Effect of Azathioprine on Lung and Skin in Scleroderma
Effect of Azathioprine on Skin and Lung Parameters in Patients with Systemic Sclerosis: A Systematic Literature Review
Sevinç Şen Torun1, Akif Bayyurt2, Bilal Uğurlu3
- University of Health Sciences, Turkey, Prof. Dr. Cemil Taşcıoğlu City Hospital, Department of Rheumatology, Istanbul, Turkey
- University of Health Sciences, Turkey, Prof. Dr. Cemil Taşcıoğlu City Hospital, Department of Internal Medicine, Istanbul, Turkey
- University of Health Sciences, Turkey, Prof. Dr. Cemil Taşcıoğlu City Hospital, Department of Internal Medicine, Istanbul, Turkey
Correspondence: Sevinç Şen Torun, E-mail: [email protected]
Received: 31 December 2024
Accepted: 31 December 2024
OPEN ACCESS
PUBLISHED: 31 December 2025
CITATION: TORUN, Ege Sinan et al. Effect of Azathioprine on Skin and Lung Parameters in Patients with Systemic Sclerosis: A Systematic Literature Review. Medical Research Archives,. Available at: <https://esmed.org/MRA/mra/article/view/6131>.
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.18103/mra.v12i12.6131
ISSN 2375-1924
Abstract
Azathioprine (AZA) is a purine analogue that acts as an immunosuppressant by inhibiting cell proliferation. Azathioprine is effective as a maintenance agent after induction therapy with cyclophosphamide (CYC) in patients with lupus nephritis and systemic vasculitis. In daily rheumatology practice, azathioprine has been largely replaced by mycophenolate mofetil (MMF) after the its successful use as a remission induction and maintenance agent in various autoimmune connective tissue diseases. Currently AZA is mainly used in special scenarios like pregnancy, where MMF is contraindicated.
Systemic sclerosis (SSc) is a disease characterized by autoimmunity, vasculopathy, and fibrosis of the skin and internal organs. The aim of this systematic review was to evaluate the effect of azathioprine in SSc patients on skin and lung parameters.
Keywords: Azathioprine, systemic sclerosis, skin parameters, lung parameters, systematic review
Introduction
There are two authors (EST and E) independently assessed the risk of bias (RoB) of each study with the Newcastle-Ottawa Scale (NOS) tool for randomized controlled trials and the Newcastle-Ottawa Scale for the cohort studies1,2. In case of disagreement, a final decision was reached by the discussion of the two reviewers.
Begga and Mazumdar rank correlation test was used to test for publication bias in the studies that were included in the meta-analysis. Cohort Q and I2 index were used to determine the heterogeneity of the studies.
Change in mean mRSS, FVC and DLCO were assessed as outcomes.
Method
The study design and brief description of the studies selected for systematic review are shown in Table 1.
| Study | Study Design | Brief Description of the Study |
|---|---|---|
| Brezend-20081 | Retrospective Multicenter Open-label Study | In this study, 22 patients received CYC, 2 patients received AZA, 3 patients received both AZA and CYC. |
| Hoda-20042 | Retrospective, Prospective, Randomized, Double-Blind Study | In this study, 22 patients received CYC, 2 patients received AZA, 3 patients received both AZA and CYC. |
| ludici-20143 | Prospective, Observational Study | In this study, 45 SSc patients with early pulmonary fibrosis were randomized to receive AZA. |
| Kiboshi-20124 | Single-center, Prospective, Observational, Open-label Study | In this study, 30 patients were treated with AZA. |
| Nadashkevich-20065 | Retrospective Cohort Study | In this study, 36 SSc patients with interstitial lung disease (SSc-ILD) were compared. |
Results
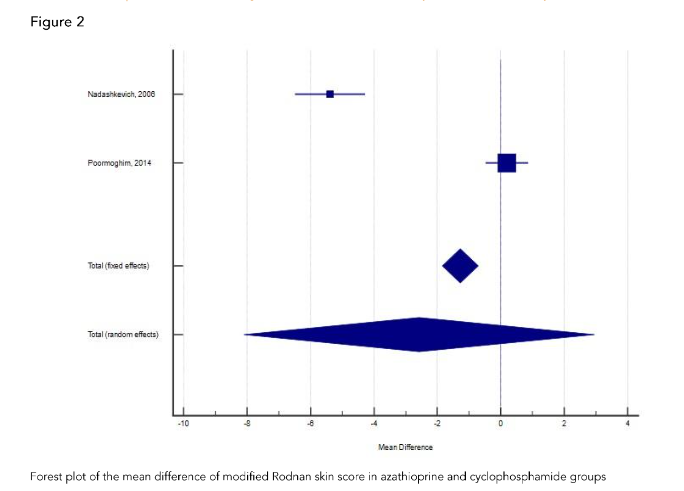
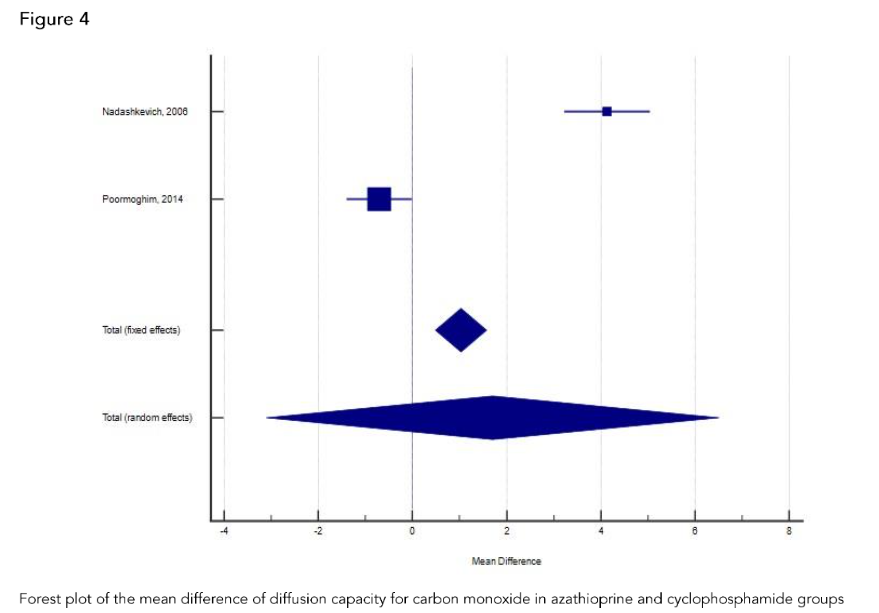
Effect of azathioprine on modified Rodnan skin score in azathioprine and cyclophosphamide groups.
In a multicenter, prospective, randomized, double-blind, placebo-controlled study by Ludici, 45 SSc patients with early pulmonary fibrosis were randomized to receive AZA (n=23) or placebo (n=22). Baseline FVC and DLCO of both arms were similar. At the end of 12 months, 8 patients received stable and at the endpoint 2 patients improved.
At the end of 12 months, 8 patients received stable and at the endpoint 2 patients improved, 9 patients were stable, only one patient improved.
Discussion
Mycophenolate mofetil and cyclophosphamide represent conventional immunosuppressive agents whose efficacy in systemic sclerosis has been explored in various studies. However, there is a need to evaluate azathioprine’s efficacy in SSc patients.
In the study by Paone, although azathioprine maintained the improvement in modified Rodnan skin score in SSc patients, the study did not show a significant change in lung function parameters.
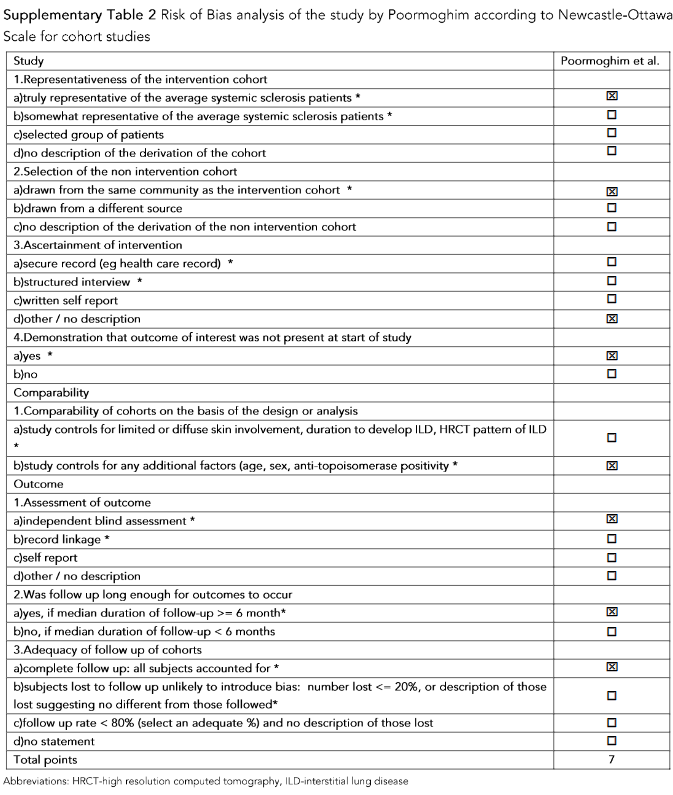
In the retrospective cohort study of 36 SSc patients with interstitial lung disease, Promoghin compared 21 patients that received oral cyclophosphamide and 15 patients that received azathioprine. Changes of mRSS at AZA group and CYC group at the end of 12th month were not significant.
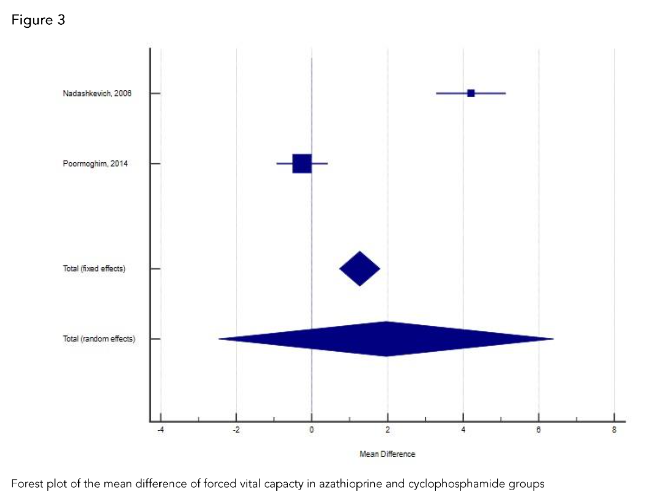
Despite neither drug causing a significant drop in lung function, it should be noted that cyclophosphamide has been considered as a promising disease modifying medication for SSc.
In the retrospective cohort study of 36 SSc patients with interstitial lung disease, Promoghin compared 21 patients that received oral CYC and 15 patients that received azathioprine. Changes of mRSS at AZA group and CYC group at the end of 12th month were not significant.
Conclusion
In conclusion, this study demonstrates that AZA can have a stabilizing effect on skin and lung parameters in SSc patients. Further studies are needed to confirm these findings.
Conflict of Interest Statement:
The authors declare no conflict of interest.
Funding Statement:
No funding was received for this study.
References
- JCA Van Riel, Mycophenolate mofetil, Ann Thorac Soc 2024; 12(1): 1-6.
- Judd IM, Choueiri O, Vettori S, et al. Low-dose cyclophosphamide in interstitial lung disease associated with systemic sclerosis (SSc-ILD): efficacy of maintenance immunosuppression in responders and non-responders. Semin Arthritis Rheum. 2015;44(4):437-444.
- Kiboshi T, Katani T, Koma J, et al. Comparison of therapeutic effects of combination therapy with prednisolone and tacrolimus or azathioprine on pulmonary involvement in systemic sclerosis. Mod Rheumatol. 2022;32(3):358-364.
- Volkman ER, Andraos K, Smith V, et al. Systemic sclerosis: a new therapeutic approach. Ann Rheum Dis. 2015;74(1): 1-6.
Supplementary Tables
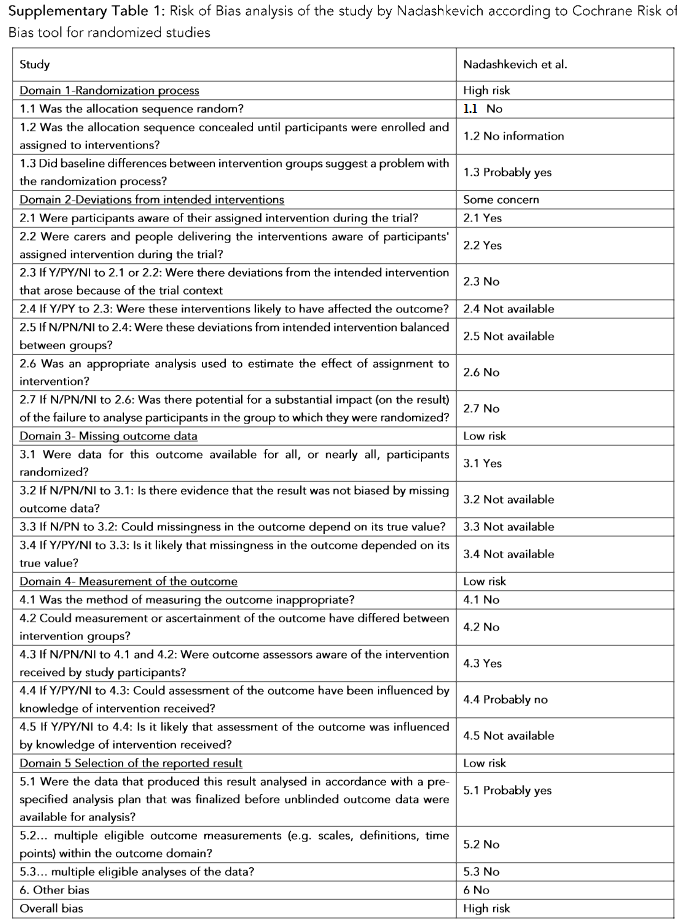
Supplementary Table 1: Risk of Bias analysis of the study by Nadashkevich according to Cochrane Risk of Bias tool for randomized studies.
Supplementary Table 2: Risk of Bias analysis of the study by Poormoghin according to Newcastle-Ottawa Scale.