can be used as a basis for the synthesis of new medicinal substances. This article is dedicated to description of THCA and CBDA nitrogen containing derivatives including their anti-inflammatory properties and anticancer activity toward various cancer cells.
2. Biological properties of cannabinolic acids
The use of cannabinoids as a new drugs has invited the attention of researchers for a long time. Among the possible ways of their activity study the main interest has their anti-inflammatory and anticancer activity due to binding with cannabinoid receptors and affect the ability to expression of pro-inflammatory cytokines like TNF-α, interleukins and INF-γ. Till now the focus was mainly concentrated on THC and CBD as the most affordable and stable products isolated from marijuana. Intensive research on THCA and CBDA in recent years has not only provided supportive evidence for their anti-inflammatory and anticancer activity but, more importantly, the absence of any psychoactive properties. This is consistent with the fact that they bind to CB1 and CB2 cannabinoid receptors: THCA binds to CB1R at the concentration range of 630 nM to 3 µM and to CB2R from 1.3nM to 10 µM. The large THCA concentration range for binding to CB1R may be indicative of a partial THCA decarboxylation event which forms THC during the course of the experiment, as THC has a significantly higher affinity for the CB1R. CBDA has been shown to bind to CB1R at >10000 nM, but has very good CB2R affinity (4.9-77 nM). Another important feature of cannabinolic acids compared to THC and CBD is their high bioavailability. A preliminary study comparing the oral bioavailability of cannabinolic acids with THC, CBD and their metabolites showed that THCA and CBDA concentrations in blood are more than 10 times higher than for THC and CBD and reach a maximum one hour after ingestion. Comparison of half-life data of these acids in the body showed the significant advantage of THCA compared to CBDA and, other things being equal, makes THCA particularly useful as a potential drug. Initially biological studies of these acids focused on their in vitro and in vivo anti-inflammatory activity. These established the ability of THCA and CBDA to suppress the proinflammatory cytokines COX-1 and COX-2, IL-2, IL-8 as well as others. THCA, unlike THC, was able to inhibit the expression of tumor necrosis factor alpha (TNF-a) in a dose dependent manner. The anticancer activity of cannabinoids and their derivatives is one of the important directions in the search for new marijuana-based drugs. This is particularly pertinent for the most common malignant diseases of the gastrointestinal tract and accessory organs, namely cancers of the small intestine, colon and pancreas. The main focus in the search for potential anticancer candidates among products derived from cannabis has so far turned to derivatives such as THC, CBD and CBG on a smaller scale. Their precursors, the cannabinolic acids and their derivatives, have been studied to a much lesser extent, even though these compounds have clear advantages in many of their characteristics such as biocompatibility, solubility in biological fluids, and the absence of psychoactive properties. Anticancer studies have shown poor CBDA activity against CEM and HL60 leukemia and human prostate LNCaP cells. In contrast CBDA has been shown to inhibit cell migration and to decrease the c-fos protooncogene expression and cyclooxygenase-2 (COX-2) in the highly aggressive MDA-MB-231 breast cancer cell line. THCA also inhibits the growth of some types of breast cancer (IC50 of 9.8 µM in MCF-7 and 18.2 µM in MDA-MB-231) and prostate cancer cell lines (IC50 of 25 µM in DU-145). Thus, although cannabinoid acids exhibit both anti-inflammatory and anti-cancer activities, their levels do not exceed those of known drugs. Moreover, the tendency of these acids to decarboxylate during heating and storage makes their use problematic. In the same time the presence of a carboxyl group in the cannabinoid acid molecules opens up a truly “Klondike” opportunity to obtain different derivatives and study their biological activity. All this leads to understanding that the main products of the cannabis plant – tetrahydrocannabinolic (THCA) and cannabidiolic (CBDA) acids can be used as the precursors for new anti-cancer drugs.
3. The in vitro anticancer activity study of some THCA and CBDA nitrogen containing derivatives
The in vitro anticancer screening toward some types of cancer cells allowed to identify the two compounds ALAM027 1 and ALAM108 2 with activity below 10 µM level. These compounds were obtained by the following synthetic routes (Figure 2).
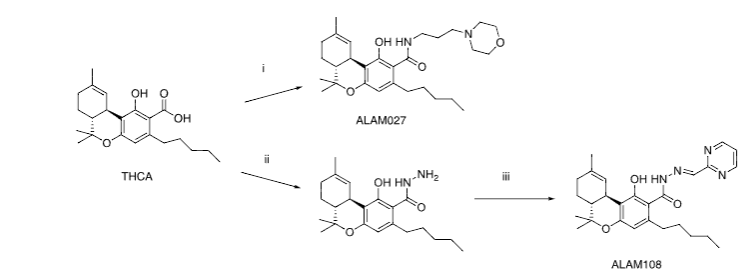
According to World Health Organization the data the most widespread types of cancers are breast, lung, colon, intestine, pancreatic, prostate tumors and blood diseases such as multiple myeloma. Brain tumors such as gliomas are also potentially interesting, particularly because of their aggressive and highly invasive characteristic so facilitate comparison of the anti-cancer activity of the natural cannabinoids THC and CBD in relation to ALAM027 1 and ALAM108 2, the current study examined the effects of these compounds on the following human cancer cell lines: T47D (breast), PC-3 (prostate), HT-29, Caco-2 (colon), A549 (lung), U87MG (brain), U266B1 (multiple myeloma) and three pancreatic cancer cell lines (PANC-1, AsPC-1, MiaPaCa-2). The current study of the activity of (1) and (2) in these selected tumor cell lines provides an estimate of their anti-cancer activity sufficient for in vivo animal experiments.
| Cancer cells | 1 Inhibition % | IC50 µM | 2 Inhibition % | IC50 µM |
|---|---|---|---|---|
| T47D breast | 97.90 | 5.52 | 47.20 | >10 |
| U251 brain | 68.11 | 8.91 | – | – |
| U-87 MG brain | 19.84 | >10 | 73.80 | 3.37 |
| A549 lung | 77.08 | 5.59 | 70.01 | 5.53 |
| TE-6 esophagus | 67.27 | 7.36 | – | – |
| Caco-2 colon | 60.81 | 8.87 | 67.16 | 6.56 |
| HT-29 colon | 86.21 | 6.27 | 88.13 | 1.99 |
| OPM-2 myeloma | 76.40 | 6.72 | – | – |
| U266B1 myeloma | 58.33 | 8.20 | 73.05 | 4.52 |
| SK-HEP-1 liver | 78.20 | 5.41 | – | – |
| PC-3 prostate | 61.13 | 9.94 | 63.61 | 7.45 |
| PANC-1 pancreas | 92.19 | 5.46 | 85.71 | 1.88 |
| AsPC-1 pancreas | 64.62 | 7.78 | 67.69 | 4.46 |
| MiaPaCa-2 pancreas | 61.41 | 8.12 | 90.65 | 1.76 |
3.1. Hormesis effect of some compounds on the in vitro study of HT-29 colon cancer cells
The in vitro activity of THCA derivative 1 in a variety of cancer cell lines was found to induce a slight increase in cell proliferation at low test substance concentrations. Although this deviates from the classical model of a concentration-dependent reduction in cell growth with increasing anticancer drug concentrations, the proliferative effect may be explained by experimental error or by a potential hormetic effect as has indeed been established for many of the currently deployed anticancer drugs. In the article E.J. Calabrese defined hormesis as “an adaptive response to low levels of stress or damage resulting in improved fitness for some physiological systems for a finite period”. For cancer cells this means activation of cell growth at specific drug concentrations and suppression of growth at increased drug concentrations. Hormetic effects have also been identified in in vitro studies of some of the cannabinoids. The article by Fowler describes results from a 66-hour incubation of four different lines of pancreatic cancer cells with THC concentrations of 3 and 4 µM, which resulted in completely inhibition of cell growth in all tested cell lines. However, a concentration of 1 µM THC on PANC-1 and 0.5 µM on Capan2 cells significantly increased cell viability, but this was not observed in BxPc3 and MiaPaCa2 cells. Stimulation of the midkine/ALK axis by low concentrations of THC followed by cell growth suppression by more concentrated samples is considered as one of possible mechanism for this phenomenon.
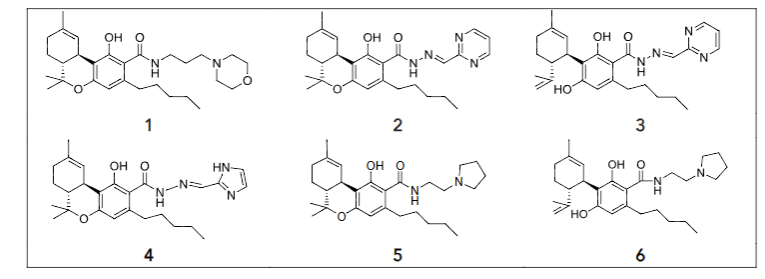
The hormesis effect study, therefore, was focused on investigating the in vitro activity of a CBD and number of cannabinolic acid derivatives 1-6 in the HT-29 colon cancer cell line using the XTT assay (Figure 3). The HT-29 cell line was chosen because it is accessible and has been previously shown to have a propensity for hormetic responses to anticancer drugs. The HT-29 cancer cell line viability curves with increasing concentrations of cannabinoid compounds 3 (A) and 1, 5, 6 and CBD (B) follow a hormetic response characterized by a growth inhibition and stimulation phase at lower compound concentrations, followed by inhibition of growth at increasing compound concentrations.
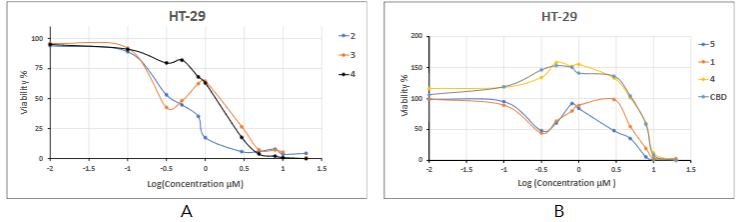
For compounds 1, 5, 6, and CBD IC50 values can be considered along with other curve parameters namely MS and h/c IC50 which are obtained by considering the 50% value as the base line. Since compounds 2 and 4 shows a very slight rise in the curve around the 0.5-1 µM concentrations, it is unclear whether this is due to hormesis or an experimental deviation, and we are omitting the MS and IC50 data points for this compound. The remaining dose-response curve parameters are shown in Table 2.
| Compounds | hIC50 µM | IC50 µM | MS % |
|---|---|---|---|
| 1 | 0.30 | 3.09 | 48 |
| 2 | – | 0.33 | – |
| 3 | 0.26 | 1.62 | 32 |
| 4 | – | 1.54 | – |
| 5 | 0.30 | 3.09 | 44 |
| 6 | – | 7.93 | 98 |
| CBD | – | 8.12 | 102 |
As shown in Table 4, the propensity of colon cancer HT-29 to hormetic effects in the presence of anticancer drugs allowed to reveal the influence of basic cannabinoid structures THCA and CBDA on the balance between their real anticancer activity and activation of the hormetic response. Paradoxically the main contribution to the cIC50 values for compounds 1, 5, 6, and CBD is the hormetic effect, whilst neither the structure of the cannabinoid moiety nor the nature of the carbonyl substituent has a large effect on the hIC50 value. The change of pyrimidine cycle in 2 to imidazole 4 resulted in an increasing of IC50. The very slight hormetic reaction of these compounds shows the advantage of a THCA cannabinoid structure in 2 over the CBDA analog 3.
This current in vitro study investigating the anticancer activity of several THCA and CBDA derivatives in the HT-29 colon cancer cell line shows the presence of a hormetic proliferative effect that is depended on cannabinolic acid and substituent structures and can be eliminated by selecting substituents in the cannabinoid carbonyl group. The results of the CBD activity study show its ability to induce cancer cell proliferation at low concentrations. This makes it necessary to treat with some caution the recommendations for its widespread use as an anti-cancer drug itself and after chemotherapy.
4. In vivo study of compounds 1 and 2 toward PANC-1 cancer cell.
The pancreatic cancer is one of the most dangerous forms of tumors due to its aggressive growth, early metastases and poor response to any known therapeutic treatments. That why the THCA derivatives 1 and 2 which showed good activity toward three pancreatic cancer cell lines were chosen to the in vivo study using the human PANC-1 pancreatic tumor xenograft model with nude female mice. The choice of PANC-1 cell line as a model for in vivo research is primarily due to its resistance, in contrast to other cell lines, to known MEK inhibitors such as trametinib and afatinib.
The compounds were administered orally as oil-based solutions, as opposed to injection as is the case for many of the established cancer drugs. The weight of the mice was monitored three times a week.
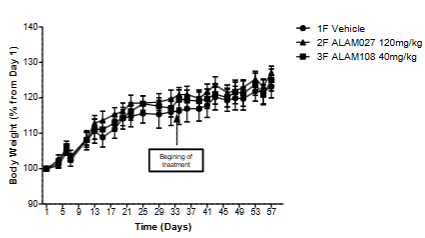
As can be seen, all mice gained weight steadily during the study, with no significant difference between cancer cells treated versus vehicle injected mice. The study results of these cannabinoids on PANC-1 cells showed that their oral administration decreased the tumor size 1.6-2 times and did not lead to any discomfort, psychotic effects and weight loss of mice.
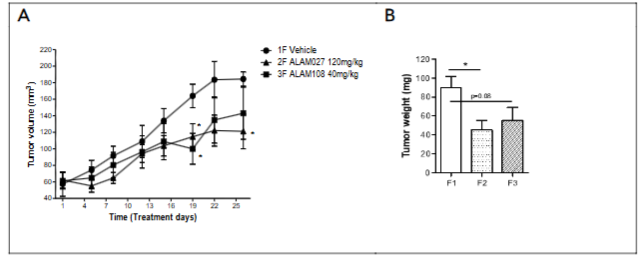
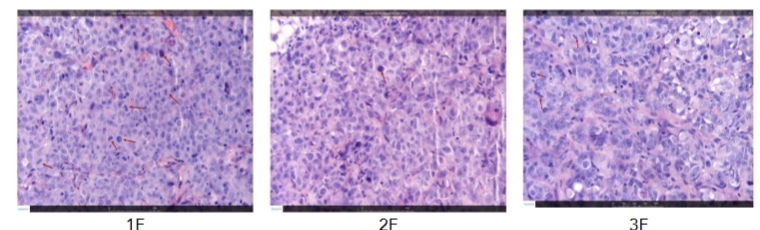
Histopathologic evaluation of tumor samples suggested that in both treated groups an apparent decrease in the mitotic figures was noted when comparing to the group treated with the vehicle. No apparent treatment related change was not in the area of necrosis and peritumoral mixed mononuclear cell infiltration. Thus the both compounds orally administration was effective in reducing tumor volume and tumor weight. Based on the data obtained, the most likely mechanism of their action is to slow down mitosis of tumor cells.
Conclusion
The decriminalization of cannabis and the increase of its use for medical and recreational purposes opens up significant opportunities for researchers to find new medicines. Cannabinoid acids are ideal drug precursors due to the carboxyl group easily converted into multifunctional aliphatic, aromatic and heterocyclic moieties, unlike traditional CBD and THC. The nitrogen containing derivatives described in this article can be compared in its synthetic availability and anticancer activity, especially compound ALAM108 2, to such widely used drugs as fluorouracil and carboplatin. A study of the cannabinoid influence on the hormesis effect in a HT-29 colon cancer cell line showed its direct dependence on the structure of both the cannabinoid moiety and the side chain substituents. Further study of these compounds will determine the mechanism of their action on cancer cells and may open the way to new therapeutic drugs. The applying of cannabinoid acids THCA and CBDA as starting materials for the synthesis of novel anticancer drugs can be considered as the next significant stage in the development of cannabinoid anticancer research. The results of this work serve as good confirmation of this statement.
Acknowledgment
The author thanks the employees of the Chempartner (China) and Pharmaseed (Israel) for their high professionalism and attention to his work.
Author Disclosure Statement
The author has no conflicts of interest, and no competing financial interests exist.
Funding Information
The work was funded from the AL&AM Pharmachem Ltd. company’s own funds.
References:
- Valizadehderakhshan M, Shahbazi A, Kazem-Rostami M, et al. Extraction of Cannabinoids from Cannabis sativa L. (Hemp). Review. Agriculture. 2021; 11 (384): 1-21.
- Sievers RE, Rebits L. US2016228385.
- Dibble CJ, Cole IB. US2017008870.
- Flockhart I, Wheatley G, Drink S, Archer L. US20150203434.
- Aizikovich A. US11472785B2.
- Bruni N, Della Pepa C, Oliaro-Bosso S, et al. Cannabinoid Delivery Systems for Pain and Inflammation Treatment. Molecules. 2018;23:2478.
- Jean-Gilles L, Gran B, Constantinescu CS. Interaction between cytokines, cannabinoids and the nervous system. Immunobiology. 2010; 215: 606–610.
- Ghezzi P, Cerami A, Tumor necrosis factor as a pharmacological target. Molecular Biotechnology. 2005; 31: 239–244.
- Moreno-Sanz G. Can You Pass the Acid Test? Critical Review and Novel Therapeutic Perspectives of D-Tetrahydrocannabinolic Acid A. Cannabis and Cannabinoid Research. 2016;1:124-130.
- Formato M, Crescente G, Scognamiglio M, et al. (‒)-Cannabidiolic Acid, a Still Overlooked Bioactive Compound: An Introductory Review and Preliminary Research. Molecules 2020; 25:2638.
- Ruhaak LR. J Felth J, Karlsson PC et al. Evaluation of the cyclooxygenase inhibiting effects of six major cannabinoids isolated from Cannabis sativa. Biol. Pharm. Bull. 2011;34:774—778.
- Pellesi L, Licata M, Verri P et al. Pharmacokinetics and tolerability of oral cannabis preparations in patients with medication overuse headache (MOH) – a pilot study. Eur J of Clinical Pharm. 2018;74(11): 1427-1436.
- Zagzoog A, Mohamed KA, Kim HJJ et al. In vitro and in vivo pharmacological activity of minor cannabinoids isolated from Cannabis sativa. Nature research. 2020;10:20405.
- Sledzinski P, Nowak-Terpiłowska A, Zeyland J. Cannabinoids in Medicine: Cancer, Immunity, and Microbial Diseases Int. J. Mol. Sci. 2021;22:263.
- Cherkasova V, Wang B, Gerasymchuk M, Fiselier A, Kovalchuk O, Kovalchuk I. Use of Cannabis and Cannabinoids for Treatment of Cancer. Cancers 2022;14:5142.
- Nigro E, M Formato M, Crescente G, Daniele A. Cancer Initiation, Progression and Resistance: Are Phytocannabinoids from Cannabis sativa L. Promising Compounds? Molecules 2021;26:2668.
- Aizikovich A. Cannabinoids and Cancer-What’s Next? Am J Biomed Sci & Res. 2021;14(3):234-236.
- Formato M, Crescente G, Scognamiglio M, et al. (-)-Cannabidiolic Acid, a Still Overlooked Bioactive Compound: An Introductory Review and Preliminary Research. Molecules 2020;25:2638.
- Takeda S, Okazaki H, Ikeda E, et al. Down-regulation of cyclooxygenase-2 (COX-2) by cannabidiolic acid in human breast cancer cells. J Toxicol Sci. 2014;39(5):711-6.
- Ligresti A, Moriello AS, Starowicz K, et al. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther. 2006;318(3):1375–1387.
- Aizikovich A. US patent 2021008759.
- Aizikovich A. In vitro Activity of Novel Cannabinoids Derived from Tetrahydrocanabinolic Acid on Various Human Tumor Cell Lines. J Oncology Res. 2021;2; 55-59.
- Calabrese EJ. Cancer Biology and Hormesis: Human Tumor Cell Lines Commonly Display Hormetic (Biphasic) Dose Responses. Toxicology, 2005;35:463–582.
- Yoshimasu T, Ohashi T, Oura S, et al. A Theoretical Model for the Hormetic Dose-response Curve for Anticancer Agents. Anticancer Res. 2015;35:5851-5856.
- Burkhard H, Robert R. Cannabinoids as anticancer drugs: current status of preclinical Research. British Journal of Cancer 2022;127:1 – 13.
- Lee H-S, Tamia G, Song H-J, Amarakoon D, et al. Cannabidiol exerts anti-proliferative activity via a cannabinoid receptor 2-dependent mechanism in human colorectal cancer cells. Int Immunopharmacol. 2022;108:108865.
- Fowler CJ. Δ9-Tetrahydrocannabinol and cannabidiol as potential curative agents for cancer. A critical examination of the preclinical literature. Clinical Pharmacology & Therapeutics. 2015;97(6):587–596.
- Lorente M, Torres S, Salazar M, et al. Stimulation of the midkine/ALK axis renders glioma cells resistant to cannabinoid antitumoral action. Cell Death Differ. 2011;18(6):959–973.
- Aizikovich A. Hormetic effects of in vitro anticancer activity of cannabinoid acid derivatives in the HT- colon cancer cell line. Int J Appl Sci & Res. 2023;6:27-30.
- Aizikovich A. Anticancer Effect of New Cannabinoids Derived from Tetrahydrocannabinolic Acid on PANC-1 and AsPC-1 Human Pancreas Tumor Cells. J. Pancreatic Cancer. 2020;6:40-44.
- Brauswetter D, Gurbi B, Varga A. Molecular subtype specific efficacy of MEK inhibitors in pancreatic cancers. PLOS ONE 2017; 28, 2017:1-13.

