Topical Spironolactone for Ocular Graft-Versus-Host Disease
Topical Spironolactone in Ocular Graft-versus-Host Disease
Rugveda R. Patil 1,3, Calvin W. Wong 2, Nathan A. Seto 3, Mitchell A. Watsky 4, Dan S. Gombos 5, Richard W. Yee 3,5
OPEN ACCESS
PUBLISHED: 30 September 2025
CITATION: PATIL, Rugveda R. et al. Topical Spironolactone in Ocular Graft-versus-Host Disease. Medical Research Archives, Available at: <https://esmed.org/MRA/mra/article/view/6822>.
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ISSN 2375-1924
DOI: https://doi.org/10.18103/mra.v13i9.6822
ABSTRACT
Ocular graft-versus-host disease (oGVHD) is a serious complication of allogeneic hematopoietic stem cell transplantation that causes chronic inflammation, fibrosis, and dysfunction across multiple ocular tissues, including the lacrimal glands, cornea, conjunctiva, and meibomian glands. Common symptoms such as dryness, visual disturbances, and ocular discomfort are often resistant to standard treatment. Current therapies provide partial relief, rarely reverse structural damage, and typically target a limited range of immune or fibrotic pathways.
Spironolactone, a mineralocorticoid receptor antagonist, has emerged as a promising candidate due to its ability to block pathological mineralocorticoid signaling. This pathway becomes abnormally active in ocular tissues with low levels of the enzyme 11β-hydroxysteroid dehydrogenase type 2, leading to unchecked inflammation and tissue remodeling.
This review outlines the immunopathology of oGVHD in four key anatomical sites and evaluates spironolactone’s potential effects. In the lacrimal glands, spironolactone reduces inflammatory cytokines, limits fibroblast activation, and helps preserve tear production. In the cornea and conjunctiva, it supports epithelial barrier integrity, reduces surface inflammation and fibrosis, and enhances healing. In the meibomian glands, it improves lipid secretion and prevents glandular dropout. Preclinical and early clinical studies show that topical spironolactone improves both objective markers, such as corneal fluorescein staining and lid margin health, and subjective symptoms, with minimal side effects.
Spironolactone’s anti-inflammatory, antifibrotic, lipid-enhancing, and epithelial-protective properties position it as a potential disease-modifying therapy for oGVHD. While additional studies are needed to confirm its protective role and long-term efficacy, current evidence suggests that spironolactone may address important unmet needs in the management of this complex condition.
Keywords: ocular graft versus host disease, spironolactone, mineralocorticoid receptor, dry eye, inflammation
1 Introduction
Graft-versus-Host disease (GVHD) is a common complication that occurs following an allogeneic hematopoietic stem cell transplantation (allo-HSCT). Allo-HSCT can be a curative measure for patients with leukemia or other hematological malignancies, but is not without considerable associated morbidity. After the transplant, donor cells may mount a response to host tissues, resulting in manifestations of GVHD in different organs. Two forms of ocular GVHD (oGVHD) have been identified based on the timing of symptom onset. Acute oGVHD typically occurs within 100 days of hematopoietic stem cell transplantation (HSCT), while chronic oGVHD presents after 100 days. Both forms are associated with substantial morbidity and a diminished quality of life.
The incidence of chronic GVHD (cGVHD) in patients with allo-HSCT has been estimated to be 30% to 70%. Of those who develop cGVHD, oGVHD has an estimated incidence of 40% to 60% with few reports as high as 90%. oGVHD can present with blurry vision, severe photophobia, chronic conjunctivitis, dry eyes, periorbital hyperpigmentation, foreign body sensation, itching, and general eye pain. However, the nonspecific nature of oGVHD symptoms and their overlap with other ocular surface diseases likely contribute to underdiagnosis, delays in treatment and worse long-term outcomes. In a survey of patients with possible oGVHD symptoms of dryness, burning, foreign body sensation, and light sensitivity, only 55.6% of those with new-onset symptoms following allo-HSCT received a diagnosis.
It is important to recognize that damage to the ocular surface may already be present before HSCT. Preexisting ocular conditions such as age-related aqueous deficiency from lacrimal gland dysfunction or meibomian gland related evaporative dry eye are common, as are systemic conditions like Sjögren’s syndrome. In addition, comorbidities from prior cancer treatments, such as chemotherapy and radiation for the underlying hematological condition, can lead to the severity of oGVHD. For this reason, comprehensive ocular surface and eye examinations should be obtained prior to HSCT to establish initial baseline parameters. Comparisons between pre- and post-HSCT assessments will allow for more accurate diagnosis and a tailored treatment plan.
The critical step in the immunopathogenesis of oGVHD involves donor-derived T cells recognizing host tissues as foreign, initiating and perpetuating a pro-inflammatory response. Initial insult to the ocular surface from post-transplant conditioning regimens, such as chemotherapy or radiation, can expose host antigens. Damage to the ocular surface activates the antigen presenting cells (APCs), which process and present the host antigens to the donor T-cells, specifically CD4+ and CD8+ T-cells. Activated CD4+ and CD8+ T cells then migrate into the ocular tissues where they initiate a pro-inflammatory response mediated by interleukin-6 (IL-6), interleukin-1β (IL-1β), interleukin-8 (IL-8), tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-y). Further, additional immune cells such as macrophages and neutrophils are recruited, leading to apoptosis of epithelial cells and stimulation of myofibroblasts, resulting in tissue fibrosis. Over time, chronic inflammation and scarring resulting from sustained immune activation disrupts the structure and function of ocular tissues.
The four anatomical locations usually affected by oGVHD are the lacrimal glands, cornea, conjunctiva, and meibomian glands, each contributing uniquely to disease burden. Dysfunction across these tissues result in compounded and complex symptomatology, which diminishes quality of life and impair visual function.
1.1 Rationale for Spironolactone in Ocular Graft-versus-Host Disease
As discussed, oGVHD affects multiple ocular surfaces which all rely on regulated inflammation and healing processes for normal function. The maintenance of normal ocular function largely depends on the local metabolism of endogenous glucocorticoids. Cortisol metabolism regulates the severity and duration of inflammation in these ocular structures by activating glucocorticoid receptors (GRs). Cortisol is the primary endogenous glucocorticoid, which binds to both GRs and mineralocorticoid receptors (MRs). In most tissues, 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) deactivates cortisol, thereby preventing mineralocorticoid receptor activation and allowing aldosterone to serve as the primary MR ligand.
However, immunohistochemical studies have demonstrated that ocular tissues express both GRs and MRs but have low levels of 11β-HSD2. Low 11β-HSD2 expression results in ineffective deactivation of cortisol signaling and consequent activation of MRs. While GR activation by cortisol generally suppresses inflammation, MR activation in low 11β-HSD2 regions promotes proinflammatory cytokine release, oxidative stress, and fibrosis. Dysregulation of cortisol metabolism allows cortisol to drive MR-mediated proinflammatory and profibrotic signaling, leading to ocular surface inflammation. MRs are also expressed on immune cells such as T-cells, macrophages, and fibroblasts are important in the deregulatory pathogenesis of oGVHD. Thus, using MR antagonists (MRA), such as spironolactone, in oGVHD inhibits pathological MR activation while restoring ocular surface homeostasis.
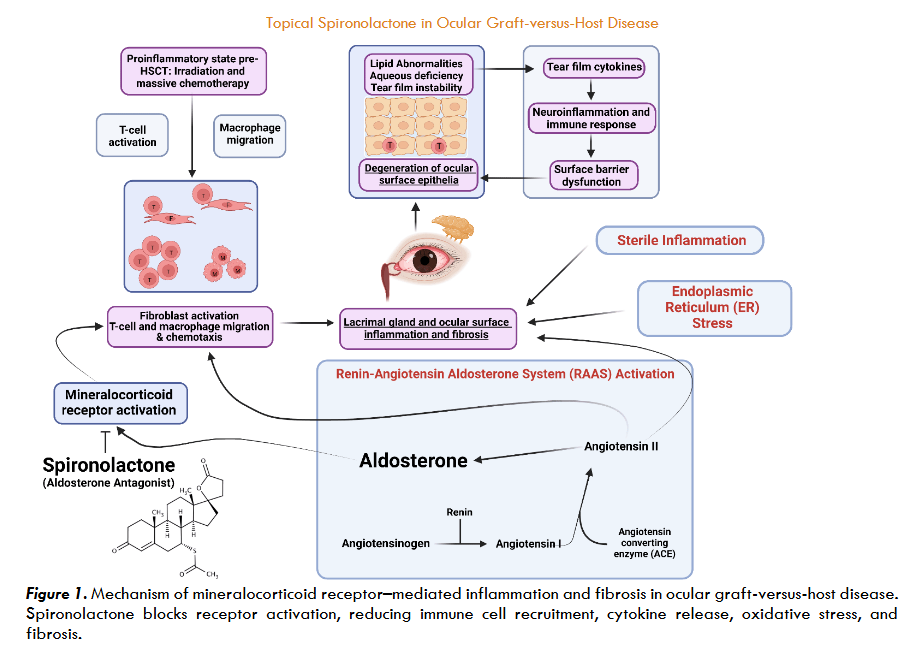
Spironolactone is an FDA approved MRA that is widely prescribed for patients diagnosed with heart failure with reduced ejection fraction, hypertension, and hyperaldosteronism. Compared to other MRAs such as eplerenone or finerenone, spironolactone offers broader receptor inhibition. Further, spironolactone is also more widely available and lower in cost than eplerenone or finerenone. Spironolactone’s mechanism of action is competitive inhibition of aldosterone at the MR, which is implicated in not only influencing water and electrolyte balance but also a host of other anti-inflammatory and novel lipid-producing signaling pathways.
Spironolactone as a topical ophthalmic agent has shown a multi-modal mechanism of action. Spironolactone possesses anti-inflammatory and epithelial-protective properties, and may exhibit anti-fibrotic activity in select contexts, though tissue-specific responses have also demonstrated pro-fibrotic signaling. The aim of this review is to evaluate spironolactone’s therapeutic treatment potential from the anatomical and pathological perspective of the four tissues commonly affected by oGVHD namely lacrimal gland, cornea, conjunctiva, and meibomian gland.
1.2 Ocular Graft-versus-Host Disease Diagnostic Criteria
The current paradigms of diagnosing and assessing oGVHD merit discussion. Accurate diagnosis of oGVHD is essential for evaluating disease severity and guiding treatment decisions. To diagnose if GVHD patients have oGVHD, several subjective and objective measurements have been proposed by the International Ocular Graft Versus Host Disease Group to generate a composite severity score and the likelihood of oGVHD is assessed. Subjective assessments are from a standardized 12 item questionnaire, the Ocular Surface Disease Index (OSDI), while objective measurements include lid margin examination, conjunctival injection grading, corneal fluorescein staining (CFS), and Schirmer’s 1 test. These standardized criteria also enable attempts to a more consistent disease grading across institutions and clinical trials.
1.2.1 Ocular Surface Disease Index
The Ocular Surface Disease Index (OSDI) is a widely used subjective 12-question patient reported outcome questionnaire specifically designed to quantify frequency of ocular discomfort, visual disturbances, and environmental sensitivity. The questionnaire allows for a comprehensive measure of how oGVHD may affect a patient’s quality of life.
Research has shown that OSDI scores are significantly higher for patients with chronic oGVHD compared to patients who are pre-transplant and patients who are post-transplant without oGVHD. A cut-off value of OSDI scores >19.4 has been identified as optimal for screening, providing high sensitivity (89.3%) and specificity (89.6%) for oGVHD detection in a clinical setting. The OSDI is therefore not only a practical screening tool for early referrals but also a valuable metric for monitoring symptom progression and treatment response over time.
The OSDI score is calculated using the following formula:
OSDI = (sum of all answered scores) × 25 ÷ number of questions answered.
This scoring method yields a total score ranging from 0 to 100, with higher scores reflecting greater symptom severity. By capturing symptoms related to ocular discomfort, visual function, and environmental sensitivity over the past week, the OSDI enables clinicians to track changes in disease burden and assess therapeutic response over time.
1.2.2 Lid Margin Examination
The eyelid margin, and especially the meibomian glands, are frequently compromised by oGVHD due to chronic inflammation. In the lid margin exam, the following are objectively evaluated: anterior blepharitis (AB), vascularity (V), obstruction of meibomian orifices (O), and turbidity of oil from the meibomian glands (T).
AB refers to the inflammation and scaling (i.e., cylindrical sleeves) at the base of the eyelashes which are commonly associated with Demodex infestation. V is indicative of the degree of vascularity and engorgement of blood vessels and telangiectasia of the lid margin which are suggestive of chronic inflammation. O refers to the characterization of internal or external obstruction at the meibomian gland orifice during expression with moderate pressure using a Q tip.
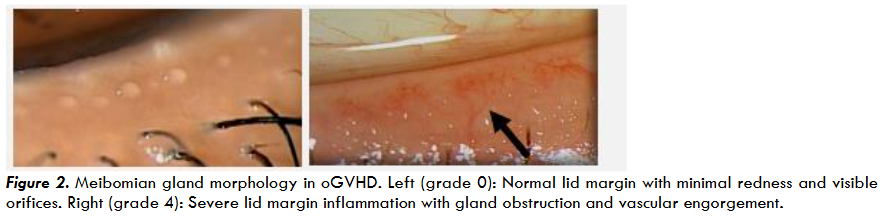
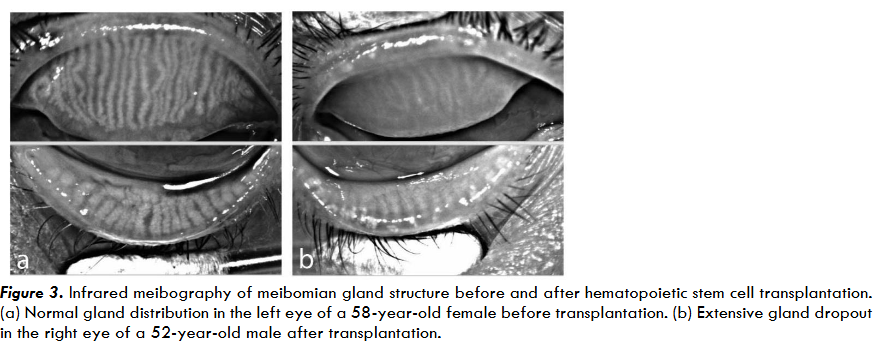
Obstruction of the meibomian gland ducts is a major contributor to evaporative dry eye and ocular surface instability in oGVHD. Chronic obstruction may result in permanent gland dropout, which can be visualized using infrared meibography.
T describes the turbidity of the meibum that is secreted from the meibomian glands, which ranges from clear, yellow and cloudy liquid to a toothpaste like secretion. Reports of abnormal meibomian gland secretion can be suggestive of abnormal systemic lipid processing necessitating ordering a lipid panel.
1.2.3 Conjunctival Injection
Conjunctival injection is characterized by the dilation of conjunctival blood vessels and visible redness of the sclera, which are clinical signs in oGVHD. Assessment of bulbar and palpebral conjunctival redness (BCR, PCR, respectively) provides an objective measure for the established clinical sign. Grading systems proposed by the NIH consensus group and the international chronic oGVHD consensus group introduce conjunctival injection as a key parameter for diagnosing and staging oGVHD severity.

Conjunctival injection serves as a general clinical indicator of ocular surface inflammation and can be further subclassified into BCR and PCR to capture the specific anatomical regions involved. BCR and PCR are independently evaluated using validated grading systems that enable more precise quantification of redness severity. The method for evaluating bulbar conjunctival injection is the BCR scale (graded 10 to 100), which quantifies bulbar redness from minimal (BCR=10) to severe (BCR=100).
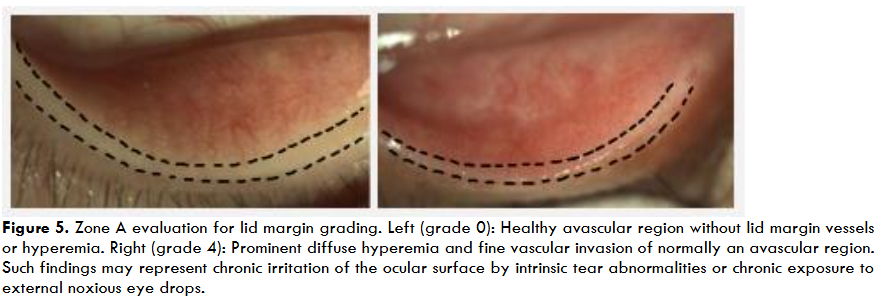
Similarly, PCR scale presents a corresponding scale for palpebral conjunctival redness (PCR), which further assists in staging conjunctival involvement in oGVHD. Although BCR remains an important feature in evaluating disease likelihood, it is not uncommon for PCR grades to be elevated even when BCR appears minimal. In such cases, PCR may reflect early or localized conjunctival involvement and may serve as a more specific indicator of oGVHD activity.
1.2.4 Corneal Fluorescein Staining
Corneal fluorescein staining (CFS) is a key diagnostic technique used to detect and quantify areas of corneal epithelial cell damage using fluorescein dye in oGVHD patients. Elevated CFS scores are common for patients with oGVHD and indicate an underlying inflammation caused by pathogenesis of oGVHD, which allows clinicians to grade the extent of compromised epithelium. Regular assessment of CFS is crucial for both diagnosis and management of oGVHD.

Conjunctival injection serves as a general clinical indicator of ocular surface inflammation and can be further subclassified into BCR and PCR to capture the specific anatomical regions involved. BCR and PCR are independently evaluated using validated grading systems that enable more precise quantification of
redness severity. The method for evaluating bulbar conjunctival injection is the BCR scale (graded 10 to 100), which quantifies bulbar redness from minimal (BCR=10) to severe (BCR=100) 46. As illustrated in Figure 7, this scale provides a visual reference for grading bulbar conjunctival injection.

Figure 7. Validated Bulbar Redness (VBR 10)/ Bulbar Conjunctiva Redness scale used to quantify conjunctival injection severity in oGVHD and other ocular surface diseases. Redness scores range from 10 to 100 in increments of 10, based on visual grading of bulbar hyperemia.
Similarly, PCR scale in Figure 8 presents a corresponding scale for palpebral conjunctival redness (PCR), which further assists in staging conjunctival involvement in oGVHD. Although BCR remains an important feature in evaluating disease likelihood, it is not uncommon for P
grades to be elevated even when BCR appears minimal. In such cases, PCR may reflect early or localized conjunctival involvement and may serve as a more specific indicator of oGVHD activity.
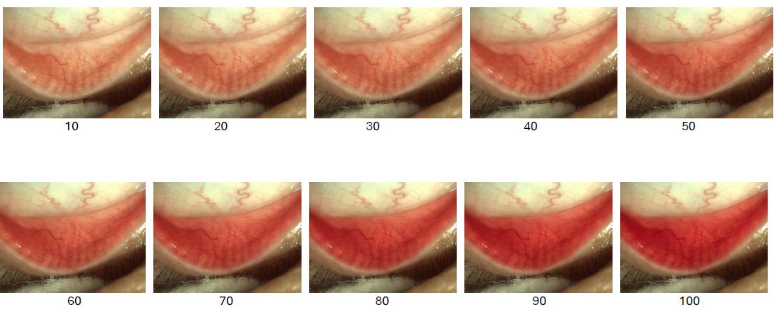
Figure 8. Palpebral Conjunctival Redness (PCR) grading scale used to quantify the severity of palpebral inflammation in oGVHD. Redness scores range from 10 to 100 in increments of 10, based on progressive vascular engorgement and erythema of the palpebral conjunctiva.
Therefore, concurrent assessment of both BCR and PCR may improve diagnostic accuracy and allow for earlier recognition, particularly in patients who do not yet report overt ocular surface symptoms.
1.2.4 Corneal Fluorescein Staining
Corneal fluorescein staining (CFS) is a key diagnostic technique used to detect and quantify areas of corneal epithelial cell damage using fluorescein dye in oGVHD
patients47. Elevated CFS scores are common for patients with oGVHD and indicate an underlying inflammation caused by pathogenesis of oGVHD, which allows clinicians to grade the extent of compromised epithelium36,48. Regular assessment of CFS is crucial for both diagnosis and management of oGVHD36,49. Figure 9 illustrates the clinical grading scale for corneal fluorescein staining, demonstrating progressive levels of epithelial damage in oGVHD.
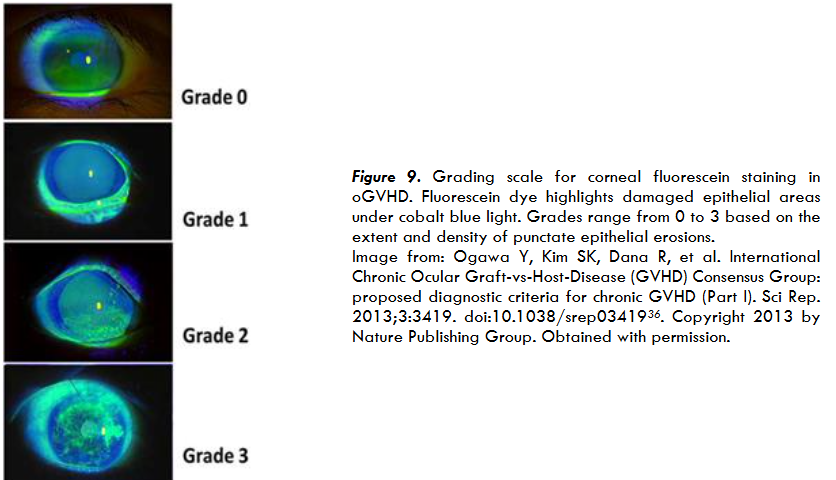
Figure 9. Grading scale for corneal fluorescein staining in OGVHD. Fluorescein dye highlights damaged epithelial areas under cobalt blue light. Grades range from 0 to 3 based on the extent and density of punctate epithelial erosions. Image from: Ogawa Y, Kim SK, Dana R, et al. International Chronic Ocular Graft-vs-Host-Disease (GVHD) Consensus Group: proposed diagnostic criteria for chronic GVHD (Part I). Sci Rep. 2013;3:3419. doi:10.1038/srep0341936. Copyright 2013 by Nature Publishing Group. Obtained with permission.
1.2.5 Tear Production
The unanesthetized Schirmer’s 1 test, which measures aqueous tear production by quantifying the wetting of a ruled paper strip (measured 0-35mm) placed in the lower eyelid for five minutes, has been a standard objective metric in dry eye diagnostics and is frequently used in the assessment of oGVHD4,36, oGVHD reduces tear output due to lacrimal gland fibrosis and T-cell mediated
destruction of acinar cells, making low Schirmer’s 1 values (<5 mm) often indicative of severe aqueous tear deficiency4,36. However, the utility of Schirmer’s test (especially when performed without anesthesia) is debated due to the specificity of the test, as interpretation of the result can be complicated by reflex tearing50. Hence, many studies consider Schirmer’s 1 test as supportive objective data in lieu of a metric sufficient
The unanesthetized Schirmer’s 1 test, which measures aqueous tear production by quantifying the wetting of a ruled paper strip (measured 0-35mm) placed in the lower eyelid for five minutes, has been a standard objective metric in dry eye diagnostics and is frequently used in the assessment of oGVHD. oGVHD reduces tear output due to lacrimal gland fibrosis and T-cell mediated destruction of acinar cells, making low Schirmer’s 1 values (<5 mm) often indicative of severe aqueous tear deficiency. However, the utility of Schirmer’s test (especially when performed without anesthesia) is debated due to the specificity of the test, as interpretation of the result can be complicated by reflex tearing. Hence, many studies consider Schirmer’s 1 test as supportive objective data in lieu of a metric sufficient to establish definitive oGVHD diagnosis. The Schirmer’s 1 test is most useful as a secondary endpoint, especially in severe cases or when combined with other objective and subjective assessments.
Thus, despite its limitations, Schirmer’s 1 test remains a practical tool in clinical practice, provided that its results are contextualized within a broader assessment of oGVHD. For example, consecutive Schirmer’s 1 values (<5 mm) should alert the clinician to the possibility of end-stage lacrimal gland dysfunction and a severely diminished ability to produce aqueous tears, which may be observed in both acute and chronic oGVHD. Repetitive low Schirmer’s 1 values may also occur in non-HSCT patients and, in either case, should prompt the clinician to consider strategies to support aqueous tear homeostasis when planning treatment.
1.2.6 Composite Scoring
Each oGVHD objective diagnostic parameter is assigned a score from 0 to 4. The OSDI score is then combined with these values to generate a composite score, which is then used for both diagnosis and severity assessment. According to the International Chronic Ocular GVHD Consensus Group criteria, a composite score of 0-3 indicates the likelihood of no oGVHD, 4-7 suggests probable oGVHD, and 8-11 is classified as definite oGVHD diagnosis. These metrics not only guide diagnosis but also serve as standardized outcome measures in clinical trials, including those evaluating novel therapies like spironolactone.
The use of both objective and subjective criteria ensures oGVHD is diagnosed accurately and consistently. Early and precise diagnosis allows for timely therapy, which helps prevent the progression of severe, sight-threatening complications and improves quality of life for patients. In clinical and research settings, the OSDI scoring system provides a process for consistent diagnosis. For future studies involving therapies, oGVHD composite scores can serve as both baseline and endpoint metrics to quantify improvement across ocular surface.
Once a diagnosis is established, understanding the specific anatomical involvement is critical for targeted therapy. The following sections discuss pathogenesis, current treatment, and spironolactone’s therapeutic potential across the four main anatomical sites affected by oGVHD.
2 Lacrimal Glands
2.1 Pathogenesis of Lacrimal Gland Dysfunction
Lacrimal gland dysfunction is a hallmark of oGVHD and is characterized by immune mediated destruction and fibrotic remodeling that results in severe aqueous tear deficiency. Pathogenesis begins with the infiltration of donor CD4+ and CD8+ T-cells into the periductal region of the lacrimal glands, where these alloreactive lymphocytes recognize host antigens as foreign and initiate cytotoxic immune responses. CD8+ T cells function as cytotoxic effectors that damage host tissues by inducing apoptosis and releasing pro-inflammatory cytokines. CD4+ T cells amplify the response by activating CD8+ T cells and recruiting other immune cells through cytokine signaling. Immune inflammatory response from CD4+ and CD8+ creates tissue injury and creates a pro-inflammatory microenvironment, which subsequently recruits additional immune cells such as macrophages, APCs, and CD34+ stromal fibroblasts.
The activation of CD34+ stromal fibroblasts plays a crucial role in the advancement of oGVHD, as almost half of these cells are from the donor and drive excessive collagen production and interstitial fibrosis around the lacrimal ducts. CD34+ stromal fibroblasts then decrease α-smooth muscle actin expression and increase expression of heat shock protein 47 (HSP47) and co-stimulatory molecules, thus allowing for enhanced migratory potential and antigen-presenting capabilities. The activation of CD34+ stromal fibroblasts triggers both the endoplasmic reticulum stress (ERS) and the renin-angiotensin systems (RAAS), leading to rapid interstitial inflammation and progressive fibrosis. Alongside interstitial inflammation and fibrosis, senescent cells within the gland adopt a senescence-associated secretory phenotype (SASP), which is associated with the release of proinflammatory factors such as IL-6, CXCL9, and osteopontin.
Another critical component to the lacrimal gland dysfunction pathology involves the epithelial–mesenchymal transition (EMT), a process in which epithelial cells lose their polarity and adhesion properties and transform into migratory, fibrogenic mesenchymal cells. EMT contributes to fibrosis by promoting extracellular matrix deposition and glandular remodeling. In oGVHD, EMT is exacerbated by decreased expression of vesicle-associated membrane protein 8 (VAMP-8), which impairs tear vesicle release from acinar cells. The resulting oxidative stress from lower levels of VAMP-8 leads to lipofuscin accumulation, acinar cell death, and progressive loss of tear production.
The combination of immune-mediated destruction, fibrotic replacement of functional tissue, and impaired cellular secretory mechanism ultimately culminates in severe lacrimal gland dysfunction, which clinically manifests as aqueous dry eye disease in oGVHD patients. Patients experience profound symptoms of dry eye disease including visual disturbances, foreign body sensations, photophobia, burning sensation, and ocular dryness that significantly impact quality of life and activities of daily living. The severity of symptoms is graded using standardized scales of Schirmer’s 1 test values and a calculated OSDI score. In severe cases, repeatable Schirmer’s 1 test values often fall below 5 mm, while OSDI scores tend to be markedly elevated. Lacrimal gland involvement in oGVHD is diagnosed by combining objective clinical findings with histopathological evidence of T-cell infiltration and fibrosis.
2.2 Management Strategies for Lacrimal Gland Dysfunction
2.2.1 Tear Supplements
Current treatments for lacrimal gland dysfunction in oGVHD primarily aim to relieve symptoms, as few available options directly reverse the underlying mechanism. The most widely used interventions include artificial tears and lubricating ointments, which provide only temporary relief by supplementing the deficient tear film but do not address the underlying immune-mediated gland destruction or fibrosis. In oGVHD, it is generally recommended to use preservative-free artificial tears. Although this approach helps retain the aqueous component of the tear film, artificial tear supplementation does not halt the ongoing inflammatory or fibrotic processes and may fail to improve natural tear production over time. While it is important to conserve existing tears, the quality and volumetric quantity may be important additional considerations when treating the aqueous related abnormalities.
2.2.2 Tear Conservation Punctal Occlusion
To improve tear retention, punctal occlusion strategies are often used. Punctal plugs are a common option and can provide quick, reversible symptom relief. However, plugs may protrude and easily dislodge and create granulomas. Additionally, plugs can cause irritation punctal fibrosis and infection. For patients who fail temporary methods, punctal cautery may be considered. While punctal cautery does last longer than punctal plugs, they still have a 58% rate of being reopened, often requiring multiple retreatments over time. In some rare cases, mild infections, entropion, or epiphoras have occurred from punctal cautery. LACRIFILL, a canalicular gel based on hyaluronic acid, offers longer occlusion with fewer discomfort complaints. It has shown good tolerability and may be more comfortable than traditional plugs, with a reduced risk of foreign body sensation. Currently, LACRIFILL has not been prospectively evaluated in oGVHD.
2.2.3 Lacrimal Gland Stimulation
Stimulation of the lacrimal gland provides an adjunctive strategy to restore tear production in oGVHD. Pilocarpine and cevimeline (Salagen) are oral muscarinic agonists that activate M3 receptors on lacrimal epithelial cells to promote endogenous tear secretion. Studies in oGVHD and dry eye populations report improved Schirmer’s 1 scores and symptom relief. While full tear fluid composition is restored, 96.8% of patients on pilocarpine have complained of its systemic side effects, such as sweating, nausea, dizziness, and visual disturbances, commonly limit use. Tyrvaya (varenicline nasal spray) activates nicotinic acetylcholine receptors in the nasal mucosa, stimulating the nasolacrimal reflex to increase aqueous secretion. Clinical trials with Tyrvaya show improvements in signs and symptoms of dry eye, including in oGVHD. However, long-term safety in oGVHD remains under investigation.
Neurostimulation devices such as iTear100 apply external oscillatory stimulation to the nasal bridge to trigger basal tear secretion. Nasal oscillation stimulates mucin, aqueous, and lipid release without medication. Early data suggest symptom relief, but effects are short-term and require repeated use. Current approval is limited to short-term prescriptions. Access may be restricted by cost or availability, and some users report mild dizziness.
2.2.4 Autologous Serum Eye Drops
Autologous serum eye drops (ASEDs) offer a supportive option for epithelial healing in oGVHD due to their content of growth factors and anti-inflammatory mediators. Their use in lacrimal gland dysfunction is largely supportive and individualized, especially given the practical limitations and lack of extensive study in oGVHD.
2.2.5 Experimental Antifibrotic Therapies
Recent research has led to the development of novel experimental therapies that target fibrotic and inflammatory mechanisms specific to lacrimal gland pathology in oGVHD. VA lip HSP47 is a vitamin A liposome formulation that delivers HSP47 siRNA within topical eye drops. Treatment with VA lip HSP47 has shown promising results in animal models by reducing HSP47 expression, decreasing collagen deposition, and restoring tear secretion. However, usage of VA lip HSP47 treatment is still in the early stages, and no published human clinical trials have yet confirmed its safety, efficacy, or optimal dosing in oGVHD. The translation of siRNA therapies from animal models to human patients is challenging, as siRNA degrades rapidly in the body and may cause unintended off target effects. Last, HSP47 plays a vital role in normal collagen processing and tissue repair. Inhibiting HSP47 may interfere with wound healing and compromise tissue structure.
Other antifibrotic agents such as valsartan, an angiotensin II receptor blocker, and tranilast, a TGF beta inhibitor, have also shown antifibrotic effects in preclinical and limited clinical studies. Valsartan’s antifibrotic effect appears to be indirect and may not fully modulate the immune and inflammatory pathways that contribute to oGVHD. Since angiotensin II signaling represents only one part of several fibrotic pathways active in oGVHD, blocking it may not be sufficient to alter disease progression. Additionally, the use of angiotensin receptor blockers in the oGVHD patient population can result in side effects such as low blood pressure, kidney dysfunction, and electrolyte imbalances. Tranilast’s mechanism of action is the inhibition of TXNIP and NF kappa B, both of which engage in inflammation and fibrosis in oGVHD. However, TGF-β is only one of many signaling molecules involved in oGVHD. While spironolactone may indirectly modulate downstream fibrotic signaling involving TGF-β, direct TGF-β inhibition is not part of its primary mechanism of action. Moreover, systemic inhibition of TGF-β can impair normal tissue repair and immune regulation, potentially increasing the risk of delayed healing and infection. Valsartan and Tranilast lack large randomized controlled trials in oGVHD populations, leaving their true clinical impact uncertain.
2.2.6 Investigational Approaches
New therapeutic strategies include Janus kinase inhibitors, spleen tyrosine kinase inhibitors, and various cell-based treatments. These therapies have shown potential to reduce inflammation and support tissue repair. However, due to the one-solve pathway of these drugs, kinase inhibitors and cell-based treatments do not deal with both immune and fibrotic mechanisms. Despite the therapies currently available, many patients with oGVHD continue to experience symptoms that are inadequately controlled. Most existing treatments are palliative, focus on a single aspect of the disease, or remain clinically inaccessible. Current deficiencies in effective treatment are the urgent need for new therapies that can simultaneously address inflammation, fibrosis, and lacrimal gland regeneration.
2.3 Impact of Spironolactone on Lacrimal Gland Dysfunction
Spironolactone has gained attention as a potential therapeutic for oGVHD due to its ability to modulate multiple pathogenic pathways within the lacrimal gland. This section outlines the mechanistic rationale for using spironolactone, supported by preclinical and early clinical findings.
2.3.1 Mechanisms/Pathways
As a mineralocorticoid receptor antagonist, spironolactone interrupts RAAS signaling, which is upregulated in oGVHD and contributes to lacrimal gland remodeling, immune-mediated injury, and fibrosis. By blocking MR signaling, spironolactone suppresses the production of pro-inflammatory cytokines such as TNF-α, IL-6, and interferon-γ by up to 70–90%, directly reducing T-cell infiltration and epithelial damage, leading to a reduction in the key drivers of lacrimal gland injury.
Spironolactone’s anti-inflammatory action is accompanied by its distinct antifibrotic properties. Landmark studies in cardiac and renal fibrosis show that spironolactone prevents excessive collagen deposition and fibroblast proliferation, even in the presence of ongoing injury, through suppression of aldosterone and MR signaling. Although spironolactone’s anti-inflammatory and antifibrotic properties are derived from cardiac and renal models, the mechanisms are conserved in glandular tissues and provide insight into spironolactone’s potential utility in oGVHD. One study also demonstrated that spironolactone inhibits the TGF-β1/Smad-2/3 signaling pathway, which plays a key role in both cardiac and glandular fibrosis. Inhibition of TGF-β1/Smad-2/3 signaling leads to reduced expression of HSP47, the same protein found in oGVHD-associated lacrimal gland fibroblasts. Mechanistic parallels between TGF-β1/Smad-2/3 signaling and HSP47 expression are particularly relevant to oGVHD, where MR-driven fibroblast activation and TGF-β signaling are critical mediators of collagen production. Spironolactone may inhibit the activation of HSP47-expressing fibroblasts and suppress TGF-β signaling, thereby preventing fibrotic replacement and preserving the gland’s secretory capacity.
Spironolactone also mitigates ER stress and oxidative injury, both of which contribute to acinar cell dysfunction and death in the lacrimal gland. Experimental studies show that spironolactone reduces ER stress markers such as IRE1 and ATF-6, which in turn suppress reactive oxygen species (ROS) production and restore mitochondrial function. Downregulation of ER stress markers help protect acinar secretory cells from apoptosis and support regenerative processes within the lacrimal gland.
The evidence shows that spironolactone simultaneously addresses inflammation, fibrosis, and cellular stress associated with oGVHD pathogenesis.
2.3.2 Clinical Efficiency
There have been a few preclinical studies and clinical case series demonstrating that topical spironolactone significantly improves key oGVHD diagnostic scores, specifically corneal fluorescein staining, lid margin vascularity, and overall severity, with minimal adverse effects. In a two-part study, Wong et al. showed that topical spironolactone can increase lipid production in corneal epithelial cells both in vitro and in vivo, which may stabilize the tear film and protect against ocular surface erosive disease, an effect not seen with other immunosuppressants or antifibrotic agents. In a retrospective clinical series of oGVHD patients, spironolactone led to improvements in corneal fluorescein staining and overall oGVHD OSDI scores after 7 to 12 weeks. Although the Schirmer’s 1 test did not reach statistical significance, there was an upward trend suggesting increased tear production. The study also emphasized that spironolactone’s inhibition of the mineralocorticoid receptor disrupts the RAAS, which aligns with findings from a mouse model showing reduced lacrimal gland fibrosis and improved ocular surface structure.
In summary, spironolactone’s ability to address lacrimal gland dysfunction makes it uniquely effective for restoring gland function and improving both objective and subjective measures of dry eye in oGVHD.
3 Cornea
3.1 Corneal Dysfunction Pathogenesis
Corneal dysfunction is a secondary complication in patients with oGVHD, often developing because of severe inflammatory responses originating in the lacrimal glands. One study found that endothelial cell density was further reduced in GVHD patients who developed oGVHD after undergoing hematopoietic stem cell transplantation, indicating that significant corneal changes occur.
Corneal dysfunction begins with infiltration of donor-derived CD4+ and CD8+ T cells into the corneal epithelium and stroma, where recipient antigens are recognized as foreign. Like lacrimal gland involvement, T cells function as cytotoxic effectors and release proinflammatory cytokines including TNF-α, IL-1β, IL-6, and IFN-γ. The immune activity induces apoptosis of epithelial cells and disrupts the corneal barrier, resulting in necrosis and atrophy. CD8+ T cells play a dominant cytotoxic role, while CD4+ T cells amplify the overall immune response.
A critical component of corneal dysfunction involves neuroinflammation and direct immune-mediated damage to corneal nerves. Studies have shown that CD4+ T cells in the cornea contribute to nerve destruction, which leads to decreased corneal sensation and neurotrophic epithelial defects. The loss of corneal innervation compromises both the blink reflex and the trophic support necessary for epithelial maintenance. In patients with oGVHD, there is increased expression of the proinflammatory marker NK1R, which is associated with the neuropeptide substance P. Substance P and NK1R signaling enhance pain sensitivity, promote cytokine release, and contribute to corneal epithelial permeability and breakdown. NK1R upregulation also reduces corneal endothelial cell density, thus compromising physiologic stromal deturgescence and corneal transparency. Corneal endothelial cell density loss can intensify epithelial inflammation, senescence, and limbal stem cell deficiency, further weakening the epithelial barrier.
This inflammatory microenvironment caused by substance P and NK1R signaling recruits additional immune cells, including macrophages and antigen-presenting cells, and activates corneal stromal fibroblasts. Under the influence of cytokines and TGF-β, fibroblasts differentiate into myofibroblasts and begin producing excess extracellular matrix, leading to stromal fibrosis and loss of corneal clarity. Matrix metalloproteinases, particularly MMP-9, are upregulated in the tear film and corneal tissue and degrade the basement membrane and extracellular matrix. MMP-9 enzymatic activity worsens epithelial defects; delays wound healing and increases susceptibility to ulceration and infection.
The combined effects of cytokine release, immune infiltration, and nerve damage manifest clinically as superficial punctate keratopathy, persistent epithelial defects, filamentary keratitis, and, in severe cases, corneal ulcers and potential vision loss. These findings are supported by slit lamp examination, fluorescein staining, and evidence of decreased corneal sensation.
3.2 Management Strategies for Corneal Dysfunction
3.2.1 Autologous Serum Eye Drops (ASEDs)
ASEDs are a specialized therapy derived from a patient’s own blood and are used to treat severe dry eye and persistent epithelial defects. The serum contains essential growth factors, vitamins, and anti-inflammatory mediators that help support corneal epithelial healing in a way that mimics natural tears. Multiple clinical studies have demonstrated that ASEDs improve corneal staining, OSDI scores, and even visual acuity in some patients.
However, while effective in theory, the practical limitations of ASEDs become more apparent in oGVHD patients. The process of creating ASEDs is labor-intensive, not universally available, and carries risks such as infection, abnormal immune responses, and the potential transmission of bloodborne diseases. These risks are amplified in oGVHD patients, who often have underlying systemic issues. For instance, up to 60–80% of HSCT recipients require central venous catheters, which over time can result in venous scarring, thrombosis, and compromised peripheral access, making consistent blood collection difficult. In addition, anemia is present in 50–70% of allogeneic HSCT patients, which not only limits the volume of blood that can be safely drawn but may also impact the effectiveness of the drops.
While ASEDs may improve some clinical outcomes, their accessibility, patient-specific limitations, and potential risks make them a less reliable long-term option for managing corneal disease in oGVHD.
3.2.2 Scleral Lenses
Scleral lenses offer a valuable therapeutic option for managing severe ocular surface disease in oGVHD. Scleral lenses provide improved hydration, mechanical protection, and visual rehabilitation in patients with persistent epithelial defects, severe dry eye, or corneal irregularity. Properly fitted scleral lenses can reduce surface inflammation but do not directly address the ongoing chronic inflammation underlying oGVHD. Limitations of scleral lenses include cost, accessibility, lens fogging, and handling challenges, particularly in patients with reduced dexterity or vision. Despite limitations, scleral lenses are often underutilized and should be considered earlier in patients unresponsive to standard therapies. MIEBO can also be used in conjunction with scleral lenses to further reduce evaporative loss.
3.2.3 Other emerging treatments under investigation
Topical immunosuppressants such as cyclosporine and tacrolimus may offer benefit for ocular surface inflammation in oGVHD, including corneal involvement. These agents are discussed in detail in Section 4.2.2. Several experimental agents have shown potential in preclinical models, though none have advanced to clinical application. Fosaprepitant is an NK1R antagonist that targets the substance P pathway, which is activated in the inflammatory pathogenesis of the cornea. In preclinical mouse models, topical fosaprepitant reduced corneal fluorescein staining by 72% and significantly decreased immune cell infiltration, indicating strong anti-inflammatory and epithelial-protective effects. However, clinical efficacy and use of fosaprepitant in human subjects has not been reported in the literature.
Cobra Venom Factor (CVF) is another unique treatment, which is a non-toxic protein derived from cobra venom. CVF closely resembles the complement component C3b, an active fragment of human complement C3. CVF forms a stable C3/C5 complex with factor B, which can help inhibit immune-mediated injury. In animal models, CVF has been shown to prevent corneal sensation loss and tissue damage by depleting complement C3. However, CVF has not yet been tested in humans. The primary concern is CVF immunogenicity. CVF’s distinct protein structure, including a glycosylation pattern not found in human proteins, may trigger potent immune responses such as allergic reactions or the production of neutralizing antibodies.
3.3 Impact of Spironolactone on Corneal Dysfunction
3.3.1 Mechanism/Pathways
Spironolactone reduces corneal staining and improves epithelial barrier integrity by preserving tight junction proteins such as ZO-1, which are essential for maintaining epithelial cohesion and preventing ulceration. By strengthening the epithelial barrier, spironolactone also suppresses the expression of key inflammatory mediators, including MMP-9 and interleukin-1β. Downregulating MMP-9 and IL-1β helps prevent cytokine-driven surface breakdown and protects against persistent epithelial defects and erosions.
In murine models of inflammatory bowel disease, spironolactone has been shown to restore goblet cell density and mucin expression in the colonic epithelium. While this finding may support a broader mucin-preserving effect, no ocular studies to date have proved a similar impact on conjunctival goblet cells. It would be of interest to investigate whether spironolactone confers comparable benefits on the ocular surface, particularly in the context of oGVHD. Goblet cell density and mucin expression are essential for protecting the ocular surface and promoting healing. In vitro and in vivo studies show that spironolactone enhances lipid synthesis in corneal epithelial cells, contributing to a more protective and stable tear film. The increase in lipid synthesis helps prevent evaporative dry eye and further epithelial injury.
Finally, spironolactone demonstrates regenerative potential. In a rat model of corneal wound healing, topical spironolactone accelerated re-epithelialization and improved cell adhesion compared to placebo. These multi-level effects support spironolactone’s role as a comprehensive therapy for corneal dysfunction.
3.3.2 Clinical Efficiency
Clinical studies reinforce the mechanistic benefits of spironolactone in dry eye patients as well as oGVHD. In a retrospective study on oGVHD patients, 0.005mg/cc topical spironolactone dosed at two to four times daily led to statistically significant improvements in corneal fluorescein staining and overall OSDI scores after 7 to 21 weeks of treatment. Importantly, spironolactone was well tolerated, with minimal adverse effects of mild application stinging for 5 to 10 seconds. Preclinical animal models further support improvement in clinical signs. In cGVHD mouse models, topical spironolactone resulted in lower CFS scores compared to controls. In rat corneal wound healing models, spironolactone accelerated epithelial closure, reduced cell infiltration, and restored epithelial cell adhesion.
4 Conjunctiva
4.1 Conjunctival Dysfunction Pathogenesis
Conjunctival dysfunction is another pathology seen in oGVHD patients, often presented as conjunctival injections or chronic conjunctivitis. Similar to previous mechanisms, the pathogenesis begins with CD8+ and CD4+ T cell infiltration of the basal conjunctival epithelium, where host antigens are recognized as foreign. Immune response from CD4+ and CD8+ T cell infiltration recruits macrophages, dendritic cells, and cytokines such as IFN-γ, IL-1β, and IL-6. These cytokines induce apoptosis and necrosis of conjunctival epithelial cells, contributing to tissue damage.
The inflammatory environment activates conjunctival fibrosis, primarily through TGF-β and other pro-fibrotic mediators. As with other oGVHD sites, excessive collagen and extracellular matrix are produced, resulting in subepithelial fibrosis, scarring, and further injury. Subepithelial fibrosis has been observed in up to 50% of oGVHD patients. In addition, inflammation may trigger epithelial-mesenchymal transition (EMT) in conjunctival epithelial cells, which further contributes to fibrotic remodeling.
Chronic inflammation and fibrosis also stimulate matrix metalloproteinase (MMP) production. MMPs degrade the extracellular matrix and basement membrane, leading to tissue remodeling and worsening conjunctival injury. A key diagnostic feature of conjunctival dysfunction is goblet cell loss. Goblet cells secrete mucins essential for tear film stability and ocular surface lubrication. Immune-mediated cytotoxicity and cytokine-induced apoptosis reduce goblet cell density, resulting in mucin deficiency, tear film instability, and increased ocular surface friction.
Clinically, these changes manifest as conjunctival hyperemia, chemosis, pseudomembrane formation, cicatricial changes, and severe dry eye symptoms.
4.2 Management Strategies for Conjunctiva Dysfunction
4.2.1 Topical Glucocorticoids
Topical glucocorticoids are used in oGVHD patients to promote lymphocyte apoptosis and suppress cell-mediated inflammation. Clinical improvement has been reported with agents such as prednisolone acetate 1% and other low-dose steroid formulations. Glucocorticoids are effective but carry significant risks with prolonged use. Risks include increased intraocular pressure, cataract formation, and heightened susceptibility to infection. oGVHD patients, who already have a compromised ocular surface, may be particularly vulnerable to these side effects. Glucocorticoids may further impair wound healing, worsen inflammation, or contribute to new vision-threatening conditions. As a result, glucocorticoid risk profile is higher compared to individuals without GVHD.
4.2.2 Lifitegrast (Xiidra)
Lifitegrast ophthalmic solution 5% (Xiidra) is a nonsteroidal, anti-inflammatory eye drop approved for the treatment of dry eye disease and has shown promise in oGVHD-associated conjunctival inflammation. Xiidra blocks the interaction between LFA-1 on T cells and ICAM-1 on ocular surface tissues, reducing T-cell activation and downstream cytokine release. Early studies suggest that Xiidra may improve both symptoms and objective signs such as Schirmer’s 1 scores and ocular surface staining in oGVHD patients. Xiidra rapid onset of action as early as two weeks and favorable safety profile make it a potential option for patients’ intolerance of steroids or calcineurin inhibitors. Xiidra is generally well tolerated, with the most common side effects being mild irritation or dysgeusia.
While current data in oGVHD remain limited to small studies, Xiidra may be considered in patients with moderate to severe conjunctival inflammation, particularly when long-term steroid use poses risk. As a steroid-sparing agent, it may play a role in early treatment to reduce chronic medication burden. However, cost and limited insurance coverage may affect accessibility.
4.2.3 Topical Calcineurin Inhibitors
Topical calcineurin inhibitors are also used to block T-cell activation and suppress cytokine release. This promotes goblet cell preservation and improves tear film stability. The two primary agents are cyclosporine A (CSA) and tacrolimus. However, use of calcineurin inhibitors may be limited by tolerability issues and ineffectiveness in severe cases. Many patients continue to experience significant symptoms despite treatment with CSA, and objective measurements of tear production often show minimal or statistically insignificant improvement, even with frequent dosing. Up to one third of patients discontinue CSA due to side effects. Additionally, CSA has been shown to improve symptoms in 62.5% of patients with conjunctival dysfunction, a rate comparable to tacrolimus. However, CSA has a delayed onset of action compared to tacrolimus, often requiring several weeks to months for full therapeutic effect.
Tacrolimus can be used in patients who are intolerant of CSA but can frequently cause discomfort or local irritation. Burning sensations following topical tacrolimus administration can last up to 15 to 20 minutes, which may contribute to nonadherence. There is also an increased risk of infections such as herpetic keratitis, particularly in patients with a prior history of herpes simplex virus infection. Furthermore, there are few large-scale randomized trials evaluating these agents specifically in patients with oGVHD, leaving their long-term safety and efficacy in this context uncertain.
Ultimately, while topical calcineurin inhibitors help to deactivate inflammatory T cells, they do not address fibrotic pathways or reverse the underlying glandular atrophy that is central to oGVHD.
4.2.4 NET-Targeted Therapies
These therapies use agents that reduce neutrophil extracellular traps (NET) proteins such as extracellular DNA (eDNA), oncostatin M (OSM), and neutrophil gelatinase-associated lipocalin (NGAL). By dismantling NETs via targeted therapies, inflammation and fibrosis can be decreased. The main medications in this category include DNase I, heparin, and antagonists targeting OSM, NGAL, and TNFSF14.
DNase I and intravenous globulin (IVIG) have been used in oGVHD patients and are considered safe and well tolerated. However, large-scale efficacy data are still lacking, particularly regarding DNase I’s effect on chronic fibrosis. Additionally, IVIG drops have limited penetration into conjunctival tissue, which may reduce their therapeutic potential.
Heparin has shown benefit in reducing NET-driven conjunctival inflammation at sub-anticoagulant doses (100 IU/mL). However, heparin may increase the risk of corneal stromal or subconjunctival hemorrhage. Heparin precise mechanism and long-term safety remain under investigation.
4.2.5 Other Therapies
Several other treatments are being explored as novel approaches. The use of 1% progesterone gel applied to the forehead has shown improvement in ocular symptoms without severe adverse effects. However, because of the novelty of administration route for 1% progesterone gel, the supporting evidence remains preliminary. Another example is oral gentamicin, an antibiotic tested in animal models. Gentamicin was shown to suppress inflammatory cell infiltration and fibrosis in cGVHD organs and reduced ocular manifestations. Although gentamicin may appear to be a conventional option, oral systemic antibiotics are not standard for conjunctival oGVHD due to the risks associated with concurrent systemic treatments.
4.3 Impact of Spironolactone in Conjunctiva Dysfunction
4.3.1 Mechanism/Pathways
In oGVHD-related conjunctival disease, goblet cell loss contributes to mucin deficiency and tear film instability. Spironolactone may help restore goblet cells, as discussed in Section 3.3.1, though evidence is limited and non-ocular. Beyond its possible ability to restore goblet cells, spironolactone exerts an anti-inflammatory effect by suppressing the production of key pro-inflammatory cytokines. This broad cytokine suppression is important in oGVHD, as T cell–mediated immune responses drive conjunctival injection and chemosis. By reducing cytokine expression and T cell activity, spironolactone interrupts the immune cascade and may provide more durable control of inflammation compared to agents that target only a single pathway.
Spironolactone also has direct anti-fibrotic properties. Spironolactone inhibits TGF-β signaling and the expression of HSP47, which are central to fibroblast activation and collagen deposition in conjunctival tissue. By targeting TGF-β signaling and HSP47 expression, spironolactone may prevent or even reverse the progression of conjunctival fibrosis and cicatricial changes, which current treatments do not achieve. Additionally, by reducing epithelial apoptosis and preserving barrier integrity, spironolactone supports the conjunctival surface and helps break the cycle of injury, inflammation, and scarring.
4.3.2 Clinical Efficiency
Early clinical and translational studies provide promising support for spironolactone’s efficacy in conjunctival oGVHD. In preclinical models, topical spironolactone reduced conjunctival IL-1β expression and improved epithelial integrity. These results are supported by another study, which demonstrated that spironolactone preserved conjunctival epithelial cell viability and reduced inflammatory cell infiltration in both in vitro and in vivo models. In the clinical setting, a case series by Gupta reported that patients with severe ocular surface disease, including those with oGVHD, experienced improvements in conjunctival hyperemia, goblet cell density, and patient-reported symptoms following spironolactone treatment. Usage of spironolactone was well tolerated, with only mild stinging lasting a few seconds in some patients.
5 Meibomian Glands
5.1 Meibomian Gland Dysfunction Pathogenesis
Meibomian gland dysfunction is a central feature of oGVHD, with a prevalence of 47.8% to 70% in affected patients. The pathogenesis of meibomian gland dysfunction is multi-phased, involving both ductal epithelial changes and acinar alterations often accompanied by lid margin neovascularization and inflammation.
Meibomian gland dysfunction begins with systemic immune activation, as previously described. Donor T cells infiltrate the eyelid margin and meibomian gland tissue, releasing inflammatory cytokines and creating a chronic inflammatory environment that contributes to tissue damage. In the meibomian glands, T-cell mediated inflammation leads to ductal epithelial hyperkeratinization, resulting in the accumulation of keratinized debris within the ducts. Obstruction of ductal epithelium causes cystic dilation and eventually glandular atrophy. Studies in oGVHD have shown changes where increased obstruction has occurred, which contribute to gland dropout and lipid layer deficiency. Additional studies have shown endothelial damage, fibroblast activation, and immune cell infiltration within the glands due to cGVHD.
Direct immune-mediated epithelial injury further exacerbates damage to the meibomian glands by destroying both the ductal and acinar epithelium, causing lymphocyte aggregation and, in some cases, pseudomembrane formation within the gland. Compared to patients without oGVHD, those with oGVHD show a lower density of meibomian gland acinar units and a shorter mean acinar diameter. This epithelial loss worsens ductal obstruction and impairs normal glandular secretion. Chronic inflammation also promotes extensive fibrosis around the gland duct due to persistent cytokine signaling. This fibrotic remodeling is largely irreversible and leads to permanent glandular atrophy and functional loss. Clinically, these pathological changes present as loss of meibomian gland tissue, reduced meibum secretion, and significant alterations in lipid composition. Patients experience tear film instability, decreased tear breakup time, and dry eye disease symptoms.
5.2 Management Strategies for Meibomian Gland Dysfunction
5.2.1 Conventional Therapies
Patients often use warm compresses and lid hygiene to manage meibomian gland dysfunction. Warm compresses help liquefy thickened meibum and promote gland expression. Lid scrubs assist in removing surface debris, and together, they are considered a foundational approach. However, recent evidence suggests treatment with warm compresses and lid scrubs has limited effectiveness. Compresses must maintain a temperature above 40°C to be effective, but heat loss through towels often prevents maintenance of necessary temperature, resulting in only transient improvement. Additionally, real-world adherence to this regimen is low, with compliance rates reported below 60%.
5.2.2 Perfluorohexyloctane (MIEBO)
Perfluorohexyloctane, a preservative-free, nonaqueous eye drop that stabilizes the tear film by forming a lipid layer over the ocular surface, reducing evaporation in patients with meibomian gland dysfunction. Phase 3 trials demonstrated early and sustained improvements in both corneal staining and dry eye symptoms, including in patients with evaporative disease. MIEBO does not rely on active gland function, making it suitable for patients with gland atrophy. MIEBO can be used alongside other topical therapies and does not require refrigeration, offering a convenient option for oGVHD-related MGD where evaporative loss is a major contributor to ocular surface disease.
5.2.3 Topical antibiotic ointments
Topical antibiotic ointments such as erythromycin or azithromycin are commonly prescribed. Occasionally, doxycycline is used for its antimicrobial properties. Doxycycline has shown some benefit in reducing lid margin inflammation and improving meibum quality. However, the overall benefits of erythromycin, azithromycin, and doxycycline remain limited, as they do not address the immune-mediated destruction or fibrotic changes in the glands. Additionally, long-term oral systemic antibiotics are not recommended for oGVHD patients, as previously discussed.
5.2.4 Intense Pulsed Light
Intense pulsed light (IPL) is a newer therapy that delivers pulses of light to the eyelids, helping to liquefy meibum within the meibomian glands. Recent studies in oGVHD patients have demonstrated statistically significant improvements in both subjective symptoms and objective clinical measures. However, as IPL remains an emerging treatment, access is limited and long-term efficacy data are still developing.
5.3 Impact of Spironolactone in Meibomian Glands Dysfunction
5.3.1 Mechanism/Pathway
Spironolactone can reduce immune cell infiltration and epithelial damage at the lid margin and within the meibomian glands by inhibiting the production of pro-inflammatory cytokines. Spironolactone’s activity as a mineralocorticoid receptor antagonist also provides benefits for sebaceous gland function. As previously discussed, the activation of mineralocorticoid receptors in ocular tissues is associated with inflammatory and fibrotic responses. By blocking these pathways, spironolactone helps preserve glandular architecture and prevent fibroblast activation, reducing the risk of irreversible gland dropout that characterizes advanced meibomian gland dysfunction (MGD).
An additional mechanistic benefit is spironolactone’s effect on lipid synthesis and secretion. Spironolactone has been shown to increase lipid droplet formation, as demonstrated by Oil Red O and BODIPY staining. Lipid-promoting action via spironolactone can support tear film stability and may help counteract the evaporative component of dry eye in oGVHD.
5.3.2 Clinical Efficiency
Clinical and translational studies support the therapeutic potential of spironolactone. In a cGVHD mouse model, topical spironolactone eye drops significantly reduced ocular surface inflammation and prevented meibomian gland depletion. Human studies have similarly shown that patients with moderate to severe MGD treated with spironolactone reported improved dry eye symptoms, enhanced meibum clarity and viscosity, and reduced lid margin inflammation. Quantitative data from these studies demonstrated statistically significant improvements in self-reported global assessment scores, turbidity scores, and lid margin vascularity. Although meibomian gland dropout scores did not reach significance during short-term follow-up, improvements in meibum quality and lid margin inflammation were consistently noted. Case reports and small series have further emphasized spironolactone’s value in refractory cases where conventional therapies failed, with patients showing marked improvement in both symptoms and clinical signs of MGD within a few weeks of treatment.
6 Limitations
While early findings on spironolactone in oGVHD are promising, several limitations remain. Most of the data stems from preclinical animal studies or small clinical case series, limiting generalizability. Large-scale, randomized controlled trials in diverse patient populations are lacking, and long-term safety data on topical ocular formulations have not yet been established. Furthermore, most studies focus on short-term outcomes, so the durability of therapeutic effects remains unknown. Finally, variability in dosing, formulation, and delivery methods of spironolactone across studies makes it difficult to standardize comparisons or treatment protocols.
7 Future Directions
Future research should aim to validate spironolactone’s therapeutic potential in oGVHD through larger, randomized clinical trials. Standardizing topical formulations and dosing regimens will be essential to optimize efficacy and patient tolerability. Comparative studies with other MRAs may help define the most effective and safest treatment strategy. Investigating long-term outcomes, including spironolactone’s ability to reverse fibrosis and promote ocular surface regeneration, will further clarify its role as a disease-modifying agent.
8 Conclusion
Spironolactone presents a compelling treatment approach for ocular graft-versus-host disease (oGVHD) due to its multi-mechanistic profile that addresses both the immune and fibrotic components of disease pathology. Unlike current therapies, which often target a single pathway or provide only symptomatic relief, spironolactone exerts anti-inflammatory, antifibrotic, and epithelial-protective effects across all four major tissues affected by oGVHD: the lacrimal glands, cornea, conjunctiva, and meibomian glands. Spironolactone’s ability to modulate mineralocorticoid receptor signaling while preserving beneficial glucocorticoid activity provides a mechanistic advantage that is especially relevant in tissues with dysregulated cortisol metabolism. Preclinical models and early clinical observations support spironolactone’s efficacy in improving key diagnostic markers such as corneal fluorescein staining, lid margin vascularity, and goblet cell density, while maintaining a favorable safety profile. Although further randomized trials are needed to establish long-term outcomes and optimize formulation and dosing, current evidence suggests that spironolactone may offer disease-modifying potential and fill a significant therapeutic gap in the management of oGVHD.
9 Conflicts of Interest Statement
Richard W. Yee is a co-inventor on a patent related to the topical ophthalmic use of spironolactone. None of the other authors have any conflicts of interest to declare.
10 Funding Statement
The authors received no specific funding for the research, authorship, or publication of this article.
11 References
- Tyndall A, Dazzi F. Chronic GVHD as an autoimmune disease. Best Practice & Research Clinical Haematology. 2008;21(2):281-289. doi:10.1016/j.beha.2008.03.003
- Atkinson K, Horowitz M, Gale R, et al. Risk factors for chronic graft-versus-host disease after HLA-identical sibling bone marrow transplantation. Blood. 1990;75(12):2459-2464. doi:10.1182/blood.v75.12.2459.2459
- Ochs LA, Miller WJ, Filipovich AH, et al. Predictive factors for chronic graft-versus-host disease after histocompatible sibling donor bone marrow transplantation. Bone Marrow Transplant. 1994;13(4):455-460.
- Inamoto Y, Valdés-Sanz N, Ogawa Y, et al. Ocular Graft-versus-Host Disease after Hematopoietic Cell Transplantation: Expert Review from the Late Effects and Quality of Life Working Committee of the Center for International Blood and Marrow Transplant Research and Transplant Complications Working Party of the European Society of Blood and Marrow Transplantation. Biology of Blood and Marrow Transplantation. 2019;25(2):e46-e54. doi:10.1016/j.bbmt.2018.11.021
- Munir SZ, Aylward J. A Review of Ocular Graft-Versus-Host Disease. Optom Vis Sci. 2017;94(5):545-555. doi:10.1097/opx.0000000000001071
- Anderson NG, Regillo C. Ocular manifestations of graft versus host disease. Current Opinion in Ophthalmology. 2004;15(6):503-507. doi:10.1097/01.icu.0000143684.22362.46
- Kezic JM, Wiffen S, Degli-Esposti M. Keeping an ‘eye’ on ocular GVHD. Clinical and Experimental Optometry. 2022;105(2):135-142. doi:10.1080/08164622.2021.1971047
- Colarusso BA, Bligdon SM, Ganjei AY, Kwok A, Brocks D, Luo ZK. Ocular Graft-versus-Host Disease Underdiagnosis: A Survey Study. Clin Ophthalmol. 2022;16:1419-1426. doi:10.2147/OPTH.S359539
- Singh RB, Cho W, Liu C, et al. Immunopathological mechanisms and clinical manifestations of ocular graft-versus-host disease following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2024;59(8):1049-1056. doi:10.1038/s41409-024-02321-3
- Cheng X, Huang R, Huang S, et al. Recent advances in ocular graft-versus-host disease. Front Immunol. 2023;14:1092108. doi:10.3389/fimmu.2023.1092108
- Hardy RS, Filer A, Cooper MS, et al. Differential expression, function and response to inflammatory stimuli of 11beta-hydroxysteroid dehydrogenase type 1 in human fibroblasts: a mechanism for tissue-specific regulation of inflammation. Arthritis Res Ther. 2006;8(4):R108. doi:10.1186/ar1993
- Onyimba CU, Vijapurapu N, Curnow SJ, et al. Characterisation of the prereceptor regulation of glucocorticoids in the anterior segment of the rabbit eye. Journal of Endocrinology. 2006;190(2):483-493. doi:10.1677/joe.1.06840
- Vassiliou AG, Athanasiou N, Vassiliadi DA, et al. Glucocorticoid and mineralocorticoid receptor expression in critical illness: A narrative review. WJCCM. 2021;10(4):102-111. doi:10.5492/wjccm.v10.i4.102
- The Multifaceted Mineralocorticoid Receptor. In: Comprehensive Physiology. 1st ed. Wiley; 2014:965-994. doi:10.1002/cphy.c130044
- Pippal JB, Fuller PJ. Structure–function relationships in the mineralocorticoid receptor. Journal of Molecular Endocrinology. 2008;41(6):405-413. doi:10.1677/jme-08-0093
- Tomlinson JW, Stewart PM. Cortisol metabolism and the role of 11β-hydroxysteroid dehydrogenase. Best Practice & Research Clinical Endocrinology & Metabolism. 2001;15(1):61-78. doi:10.1053/beem.2000.0119
- Van Uum S. The role of 11β-hydroxysteroid dehydrogenase in the pathogenesis of hypertension. Cardiovascular Research. 1998;38(1):16-24. doi:10.1016/s0008-6363(97)00299-x
- Suzuki T, Sasano H, Kaneko C, Ogawa S, Darnel AD, Krozowski ZS. Immunohistochemical distribution of 11β-hydroxysteroid dehydrogenase in human eye. Molecular and Cellular Endocrinology. 2001;173(1-2):121-125. doi:10.1016/s0303-7207(00)00403-2
- Sato S, Ogawa Y, Wong CW, et al. Mineralocorticoid receptor expression and the effects of the mineralocorticoid receptor antagonist spironolactone in a murine model of graft-versus-host disease. The Ocular Surface. 2024;34:477-488. doi:10.1016/j.jtos.2024.10.004
- Zhao M, Célérier I, Bousquet E, et al. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest. 2012;122(7):2672-2679. doi:10.1172/jci61427
- Kadmiel M, Janoshazi A, Xu X, Cidlowski JA. Glucocorticoid action in human corneal epithelial cells establishes roles for corticosteroids in wound healing and barrier function of the eye. Exp Eye Res. 2016;152:10-33. doi:10.1016/j.exer.2016.08.020
- Sun XN, Li C, Liu Y, et al. T-Cell Mineralocorticoid Receptor Controls Blood Pressure by Regulating Interferon-Gamma. Circulation Research. 2017;120(10):1584-1597. doi:10.1161/circresaha.116.310480
- Usher MG, Duan SZ, Ivaschenko CY, et al. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest. 2010;120(9):3350-3364. doi:10.1172/jci41080
- Barrera-Chimal J, Lima-Posada I, Bakris GL, Jaisser F. Mineralocorticoid receptor antagonists in diabetic kidney disease — mechanistic and therapeutic effects. Nat Rev Nephrol. 2022;18(1):56-70. doi:10.1038/s41581-021-00490-8
- Mirshahi M, Mirshahi S, Golestaneh N, et al. Mineralocorticoid Hormone Signaling Regulates the ‘Epithelial Sodium Channel’ in Fibroblasts from Human Cornea. Ophthalmic Res. 2001;33(1):7-19. doi:10.1159/000055635
- Patibandla S, Heaton J, Kyaw H. Spironolactone. In: StatPearls. StatPearls Publishing; 2025. Accessed July 9, 2025. http://www.ncbi.nlm.nih.gov/books/NBK554421/
- Zhai S, Ma B, Chen W, Zhao Q. A comprehensive review of finerenone-a third-generation non-steroidal mineralocorticoid receptor antagonist. Front Cardiovasc Med. 2024;11:1476029. doi:10.3389/fcvm.2024.1476029
- Watson AR, Ngo P. Spironolactone cost. Medical News Today. https://www.medicalnewstoday.com/articles/drugs-spironolactone-cost. June 27, 2025.
- Woodward D, Murdock J.

