Decolonization Strategies for Preventing HAIs in Hospitals
Decolonization to Prevent Healthcare-Associated Infections 2024 State-of-the-Art Review
Edward J. Septimus, MD1
- Department of Population Medicine, Harvard Medical School and Harvard Medical School Affiliates, Boston, Massachusetts, USA
OPEN ACCESS
PUBLISHED: 31 October 2024
CITATION: Septimus, E.J., 2024. Decolonization to Prevent Healthcare-Associated Infections 2024 State-of-the-Art Review. Medical Research Archives, [online] 12(10).
https://doi.org/10.18103/mra.v12i10.5782
COPYRIGHT: © 2024 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI https://doi.org/10.18103/mra.v12i10.5782
ISSN 2375-1924
ABSTRACT
Colonization with healthcare-associated pathogens such as Staphylococcus aureus, enterococci, Gram-negative organisms, and Clostridioides difficile is associated with increased risk of infection. Decolonization is an evidence-based intervention that can be used to prevent healthcare-associated infections (HAIs). The Centers for Disease Control and Prevention (CDC) report recommends attempting to prevent these infections through appropriate antibiotic use and infection prevention practices. The micro-organisms most frequently isolated from HAIs were, in decreasing order, Escherichia coli (12.7%), Klebsiella spp. (11.7%), Enterococcus spp. (10.6%), SARS-CoV-2 (9.1%), and others. The report demonstrated about 92% decrease in CLABSI between 2001 and 2022, and significant changes in SSI rates. The authors concluded that decolonization strategies should be implemented in high-risk populations.
Keywords: decolonization, healthcare-associated infections, Staphylococcus aureus, enterococci, Gram-negative organisms, Clostridioides difficile
Introduction
Healthcare-associated infections (HAIs) are a leading cause of preventable harm in hospitals. HAIs burden patients, complicate treatments, prolong hospital stays, increase costs, and can be life-threatening. Up to 15% of patients develop an infection while hospitalized. The Centers for Disease Control and Prevention (CDC) report recommends attempting to prevent these infections through appropriate antibiotic use and infection prevention practices. HAIs are now the fifth leading cause of death in United States (U.S.) acute-care hospitals¹. The human suffering and financial burden associated with these infections are significant. Recent reports have estimated that U.S. health care system direct costs that can be attributed to HAIs range from $9.8 billion to $45 billion per year². Beyond direct financial costs, HAIs also contribute significantly to increased patient length of stay in the hospital, which results in both financial and patient dissatisfaction. HAIs are not only a strain on patients and health care systems, but also the antimicrobial use associated with their treatment can accelerate antimicrobial resistance and increase adverse events. HAIs also represent one quarter of sepsis cases in hospitals³.
Over the past few decades, large changes in U.S. health care have had an impact on HAI prevention. First, we now know that a significant percentage of HAIs can be prevented by use of evidence-based strategies⁴. Second, there are now coordinated efforts among federal agencies aimed at HAI prevention, including public reporting of hospital-specific HAI rates and linking hospital-specific HAI performance measures to financial reimbursement in order to stimulate HAI prevention efforts. Since 2011, hospitals have been required to report to the CDC’s National Healthcare Safety Network (NHSN) all of their central-line associated bloodstream infections (CLABSIs) in order to qualify for annual payment updates. The Centers for Medicare and Medicaid (CMS) also requires hospitals to report new data to NHSN, including surgical site infection (SSI) rates for colon surgery and abdominal hysterectomy, methicillin-resistant Staphylococcus aureus (MRSA) bloodstream infections, Clostridioides difficile infections (CDI), catheter-associated urinary tract infections (CAUTIs), and influenza vaccination among health care workers. This data, as well as other quality metrics, will be used to determine CMS reimbursement levels for each hospital as a component of value-based purchasing, thus creating performance-driven reimbursement⁵. Therefore, hospitals now have a financial incentive to implement prevention strategies to control HAIs.
In the U.S. each day, approximately one in 31 U.S. patients and 1 in 43 nursing home residents contracts at least one HAI. In the last point-prevalence survey a total of 12,299 patients in 199 hospitals were surveyed. Fewer patients had HAIs in 2015 (394 patients [3.2%; 95% confidence interval, 2.9 to 3.7]) than in 2011 (452 [4.0%; 95% CI, 3.7 to 4.4]) (P<0.001), largely owing to reductions in the prevalence of SSIs and CDIs, which were due to CDI), and SSIs were the most common health care-associated infections⁶.
In 2022–2023, 28 European Union/European Economic Area (EU/EEA) countries and three Western Balkan countries (Kosovo, Montenegro and Serbia) participated in the third European CDC (ECDC) point prevalence survey (PPS) of HAIs and antimicrobial use in European acute care hospitals⁷. The prevalence of patients with at least one HAI in the EU/EEA sample was 7.1%. When extrapolated to the average daily number of occupied beds per country, the weighted HAI prevalence was 6.3%. After adjustment for the one non-participating EU/EEA country (Denmark), this corresponded to an estimated total of 93,305 patients with at least one HAI on any given day, 4.3 million (95% CI: 3.1–5.8 million) patients with at least one HAI and 4.8 million (95% CI: 3.1–5.8 million) HAIs (infection episodes) per year in the period 2022 to 2023 in acute care hospitals in the EU/EEA.
Of a total of 22,806 reported HAIs in the European Union/European Economic Area EU/EEA, the most. The frequently reported types of HAI were respiratory tract infections (29.3% of the total, including pneumonia 19.0%, COVID-19 7.0% and other lower respiratory tract infections 3.3%), urinary tract infections [UTIs] (19.2%), SSIs (16.1%), bloodstream infections [BSIs] (11.9%) and gastrointestinal infections (9.5%), with CDIs accounting for 62.1% of the latter and 5.9% of all HAIs. The microorganisms most frequently isolated from HAIs were, in decreasing order, Escherichia coli (12.7%), Klebsiella spp. (11.7%), Enterococcus spp. (10.0%), SARS-CoV-2 (9.5%), S. aureus (9.0%), C. difficile (8.0%), P. aeruginosa (7.9%), coagulase-negative staphylococci (5.8%), Candida spp. (4.7%), Proteus spp. (3.2%), Acinetobacter spp. (3.2%) and Enterobacter spp. (3.0%).
Up until the COVID-19 pandemic, there was significant progress in reducing HAIs. However, the pandemic resulted in extraordinary challenges for hospitals: Increased HAIs were observed throughout 2020 and 2021 as hospitals responded to increased patient volumes, increased patient acuity levels, and staffing challenges⁸. However, in the 2022 National and State Healthcare-Associated Infections Progress Report showed an improvement. The report demonstrated about 9% decrease in CLABSI between 2021 and 2022, an overall, 12% decrease in CAUTI between 2021 and 2022, a 19% decrease in ventilator associated events (VAE) between 2021 and 2022, and no significant changes in SSI related to 9 of the 10 select procedures tracked in the report between 2021 and 2022. However, hip arthroplasty SSIs increased 8%. There was a 16% decrease in hospital onset MRSA bacteremia between 2021 and 2022, There was a 3% decrease in hospital onset CDI infections between 2021 and 2022⁹.
HAIs are frequently due to endogenous pathogens, originating from the patients’ own microbial flora. This fact promotes innovative approaches to HAIs prevention including decolonization of HAIs pathogens carriers and optimizing skin preparation. The main aim of this paper is to summarize data about nasal topical decolonization, topical (e.g., skin) decolonization, selective digestive and oropharyngeal decontamination. (Table 1)
Table 1. Decolonization Strategies to Prevent Health Care-Associated Infections
Nasal Decolonization
• Mupirocin
• Povidone-Iodine
• Nasal alcohol antiseptic
• Photodynamic Therapy
Skin Decolonization
• Chlorhexidine
• Povidone-Iodine
Selective Digestive Decontamination
• Polymyxin
• Tobramycin
• Amphotericin B
Selective Oropharyngeal Decontamination
• Polymyxin
• Tobramycin
• Amphotericin B
• Cefotaxime IV
Fecal Microbiota Transplant
Bacteria is part of the normal human microflora and usually do not cause infection. Colonization is most common in body sites such as the nose, skin, and gastrointestinal tract. The body sites of colonization are usually specific to the type of bacteria. S. aureus and other commensal Gram-positive organisms (e.g., coagulase-negative staphylococci [CNS]) most commonly colonize the skin and mucosal membranes of the nose¹⁰. Both Gram-positive (e.g., Streptococcus pneumoniae) and Gram-negative organisms can colonize the pharynx¹¹,¹². Other organisms, such as enterococci, C. difficile, and Gram-negative organisms (e.g., Enterobacterales), commonly colonize the gastrointestinal tract¹³.
Bacterial colonization can occur among both healthy and ill populations. Between 15 and 30% of healthy adults are nasally colonized with methicillin-susceptible S. aureus (MSSA), and 1% to 3% are nasally colonized with MRSA¹⁴,¹⁵. Increases in S. aureus colonization at other body sites, including the pharynx, groin, perianal region, or axilla, is also associated with development of S. aureus infections. This is most common among high-risk groups such as ICU patients, men who have sex with men, HIV-infected patients, insulin dependent diabetes mellitus, and certain chronic skin conditions¹⁶,¹⁷. Similarly, gastrointestinal colonization with VRE is associated with increased risk of VRE infection¹⁸,¹⁹. Recent studies have highlighted the role of rectal colonization with carbapenem-resistant gram-negative bacteria as an important source of HAIs²⁰. MDRO colonization in solid organ transplants is also associated with increased risk of infection and death. The risk on mortality was higher with carbapenem resistant Enterobacteriaceae (CRE) colonization among liver transplant recipients²¹.
Hospitalized patients and long-term-care facility residents are at higher risk of colonization with health care-associated pathogens especially MDROs. There is clearly an increased risk of colonization and infection with multidrug resistant organisms (MDROs) in long term care in the range of 40% to as high as 80%²²,²³. This is far higher than the typical hospital with a prevalence of 10%-15%. Infections caused by MDROs are estimated to cause over 35,000 deaths in the U.S. every year and increase medical costs by $4.6 billion²⁴. Efforts to reduce spread of MDROs has focused on infection prevention primarily in acute care facilities and more recently in long-term care as well²⁵. MDRO colonization is an important risk factor for MDRO infection and is directly related to bacterial load on the body. Carriers with high bacterial loads are at higher risk of infection and are more likely to transmit the bacteria to their environments¹⁵,²⁶.
Since colonization often leads to infection, two overarching approaches to HAI prevention have emerged: (i) horizontal strategies to broadly reduce the burden of all pathogens and (ii) vertical approaches to reduce colonization or infection due to specific pathogens (Table 2)²⁷.
Table 2. Vertical and horizontal approaches
Vertical (substantially reduces one pathogen; is pathogen specific)
• Active surveillance (e.g., for MRSA, VRE, C. difficile, Gram-negative MDROs)
• Contact precautions (e.g., for MRSA/VRE colonization or MRSA/VRE infection, C. difficile infection, Gram-negative MDROs)
• Decolonization (e.g., for MRSA)
Horizontal (substantially reduces all infections; is not pathogen specific)
• Standard precautions (HH, cough etiquette, PPE, universal gloving)
• Bundles of care (e.g., CLABSI, SCIP, ventilator bundle)
• CHG bathing
• Selective digestive tract decontamination
• Fecal Microbiota Transplant
HH, hand hygiene; PPE, personal protective equipment; SCIP, surgical care improvement project. Vertical approaches are directed at a single pathogen and often utilize active surveillance testing. This is important because multidrug-resistant organisms (MDROs), such as VRE, multidrug-resistant Gram-negative organisms, MRSA, and C. difficile, are similar in that colonization often precedes infection, transmission occurs by direct or indirect contact, and there are many more asymptomatic patients than infected patients. In addition, unrecognized colonized patients can serve as a source of transmission²⁸.
Horizontal decolonization approaches can target all clinically meaningful health care-associated bacteria, including S. aureus, enterococci, Candida, and Gram-negative bacteria. Chlorhexidine gluconate (CHG) skin decolonization of all high-risk patient populations is an example of a horizontal strategy. Since CHG has broad-spectrum activity, it has been shown to reduce infections due to Gram-positive, Gram-negative, and Candida spp., including MRSA. In addition, the use of CHG can also prevent blood culture contamination caused by the skin commensals (e.g., coagulase-negative staphylococci), which may reduce the additional costs and unnecessary antibiotic treatment associated with blood culture contamination²⁹. Selective digestive tract decontamination (SDD) and fecal microbiota transplant (FMT) are additional horizontal decolonization strategies to prevent HAIs to be discussed later.
Decolonization is the most effective among patient populations who are at risk of infection for only a short period of time³⁰. These include populations such as surgical patients, who may be at a lower risk of infection after surgical closure and surgical wound healing, and ICU patients, who are at a much lower risk once they are discharged from the ICU. This window of time is important because of concern regarding both recolonization and resistance to colonizing agents. Thus, patient populations who are at risk for only short periods of time can achieve short-term success with decolonization.
This is important since studies have found that patients tend to become recolonized with S. aureus within weeks or months of being decolonized³¹,³². In fact, S. aureus recolonization rates at 1 year approached 50% for health care workers and 75% for patients on peritoneal dialysis³³. Similarly, one study found that the S. aureus recolonization rate at 4 months was 56% in patients on hemodialysis³⁴. The goal of this paper is to review the current evidence for different decolonization strategies on preventing HAIs.
Nasal topical decolonization strategies
Mupirocin
Nasal mupirocin is the most widely used topical antibacterial agent. Mupirocin inhibits synthesis of bacterial proteins by reversibly binding to bacterial isoleucyl-tRNA synthetase. It has excellent activity against staphylococci, most streptococci, and some gram-negative organisms, including Neisseria gonorrhoeae, Haemophilus influenzae, and Moraxella catarrhalis³⁵. There are two different formulations of mupirocin, depending on the vehicle. The first is a nasal ointment in petrolatum. The second is a generic topical ointment that utilizes a polyethylene glycol vehicle. Both have been used for nasal decolonization; however, the generic topical ointment may be used more frequently due to its lower cost. Side effects are uncommon and are mostly local site reactions such as stuffy nose or burning or stinging of the nose.
A randomized control trial (RCT) comparing mupirocin against a placebo found that 83% of the mupirocin group were decolonized, compared with only 27% of the placebo group (P =0.001). That trial also found that 81% of carriers who received three to five doses of mupirocin were decolonized, compared with 93% of carriers who received six or more doses of mupirocin (P =0.001)³⁴. Currently, mupirocin is recommended to be applied to the anterior nares twice daily for 5 days.
A systematic literature review evaluated 23 clinical trials, including 12 trials that evaluated topically applied antibiotics. The authors concluded that short-term nasal mupirocin was the most effective treatment for MRSA decolonization, with success rates of 90% at 1 week after treatment and approximately 60% after a longer follow-up time¹⁰. The effectiveness of mupirocin was similar for both MSSA and MRSA carriers. A Cochrane review aimed to determine whether the use of mupirocin among S. aureus carriers reduced S. aureus infections. Only RCTs comparing a mupirocin group with a control group that received either no treatment, placebo, or an alternative nasal treatment were included. The authors found that mupirocin was associated with a significant reduction in S. aureus infections (relative risk [RR] 0.55; 95% confidence interval [CI], 0.43 to 0.70)³⁷. Lastly, two systematic literature reviews and meta-analyses of published studies found a protective effect of mupirocin decolonization against surgical site infections (SSIs), especially among nonsurgical patients such as cardiac surgery, orthopedic surgery, and neurosurgery³⁸,³⁹.
Although mupirocin appears to be a very effective topical agent, resistance among S. aureus has now been identified in multiple studies, especially with widespread use over prolonged periods⁴⁰,⁴¹. More importantly, studies have shown that high-level mupirocin-resistant (HL-MR) S. aureus results in decolonization failure. The association between low level mupirocin (LL-MR) and failure of mupirocin decolonization is unclear. Walker et al.⁴² published a prospective study to determine the efficacy of nasal mupirocin in decolonizing patients with mupirocin-susceptible MRSA (MS MRSA) and mupirocin resistant MRSA, both LL-MR MRSA and HL-MR MRSA. Patients received 2% mupirocin nasally twice daily for 5 days. They were then cultured at day 3 and weeks 1, 2, and 4 after treatment. Nares cultures at day 3 posttreatment were negative for 79% of patients who had MS MRSA, 80% of patients who had LL-MR MRSA, and 28% of patients who had HL-MR MRSA. However, at the follow-up 1 to 4 weeks later, the sustained decolonization for patients with HL-MR MRSA and LL-MR MRSA was low (25% each, compared to 91% in patients colonized with MS MRSA). This result suggests that mupirocin probably temporally suppresses growth of LL-MR MRSA but does not result in sustained decolonization. Posttreatment cultures usually had the same genotype and susceptibility phenotypes as the corresponding baseline cultures. This appears to show endogenous recolonization rather than exogenous acquisition.
Perl et al. treated over 2,000 patients with mupirocin, performed mupirocin susceptibility testing, and found that only 6 of the 1,021 isolates (0.6%) were mupirocin resistant³⁶. Another study described the results of repeated point-prevalence surveys over 4 years to determine if mupirocin resistance had emerged in surgical units using preoperative prophylaxis with 5 days of nasal mupirocin. They found no evidence of sustained emergence or spread of mupirocin resistance. No HL-MR strains were identified⁴³. Finally, a Dutch study evaluated over 20,000 patients who received mupirocin prophylaxis for S. aureus decolonization. No difference in the rates of mupirocin were done over a decade ago. More recently Hayden et al.⁴⁴ evaluated mupirocin susceptibility of MRSA in the REDUCE-MRSA Trial. Isolates from the baseline and intervention periods were collected and tested for susceptibility to mupirocin by Etest. At baseline, 7.1% of MRSA isolates expressed low-level mupirocin resistance, and 7.5% expressed high-level mupirocin resistance. The study found the odds of mupirocin-resistance were no different in the intervention versus baseline periods across arms, but confidence limits were broad and therefore, results should be interpreted with caution. However, a recent meta-analysis described a global increase in the prevalence of high-level mupirocin resistance among clinical S. aureus isolates over time⁴⁵. In another study Semret et al. found topical mupirocin was able to interrupt colonization of 52% and 68% of methicillin-resistant Staphylococcus aureus (MRSA)-colonized patients carrying mupirocin-resistant and -sensitive strains, respectively, including 44.4% and 85.7% of those colonized only in the nares⁴⁶. Although a trend decreased effectiveness was seen for clearing mupirocin-resistant MRSA, this agent can still decolonize many patients with resistant strains. They used higher doses of mupirocin, four times daily for 2 weeks, or until discharge.
In a new large-scale pragmatic study, the Mupirocin-Iodophor ICU Decolonization Swap Out Trial, the investigators compared nasal mupirocin to nasal iodophor along with universal CHG bathing⁴⁷. In this noninferiority, cluster-randomized trial of 801,668 admissions at 137 hospitals, exposure to nasal mupirocin significantly reduced S. aureus clinical cultures by 18.4% compared with iodophor in adult ICUs in the setting of universal daily chlorhexidine bathing. The sustained effectiveness of mupirocin plus CHG bathing over 7 years of use since the REDUCE MRSA trial⁴⁸, even in the setting of reports of rising and high rates of mupirocin resistance is reassuring. In summary, although mupirocin nasal therapy may be an option for S. aureus nasal decolonization, the use of mupirocin may lead to mupirocin resistance and treatment failures. Thus, alternatives to mupirocin for eradication of patients colonized with S. aureus may be needed, however, mupirocin remains the gold standard.
POVIDONE-IODINE
Povidone-iodine (PI) is a complex of polyvinylpyrrolidine and tri-iodine ions that has been widely used as an antiseptic on skin, wounds, and mucous membranes. PI has activity against both gram-positive and gram-negative bacteria. Specifically, PI has activity against both MSSA and MRSA. Hill and Casewell⁴⁹ assessed the in vitro activity of 5% PI as an alternative to mupirocin for the nasal decolonization of S. aureus. In that study, PI was able to eliminate 11 test organisms, including both mupirocin sensitive and mupirocin-resistant MRSA. The results suggested that PI may be a good decolonizing agent for the prevention of infections due to S. aureus, including MRSA and mupirocin-resistant strains, however, the addition of nasal secretions in vitro reduced the PI activity. This concern was confirmed by Rezapoor et al.⁵⁰ who published a randomized, placebo-controlled study comparing 10% off-the-shelf PI, 5% PI-based nasal antiseptic (PINA), or saline (placebo) for nasal decolonization. Four hundred and twenty-nine patients undergoing primary or revision total joint arthroplasty, femoroacetabular osteoplasty, pelvic osteotomy, or total shoulder arthroplasty were included. Baseline nasal cultures were taken immediately preoperatively, followed by treatment of both nares twice for 2 minutes with 4 applicators. Reculturing of the right nostril occurred at 4 hours and the left at 24 hours. Ninety-five of the 429 patients (22.1%) had a positive culture result for S. aureus. Of these 95, 29 were treated with off-the-shelf PI, 34 with PINA, and 32 with saline swabs. At 4 hours post-treatment, S. aureus culture was positive in 52% off-the-shelf PI patients, 21% PINA patients, and 59% saline patients. After 24 hours posttreatment, S. aureus culture was positive in 72% off-the-shelf PI patients, 59% PINA patients, and 69% saline patients. PI was significantly better than saline at 4 hours, but there was no significant difference at the 24-hour time interval (P = .003). The authors concluded off-the-shelf PI swabs may not be as effective at 4 hours as the specifically manufactured product for nasal S. aureus decolonization. There are now currently 3 nasal iodophor antiseptics (5% and 10%) with properties which enable better adherence to nasal mucosa.
Phillips et al. performed a prospective, open-label trial of twice-daily nasal mupirocin for 5 days before surgery compared to two applications of a 5% nasal PI solution within 2 hours of surgical incision in patients undergoing arthroplasty or spine fusion surgery. Both groups also received CHG baths, with 2% cloths, the night before and the morning of surgery. If the preoperative nasal culture was positive for S. aureus, another nasal culture was obtained within 1 to 3 days after surgery. The proportion of postoperative negative nasal cultures was 92% (78 of 85 patients) for those assigned to mupirocin versus 54% (45 of 84 patients) for those assigned to PI⁵¹. In a recent study Saidel-Odes et al.
the evening before and the morning prior to surgery was very effective in achieving S. aureus eradication. However, when there was a high S. aureus nasal colony count preoperatively, nasal PI application on the evening before and the morning prior to surgery was less effective in achieving S. aureus eradication⁵². In the study discussed under mupirocin, exposure to nasal mupirocin significantly reduced S. aureus clinical cultures by 18.4% compared with iodophor in adult ICUs in the setting of universal daily chlorhexidine bathing. However nasal iodophor was better than no intranasal intervention⁴⁷. Lastly, in a proof-of-concept study, the investigators set out to determine whether nasal decolonization with PI diminishes the utility of polymerase chain reaction (PCR)–based nasal MRSA screening. All baseline PCR-positive results were confirmed via culture. Follow-up MRSA PCR tests were obtained after 4–6 days, immediately prior to intranasal PI application, or at least 8 hours after a subsequent application. All follow-up PCRs remained positive. They concluded that PCR remained highly sensitive for nasal MRSA colonization even after multiple applications of PI and most patients remained culture positive after 4–13 applications raises concerns that PI may be less effective than mupirocin for clearing nasal colonization⁵³. In summary, PI may be an alternative to mupirocin, but PI may not be as effective as mupirocin at eradicating intranasal S. aureus.
ALCOHOL-BASED NASAL ANTISEPTIC
Alcohols are antimicrobial by denaturing proteins. Alcohol has bactericidal activity against most gram-positive and gram-negative bacteria, including MDROs. Alcohol concentrations between 60 and 90% are most effective, but duration of activity is short. Most alcohol-based hand antiseptics contain either isopropanol or ethanol⁵⁴. Steed et al. published a double-blinded, placebo controlled RCT testing the effectiveness of an alcohol-based nasal antiseptic in reducing S. aureus nasal colonization in colonized health care workers. Health care workers testing positive for nasal S. aureus colonization were treated three times during the day with a nasal alcohol-based antiseptic or placebo. The antiseptic formulation contained 70% ethanol combined with natural oil emollients and the preservative benzalkonium chloride. Nasal S. aureus and total bacterial colonization levels were determined before and at the end of a 10-hour shift. Antiseptic treatment reduced S. aureus colony forming units (CFUs) from baseline by 82% (mean) and 99% (median) (P= 0.001)⁵⁵.
In research brief Kanwar et al. conducted 2 nonblinded trials to determine effectiveness of single application of two different doses of a commercially available alcohol product (Nozin® Nasal Sanitizer) to reduce nasal MRSA in colonized patients⁵⁶. In the first trial a single application was applied in each nostril. In the second trial 3 separate applications were applied intranasally over 3 minutes. The control group received intranasal saline. For all participants cultures were collected from the nares prior to and 10 minutes, 2 hours, and 6 hours after treatment. A separate group of participants (n= 23) also applied 3-single dose applications at 0, 4, and 8 hours. Nares cultures were collected at baseline and 2 hours after the third dose. The single-dose application was associated with a nonsignificant trend toward reduced mean MRSA concentrations in the treatment group versus the control group at 10 minutes and at 2 hours after dosing. The triple-dose application significantly reduced mean MRSA concentrations in comparison to controls at 10 minutes and at 2 hours after dosing, but not at 6 hours after dosing. MRSA was frequently recovered from the clothing (19 of 23, 83%) and skin (17 of 23, 74%) of participants. For repeated dosing of the alcohol-based nasal sanitizer over 8 hours there was no reduction in MRSA 2 hours after the final dose in comparison to baseline (mean ±SE, 2.3 ±0.43 vs 2.3 ±0.78 log₁₀ colony forming units per swab; P > .05). Bottom line: single-dose application of alcohol-based sanitizer did not significantly reduce nasal MRSA and triple-dose application only transiently reduced MRSA.
daily application of single dose postoperatively and for ICU decolonization. Based on the Kanwar study, it may be possible that frequent high dose alcohol may be adequate to suppress S. aureus. Therefore, more studies are needed to determine the optimal concentration and frequency to eradicate colonization of S. aureus.
PHOTODYNAMIC THERAPY (PDT)
The use of a light source, such as a laser, has been suggested as an alternative method to eliminate S. aureus nasal carriage. Photodynamic therapy (PDT) consists of the combination of a light-activated chemical and UV or infrared wavelengths. This combination creates free radicals that damage bacterial cell walls and membranes. In preliminary human testing, PDT eradicated nasal MRSA, with total treatment times of less than 10 min⁵⁷. In a small cohort study, Bryce et al.⁵⁸ found that the colonization rates for MSSA and MRSA were 24.4% and 0.9%, respectively, before PDT therapy. Of those who received PDT (0.1% methylene blue gel applied to anterior nares as measured by semiquantitative colony counts. Street and colleagues used a methylene blue- and CHG–based photosensitizer formulation⁵⁷. That study evaluated the efficacy of using PDT for nasal MRSA decolonization at the preclinical and clinical levels. Preclinical testing was done in a custom nasal reservoir model and on human skin cultures colonized with MRSA. Human clinical testing was also performed. Using full-thickness skin cultures, they performed photodynamic treatment comparisons with either methylene blue or CHG alone or the combination of methylene blue and CHG. They found that the combination formulation using both methylene blue and CHG was much more effective than either methylene blue or CHG alone. Application of methylene blue or CHG alone with illumination led to some reduction in MRSA viability compared with that for the control (0.2-log10 and 1.1-log10 reductions, respectively) immediately posttreatment. In contrast, PDT treatment using a combination of methylene blue and CHG produced a statistically significant 5.1-log10 reduction compared with the nontreated control and a rapid antibacterial effect. In addition, the combination produced sustained decolonization that persisted for up to 5 days. Embleton et al. demonstrated that a monoclonal antibody conjugate targeting MRSA, when exposed to red light, selectively eliminated MRSA in all growth phases while not harming Escherichia coli and Staphylococcus epidermidis. This suggested that PDT may protect normal nasal microbiota while eliminating the target organism⁵⁹. Lastly in a small study, patients on maintenance hemodialysis who were nasal carrier of S. aureus were randomly assigned to decolonization with a single application of PDT or with a topical mupirocin regimen (twice a day for 5 days)⁶⁰. Nasal swabs were collected at time 0, directly after treatment completion, 1 month after treatment, and 3 months after treatment. Participants receiving photodynamic therapy had cultures that were negative for S. aureus (P=0.9). Of the patients who had negative culture directly after completion of the mupirocin therapy, 67% were recolonized within 3 months and 54% of mupirocin patients were recolonized after 3 months. PDT is another promising approach for nasal S. aureus decolonization, but larger clinical trials are needed to evaluate the impact on clinically significant infections.
Topical Agents
CHLORHEXIDINE GLUCONATE
Chlorhexidine, a topical antiseptic, has been used throughout the world for decades. Chlorhexidine gluconate (CHG) is a cationic biguanide that works by binding to bacterial cell walls, which alters the osmotic equilibrium of the bacterial cell. CHG has activity against Gram-positive and Gram-negative bacteria and yeasts. CHG has an excellent safety record. Adverse events associated with CHG are mild skin irritation and rare serious allergic reactions⁶¹.
CHG efficacy has been documented for diverse indications, including handwashing, procedure skin preparation, vaginal antisepsis, gingivitis treatment, and body washes for infection prevention. CHG is available in a wide range of concentrations (0.5% to 4%) and formulations. CHG can be used on its own or combined with ethanol or isopropyl alcohol. Some CHG products are also sold over the counter. This review focuses only on the use of CHG to prevent HAIs.
In 1991, a study demonstrated that CHG alcohol disinfection of the central line site before insertion was associated with a significant reduction in central-line-associated infections compared with 10% PI or 70% alcohol⁶². The use of CHG alcohol has now become the standard of care for site preparation and maintenance.
Recently, multiple studies have evaluated the use of CHG bathing to decrease the bacterial burden on the skin of ICU patients to reduce HAIs. CHG bathing can decrease the bioburden of bacteria and yeasts on patients, the hospital environment, and the hands of health care workers⁶³. Bleasdale et al. observed a 60% reduction in BSIs among medical ICU patients who were bathed with 2% CHG cloths compared to those who received soap and water⁶⁴.
Borer et al. examined the association between 4% CHG liquid body wash use and multidrug-resistant Acinetobacter baumannii skin colonization and BSIs in the medical ICU. Patients underwent CHG bathing immediately after obtaining initial cultures. Seventeen percent of patients were colonized with Acinetobacter baumannii on admission, 5.5% at 24 h, and 1% at 48 h (P = 0.002). The prevalence of Acinetobacter baumannii BSIs decreased from 4.6 to 0.6 per 100 patients, and the incidence decreased from 7.6 to 1.25 (85% reduction)⁶⁵.
In 2013 three randomized cluster trials on the topic of CHG bathing among ICU patients were published. One cluster-crossover study reported that daily 2% CHG cloth bathing in the ICU resulted in a 23% reduction of VRE and MRSA acquisition and a 28% reduction in BSIs⁶⁶. In another study of pediatric ICU patients, Milstone et al. found a significant association between 2% CHG cloth bathing and a decline in BSIs compared with standard bathing⁶⁷. Another trial, called the REDUCE MRSA study, cluster randomized 74 adult ICUs to evaluate three MRSA prevention interventions: the first cluster implemented MRSA screening and isolation, the second cluster included screening, isolation, and decolonization of MRSA carriers with CHG bathing and nasal mupirocin (i.e., targeted decolonization), and the ICUs in the third cluster did not screen any patients but instead all patients were decolonized with CHG cloth bathing and nasal mupirocin (i.e., universal decolonization). Universal decolonization was found to be associated with the greatest decrease in all-cause BSIs (44%; P <0.001) and rates of MRSA clinical cultures (37%; P = 0.01)⁶⁸. In a secondary analysis, CHG bathing was also shown to reduce blood culture contamination by 45% (P = 0.02), confirming earlier studies²⁹. In a follow-up study, implementation of universal decolonization of ICU patients across a large health care system resulted in a decrease rate of CLABSIs by 23.5% (P<.001)⁶⁹.
In 2014, a European quasi-experimental study found that universal daily CHG cloth bathing in the medical ICU could decrease acquisition of MDROs. That study found that this intervention was associated with a significant decline in MDROs. Then, in a subsequent cluster randomized trial, they found that the addition of rapid screening and isolation did not lead to a further decline in MDROs⁷⁰.
In 2015, Noto et al. published a cluster-randomized crossover study of five different ICUs in a single academic institution⁷¹. ICUs were randomized to bathing with either CHG or nonantimicrobial cloths for 10 weeks, and then there was a 2-week washout period, after which ICUs were crossed over to 10 weeks of the other bathing treatment. The study evaluated a composite outcome of CLABSIs, VAP, CAUTIs, and CDI. This study also evaluated MDRO clinical culture rates, blood culture contamination, and health care-associated BSIs. Unlike in the previous trials, CHG did not reduce the incidence of HAIs. The findings in this study need to be interpreted considering several limitations. For one, the study did not monitor adherence to the bathing protocol, so it is possible that the lack of benefit reflected inadequate bathing. Second, two of five units were already using CHG. Third, the intervention was only 10 weeks long. It takes a minimum of several weeks to ramp up to ensure adequate training and compliance; thus, many patients may not have received adequate CHG bathing during the intervention periods. Fourth, for two of the HAIs in the composite outcome, VAP and CDI, one would not expect reductions due to the use of CHG. Fifth, the study was conducted at a single center. Lastly, the baseline rates of hospital-acquired infections were low before the study was started, so it may not have been statistically powered to see a difference.
The role of CHG bathing among patients in non-ICU settings has been addressed in two recent trials. The first was the ABATE Infection (active bathing to eliminate infection) trial which was a cluster-randomized trial of 53 hospitals comparing routine bathing to decolonization with universal chlorhexidine and targeted nasal mupirocin in noncritical care units⁷². They found that universal decolonization did not reduce infection in the overall population, but in post-hoc analyses of patients with medical devices (central lines and midlines) the regimen was associated with significant reductions in all-cause BSIs and MRSA or VRE clinical cultures. In the second trial adults with hematological malignancies hospitalized for chemotherapy in noncritical care units were offered daily 2% CHG bathing⁷³. They compared outcomes of patients who chose CHG bathing (CHG group) with outcomes of those who did not choose CHG bathing (usual-care group). The primary outcome was gram-positive cocci–related, skin flora–related, or CLABSIs. The CHG bathing group was associated with a 60% decrease in the primary outcome (adjusted hazard ratio [HR], 0.4; P < .001). However, in a multicenter cluster-randomized controlled trial examining the effect of daily bathing with CHG, octenidine, or soap and water demonstrated a lack of significant preventive effect on CLABSIs in ICUs. However, the trial was underpowered due to a CLABSI rate in the routine care group that was approximately 40% lower than initially assumed⁷⁴.
Recently the CDC has proposed a new measure Hospital-Onset Bacteremia and Fungemia (HOB) which would require hospitals to expand surveillance with the intent on broader reduction of BSIs regardless of causative organism or association with a medical device. Investigators have suggested that HOB is a more inclusive measure than CLABSIs⁷⁵,⁷⁶. This is supported by a recent study that demonstrated non-CLABSI HOB events occur in substantially greater numbers than CLABSI events occurring in a 79% to 21% ratio, respectively, in an observational study of patients in 41 acute-care hospitals conducted over a four-year period; additional findings indicated that 68% of the non-CLABSI HOB events were not related to secondary culture sources such as urinary or respiratory sites, and therefore may have attribution to use of vascular access devices⁷⁷. There has been a growing awareness that peripheral IV catheters (PIVCs) can also cause significant morbidity and mortality. In a recent review, investigators estimated that more than a billion PIVCs are inserted annually worldwide and have found that PIVCs placed in the upper extremity rather than central lines. It is unclear whether CHG bathing with or without an intranasal agent can impact PIVCs BSIs⁷⁸. Just published is a review on prevention of vascular-associate HOB⁷⁹. The review outlines the evidence-based interventions including compliance with appropriate proper skin antisepsis and CHG bathing for high-risk patients.
Based on current evidence, the updated Compendium on “Strategies to Prevent Central Line Associated Bloodstream Infections in Acute-care Hospitals: 2022 Update” continues to recommend CHG bathing in the ICU for patients 2 months and older⁸⁰. The Compendium stated the role of CHG bathing among patients in non-ICU settings was considered unclear despite several good studies discussed above. There is also a need to determine if the widespread use of CHG-based products promotes reduced CHG activity. Testing for CHG susceptibility is currently not standardized and the clinical impact of reduced chlorhexidine susceptibility in bacteria is unknown and not yet well-defined. The future Compendium will hopefully address prevention of HOB not just CLABSI.
POVIDONE-IODINE
PI has broad activity against both Gram-positive and Gram-negative bacteria. PI is applied topically in concentrations from 4% to 10%. It is well tolerated; however, it may cause mild skin irritations and most preparations are inactivated by organic matter. PI has a more rapid bactericidal effect than CHG, but povidone-iodine has not been shown to have a persistent effect like CHG⁶¹. CHG is recommended over 10% iodine solutions for catheter placement because CHG is associated with a lower risk of infection⁶². Another study compared a CHG preparation to a povidone-iodine preparation for surgical scrub use. The authors found that CHG had more persistent activity than povidone-iodine⁸². In a recent trial patients who were undergoing surgical fixation of a closed fracture of a lower limb or the pelvis, found that the risk of SSIs was lower with skin antisepsis provided by iodine povacrylex in alcohol than with antisepsis provided by chlorhexidine gluconate in aqueous alcohol. Iodine povacrylex is an iodophor that is available in alcohol and unique due to its copolymer, povacrylex and is not inactivated by organic matter. In another recent trial PI in alcohol as preoperative skin antisepsis was noninferior to chlorhexidine gluconate in alcohol in preventing SSIs after cardiac or abdominal surgery⁸⁴. The recent Compendium Strategies to Prevent Surgical Site Infections in Acute-care Hospitals: 2022 Update recommended using alcohol-containing preoperative skin preparatory agents if no contraindication exists⁸⁵. Although PI has broad-spectrum properties, it may not be the ideal agent for bathing due to a lack of evidence for persistence, but an iodophor alcohol preparation may be a good alternative agent for preoperative skin site preparation.
Selective Digestive or Oropharyngeal Decontamination
SELECTIVE DIGESTIVE DECONTAMINATION AND SELECTIVE OROPHARYNGEAL DECONTAMINATION
Decontaminations of the upper respiratory and digestive tracts are interventions designed to decrease colonization with pathogenic Gram-negative organisms and infections in critically ill patients. These interventions include selective digestive decontamination (SDD) and selective oropharyngeal decontamination (SOD). SDD is performed by application of nonabsorbable antibiotics to the oropharynx and digestive tract. These nonabsorbable antibiotics include tobramycin, polymyxin, and amphotericin, as well as a short course of intravenous antibiotics such as cefotaxime. Oropharyngeal antibiotics are administered as a paste, while the gastric antibiotics are administered as a suspension down the nasogastric tube. SOD consists of the application of topical antibiotics to the oropharynx alone, and intravenous antibiotics are not given.
A 2007 meta-analysis evaluated the association between oral decontamination and the incidence of VAP⁸⁶. It included 11 trials involving 3,242 mechanically ventilated patients that used an antiseptic or antibiotic application of either antibiotics or antiseptics or pooled, it was found that both methods of oral decontamination were associated with decreased risk of VAP (RR 5 0.61; 95% CI, 0.45 to 0.82); however, significant differences for duration of mechanical ventilation, ICU length of stay, or mortality were not seen. In 2009, a cluster randomized crossover study was performed in 13 ICUs located in the Netherlands to evaluate the effectiveness of SDD and SOD⁸⁷. Each ICU was randomized to implement SDD, SOD, and standard care in a random order over a 6-month period. In a logistic regression model, the SOD and SDD groups had lower odds of death at 28 days than the group that received standard care (SOD: OR 5 0.86; 95% CI, 0.740 to 0.99; and SDD: OR 5 0.83; 95% CI, 0.72 to 0.97). For patients receiving SDD or SOD, ICU BSIs were statistically significantly lower for S. aureus and nonfermenting Gram-negative organisms, especially Pseudomonas aeruginosa and Enterobacteriaceae, than with standard care. In another follow-up study, SOD and SDD were also associated with decreased rates of bacteremia and colonization of the respiratory tract.
with antibiotic-resistant Gram-negative bacteria among patients admitted to the ICU for greater than 3 days⁸⁸. That study included 47 episodes of acquired BSI that were caused by highly resistant organisms. BSIs acquired in ICUs and caused by highly resistant pathogens were 59% less frequent with SDD than with standard care and 63% less frequent with SDD than with SOD. In a later large trial also in the Netherlands, the authors compared SDD to SOD in regard to antibiotic resistance and patient outcomes⁸⁹. They reported that both SDD and SOD were associated with low levels of antibiotic resistance and that there was no difference in 28-day mortality. Compared with SOD, SDD was associated with decreased rates of BSIs acquired in the ICU and rectal colonization of multidrug-resistant Gram-negative organisms. However, SDD was also associated with an increase in aminoglycoside-resistant Gram-negative colonization. The reduction in BSIs was more pronounced for Enterobacteriaceae (OR 5 0.42; 95% CI, 0.31 to 0.97). Price et al. published a systematic review and meta-analysis evaluating SDD, SOD, and topical CHG compared to standard care or placebo to determine the association with mortality in adult patients in general ICUs⁹⁰. SDD was protective against mortality, with a pooled OR of 0.73 (95% CI, 0.64 to 0.84). SOD was also associated with decreased mortality with a pooled OR of 0.895 (95% CI, 0.74 to 0.97). CHG was associated with higher mortality (OR 5 1.25; 95% CI, 1.05 to 1.5) which in part is why in the recent Compendium update on “Strategies to Prevent Ventilator-associated Pneumonia, Ventilator-associated Events, and Nonventilator Hospital-acquired Pneumonia in Acute-care Hospitals: 2022 Update” states that oral care with CHG is no longer recommended in prevention of ventilator-associated pneumonia (VAP)⁹¹.
There remains concern that SDD or SOD may result in selection of resistant organisms. Daneman et al. published a systematic review and meta-analysis on the effect of selective decontamination on antimicrobial resistance⁹². They were unable to detect an association between SDD or SOD and antimicrobial resistance in ICU patients. However, they did admit that the association between decolonization and antimicrobial resistance in the ICU setting needs more research.
More recently, a meta-analysis of 6 cluster randomized trials performed in countries with low levels of antibiotic resistance reported that SOD was associated with a 16% reduction in hospital mortality, and SDD was associated with an 18% reduction in hospital mortality⁹³. SDD was more effective than SOD (OR, 0.90; 95% CI, 0.82–0.97 for hospital death).
Finally, a recent systematic review and meta-analysis reviewed the use of SDD in prevention of VAP⁹⁴. Among adults in the ICU treated with mechanical ventilation, the use of SDD compared with standard care or placebo was associated with significantly lower ICU BSI rates, especially Pseudomonas aeruginosa and Enterobacteriaceae, than with standard care. In another follow-up study, SOD and SDD were also associated with decreased rates of bacteremia and colonization of the respiratory tract.
On a different note, several studies have examined the role of SDD in reducing colonization, infections, and outbreaks caused by multidrug resistant Gram-negative bacteria. Huttner et al. performed a double-blinded, placebo-controlled RCT to evaluate the efficacy of oral colistin, neomycin, and nitrofurantoin to reduce intestinal colonization with ESBL-producing Enterobacteriaceae⁹⁵. This regimen temporarily suppressed ESBL-producing Enterobacteriaceae during and immediately after treatment, but the authors documented a rebound only 1 week after ending treatment.
Saidel-Odes et al. performed a blinded RCT comparing placebo to oral gentamicin and oral polymyxin gel plus oral solutions of gentamicin and polymyxin for 7 days to eradicate carbapenem-resistant Klebsiella pneumoniae (CRKP)
oropharyngeal and gastrointestinal carriage⁹⁶ After 2 weeks, the proportion of rectal cultures that were negative for CRKP was significantly improved in the intervention group (16% in the placebo group versus 61% in the intervention group [OR = 0.13; 95% CI, 0.02 to 0.74; P <0.0016]). A difference was still maintained at 6 weeks (33.3% in the placebo arm and 58.5% in the intervention arm), but it was not statistically significant. Secondary resistance to gentamicin or colistin was not observed in any of the SDD-treated patients. In another study, nonabsorbable oral antibiotics were administered for up to 60 days or until decolonization was documented in patients colonized with CRE⁹⁷. Oral gentamicin or oral colistin was used based on the susceptibility of the isolate. Patients with isolates sensitive to both colistin and gentamicin were randomized to receive either colistin or gentamicin or both. Patients with isolates resistant to one of the agents were not provided with SDD but were followed for spontaneous clearance of colonization. Rates of eradication across the treatment groups (gentamicin, colistin, or both) were 42%, 50%, and 37.5%, respectively, each significantly higher than the 7% spontaneous clearance in the control group (P <0.001, P<0.001, and P=0.004, respectively). However, there was no significant difference between the three treatment groups. Mortality in patients who achieved eradication (either spontaneously or by SDD) was significantly lower than that in patients where eradication failed (17% versus 49%, respectively; P = 0.002). Secondary resistance developed in 7 of the 50 SDD-treated patients, gentamicin resistance in 6 of 26 gentamicin-treated patients, and colistin resistance in 1 of 16 colistin-treated patients.
FECAL MICROBIOTA TRANSPLANT
Small studies have shown some effect of fecal microbiota transplant (FMT) for MDRO decolonization. A systematic review in 2021 including found FMT decolonization rates between 20%–90%, and they were slightly higher for carbapenem-resistant Enterobacteriaceae than for VRE; this review further noted decreased MDRO infections with FMT even with decreased MDRO colonization⁹⁸. However, they conclude questions remain regarding the true efficacy of FMT and observed decrease in MDRO infections post. They recommend that FMT warrants further research. In 2022 the authors did a systematic review on use of FMT for CRE⁹⁹. FMT was effective to eradicate CRE in 78% of cases and FMT was safe and well tolerated even in immunocompromised patients. They also recommended more studies, especially randomized trials, are needed to validate the safety and clinical utility of FMT for CRE eradication. Huttner and colleagues randomized 39 patients to either 5 days of oral nonabsorbable antibiotics followed by frozen FMT or control in patients colonized with extended-spectrum β-lactamase (ESBL-E) and/or carbapenemase-producing Enterobacteriaceae (CPE)¹⁰⁰. Non-absorbable antibiotics followed by FMT slightly decreased ESBL-E/CPE carriage compared with controls, but this difference was not statistically significant. Study drugs were well tolerated overall but three patients in the antibiotic-only group discontinued the oral antibiotics because of diarrhea (all received FMT).
Lastly Woodworth et al. conducted a randomized, controlled trial of FMT for MDRO decolonization in renal transplant recipients¹⁰¹. Eleven participants were enrolled and randomized 1:1 to FMT or an observation period followed by delayed FMT if stool cultures were MDRO positive at day 36. Participants who were MDRO positive after one FMT were treated with a second FMT. At the last visit, eight of nine patients who completed all treatments were MDRO culture negative. FMT-treated participants had longer time to recurrent MDRO infection versus controls who were not treated with FMT. Bottom line: More studies, especially randomized trials, are needed to validate the safety and clinical utility of FMT for MDRO eradication.
Decolonization to prevent surgical site infections
There is strong evidence that nasal and skin decolonization prior to cardiac and orthopedic
There is strong evidence that nasal and skin decolonization prior to cardiac and orthopedic surgery is effective at preventing SSIs especially caused by Gram-positive organisms that are susceptible to mupirocin and CHG. This is because SSIs are often endogenous, spreading from one body site (e.g., nose or skin) to the surgical wound of the same patient. Multiple studies have demonstrated that the genotypes of S. aureus colonizing and infecting isolates are identical in 75% to 85% of surgical patients¹⁰². A meta-analysis of 17 RCTs or quasi-experimental studies that included cardiac and orthopedic surgery patients evaluated the effectiveness of preoperative decolonization³⁸. The meta-analysis found that decolonization was significantly protective against Gram-positive SSIs, specifically S. aureus SSIs. Decolonization has been effective against both MRSA and MSSA SSIs. One of the larger RCTs included in that meta-analysis was performed in the Netherlands, which has a low incidence of MRSA¹⁰². That study used PCR to rapidly identify S. aureus carriers and nasal mupirocin ointment for those carriers via an intranasal application for 5 days. They found a greater-than-2-fold decline in S. aureus infections and more than a 4-fold decline in S. aureus deep SSIs.
Another large quasi-experimental study included in the meta-analysis prospectively evaluated 992 consecutive open heart surgery patients who did not receive mupirocin prophylaxis in the 22-month preintervention period. They then began providing open heart surgery patients with intranasal mupirocin and CHG bathing on the night before and morning of surgery, as well as mupirocin twice daily for 5 days postoperatively. This intervention group of 854 consecutive patients was followed prospectively for the 16-month intervention period. The rate of sternal wound infections decreased significantly from 2.7% (27 of 992) in the preintervention group to 0.9% (8 of 854) in the intervention group (P = 0.005)¹⁰³.
A more recent pragmatic quasi-experimental study implemented a bundled intervention in 20 hospitals in order to prevent S. aureus SSIs after cardiac surgery and hip and knee arthroplasty¹⁰⁴. The bundle included CHG bathing for all patients, screening for MRSA and MSSA nasal colonization, and providing vancomycin and cefazolin perioperative prophylaxis for MRSA carriers. (Figure 1)
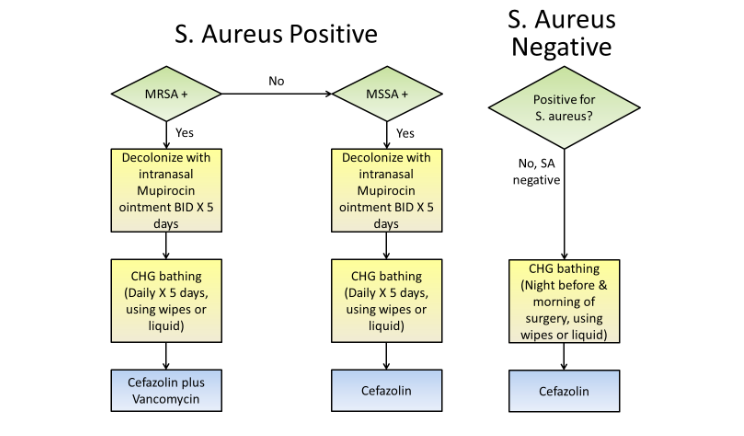
The mean rate of complex S. aureus SSIs significantly decreased from 36 infections per 10,000 operations during the baseline period to 21 infections per 10,000 operations during the intervention period (rate ratio = 0.58; 95% CI, 0.37 to 0.92). A meta-analysis by Kallen et al. aimed to determine whether intranasal mupirocin decolonization could prevent SSIs caused by any
pathogen³⁹. They categorized surgery into nongeneral surgery and general surgery. They hypothesized that general surgical procedures, especially those that involve the bowel, would be more likely to be associated with SSIs caused by organisms that are not susceptible to mupirocin (e.g., Gram negative or anaerobic organisms), and thus attenuate the effect of mupirocin. Mupirocin use among nongeneral surgery patients (e.g., those undergoing cardiothoracic surgery, neurosurgery, or orthopedic surgery) was associated with a reduction in SSIs. Conversely, mupirocin use among general surgery patients (e.g., those undergoing gastrointestinal, oncologic, or gynecologic surgery) did not reduce SSIs. Thus, mupirocin decolonization is recommended for clean nongeneral procedures but not for general surgical procedures that are associated with contamination from the gastrointestinal tract during the procedure.
Phillips et al.⁵¹ performed a prospective, open-label trial of twice-daily nasal mupirocin for 5 days before surgery compared to two applications of a 5% nasal PI solution within 2 hours of surgical incision in patients undergoing arthroplasty or spine fusion surgery. Both groups also received CHG baths, with 2% cloths, the night before and the morning of surgery. A total of 763 surgical procedures were evaluated among patients who received mupirocin and 776 surgical procedures among patients who received PI. In the per-protocol analysis, S. aureus deep SSIs developed in five patients (0.66%) who received mupirocin and zero patients (0.00%) among those who received PI (P < 0.03). In addition, if the preoperative nasal culture was positive for S. aureus, another nasal culture was obtained within 1 to 3 days after surgery. The proportion of postoperative negative nasal cultures was 92% (78 of 85 patients) for those assigned to mupirocin versus 54% (45 of 84 patients) for those assigned to PI. The authors commented that this was not unexpected, since mupirocin was intended to eradicate colonization while PI was intended only to suppress S. aureus around the perioperative period.
This study has several limitations. First, the investigators could not perform multivariate analysis due to the small sample size. Second, patients were not followed after discharge to identify late infections.
Bebko and colleagues¹⁰⁵ published a second study using a preoperative decontamination protocol to reduce SSIs in orthopedic patients undergoing elective hardware implantations. This was a quasi-experimental, retrospective, nonrandomized trial comparing a bundled intervention to historical controls. The intervention consisted of application of 2% CHG and oral CHG the night before and morning of surgery plus an intranasal PI solution the morning of surgery. Patients were evaluated for SSI for the 30 days after their surgery date. Rates of SSIs were statistically significantly lower in the intervention group than in the control group (1.1% versus 3.8%; P <0.02). However, that study was limited because it was not a randomized trial, patients were only followed for 30 days, and screening regarding the MRSA carrier status of patients before and after decontamination was not collected; therefore, the study did not allow for evaluation of the effect of nasal decolonization alone versus other interventions.
In a study published earlier this year, Saidel-Odes et al. in a retrospective case control study studied preoperative intranasal PI application in conjunction with CHG bathing to reduce S. aureus colonization and SSIs⁵². On admission, the evening before surgery, a nasal swab for S. aureus colonization was performed by semiquantitative culture analysis and repeated within 24-hour after surgery. There was a 39.6% eradication of S. aureus nasal colonization post PI solution intranasal application similar to the Phillips trial. They did confirm pre-surgery S. aureus nasal colonization was a risk factor for subsequent SSI in orthopedic surgery and they also demonstrated a reduction in SSI rate in the intervention versus nonintervention period for knee arthroplasty, hip arthroplasty, and spine surgery. Of interest patients who were still positive for S. aureus on a nasal culture postoperatively were three-fold more likely
to develop an SSI compared to patients who were culture negative postoperatively. Nasal PI application on the evening before and the morning prior to surgery was less effective in achieving S. aureus eradication if initial nasal presurgery culture demonstrated higher colony counts.
Nasal alcohol products have bactericidal activity against most gram-positive and gram-negative bacteria but have a very short duration of activity. There are only two peer reviewed studies on the use of nasal alcohol in prevention of SSIs. Mullen et al.¹⁰⁶ published a brief report in 2017 using an alcohol-based nasal antiseptic decolonization to reduce Staphylococcus species surgical site infections. All patients scheduled for spine surgery were included in the study. Records from 1,073 spine surgical patients undergoing inpatient or outpatient procedures (400 and 673 in the baseline and intervention periods, respectively) were part of the study. In the intervention period, a comprehensive pre and postoperative decolonization protocol. After surgery, patients were expected to follow the regular 3 times daily cycle of staff-applied alcohol-based application in the postsurgical units until discharge, at which time the patient and family coach were instructed to continue applications for an additional 5–7 days with the remaining antiseptic. Mean infection rates were significantly decreased by 81% from1.76 to 0.33 per 100 surgeries during the 15-month trial, when compared with the prior 9-month baseline (P =.036). This is a single-center quasi-experimental intervention. The investigators did comment that their spine population was not sufficiently large to do a randomized control trial so they decided to do a before-and-after trial design.
In the second study, Bostian et al. did a retrospective study comparing an 18-month cohort of elective primary total joint arthroplasty patients treated perioperatively with an alcohol-based nasal agent to historical controls¹⁰⁷. The alcohol-based agent was administered pre-operatively on the day of surgery and for up to 5 days after surgery. They reported patients who used the alcohol-based nasal decolonization had a lower rate of SSI compared with controls not receiving nasal decolonization (0.64% [5/779] vs. 1.55% [10/647]; p = 0.048; odds ratio, 2.43). This study has several limitations. First this was another quasi-experimental retrospective study. Second, the difference found regarding infection rates between groups was found when performing a chai-square analysis, but not when performing regression analysis. Much larger control studies involving patients colonized with S. aureus will be necessary to determine if decolonization with a nasal ethanol antiseptic can reduce S. aureus infections.
PDT
PDT is another promising approach for nasal S. aureus decolonization. Bryce et al. studied patients undergoing elective cardiac surgery, orthopedic surgery, spinal surgery, vascular surgery, thoracic surgery, or neurosurgery who were asked to bathe with 2% CHG cloths the 24 h prior to surgery and then receive intranasal PDT (0.1% methylene blue plus laser) in the preoperative area¹⁰⁸. There was a statistically significant decrease in the SSI rate when comparing treated patients to a historical control group (1.6% versus 2.7%; P = 0.0004; OR 1.73; 95% CI, 1.28 to 2.34). However, the study was limited, since the benefits of CHG alone compared to PDT alone were not evaluated. Larger clinical trials are needed to evaluate the impact on clinically significant infections.
The Compendium on “The Strategies to Prevent Surgical Site Infections in Acute Care Hospitals: 2022 Update” now recommends as an essential practice to decolonize surgical patients with an antistaphylococcal agent in the preoperative setting for high-risk procedures, especially orthopedic and cardiothoracic procedures⁸⁵. This includes patients colonized with either methicillin susceptible S. aureus (MSSA) or MRSA. They believe the strongest data recommends up to 5 days of intranasal mupirocin (twice daily) and daily CHG bathing for decolonization. They state there is some preliminary data on intranasal PI administered immediately before surgery. They admit this approach may have some practical advantages, but more data is needed. There is even less evidence for other alternative strategies such as intranasal alcohol and PDT.
Moving forward, challenges relating to decolonization include expansion of these strategies for additional surgical patient populations, as well as nonsurgical invasive procedures. For example, interventional radiology patients receiving implants such as cardiac implantable electronic devices (CIED) have been associated with significant numbers of postimplant infections, often caused by S. aureus¹⁰⁸. For patients known to be colonized with MRSA or MSSA prior to a CIED procedure, a multigroup British Working Party guideline recommends the use of nasal and topical antimicrobial agents preprocedure in order to suppress carriage¹⁰⁹.
Conclusion
In summary, colonization with health care-associated pathogens such as S. aureus, enterococci, Gram-negative organisms, and C. difficile is associated with increased risk of infection. Many of these HAIs may be preventable by evidence-based interventions. Based on the evidence described here, decolonization is one such intervention that can reduce rates of HAIs.
Decolonization prevents both vertical and horizontal transmission, depending on the method. There are several decolonization methods, such as nasal, topical, and oral decontamination, with many different agents. Mupirocin remains the gold standard agent for nasal decolonization of S. aureus, but there is concern about mupirocin resistance, and alternative agents are needed. CHG is the skin decolonization agent that has the strongest evidence for reducing HAIs. There is also evidence to support decolonization with SDD and SOD to prevent VAP in countries and ICUs with low prevalence of antibiotic-resistant organisms. FMT may be a new promising intervention in decolonizing patients with gram-negative MDROs, but more studies are needed.
Conflict of Interest:
None.
Funding Statement:
None.
Acknowledgements:
None.
Disclosures:
None.
References
1. Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. The New England journal of medicine. Mar 27 2014;370(13):1198-208. doi:10. 1056/NEJMoa1306801
2. Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA internal medicine. Dec 9-23 2013;17 3(22):2039-46. doi:10.1001/jamainternmed.2013.9763
3. WHO Global report on the epidemiology and burden of sepsis. accesseed September 16, 2024 doi:https://iris.who.int/bitstream/handle/10665/334216/9789240010789-eng.pdf?sequence=1
4. Schreiber PW, Sax H, Wolfensberger A, Clack L, Kuster SP. The preventable proportion of healthcare-associated infections 2005-2016: Systematic review and meta-analysis. Infection control and hospital epidemiology. Nov 2018;39 (11):1277-1295. doi:10.1017/ice.2018.183
5. National Action Plan. Accessed June 19, 2024. https://health.gov/about-odphp/previous-initiatives#SSI
6. Magill SS, O’Leary E, Janelle SJ, et al. Changes in Prevalence of Health Care-Associated Infections in U.S. Hospitals. The New England journal of medicine. Nov 1 2018;379(18):1732-1744. doi:10. 1056/NEJMoa1801550
7. EU HAI Report 2022/3. Accessed June 19, 2024. https://www.ecdc.europa.eu/sites/default/files/documents/healthcare-associated-point-prevalence-survey-acute-care-hospitals-2022-2023.pdf
8. Lastinger LM, Alvarez CR, Kofman A, et al. Continued increases in the incidence of healthcare-associated infection (HAI) during the second year of the coronavirus disease 2019 (COVID-19) pandemic. Infection Control & Hospital Epidemiology. 2022:1-5. doi:10.1017/ice.2022.116
9. 2022 National and State Healthcare-Associated Infections Progress Report. Accessed June 19, 2024. https://www.cdc.gov/healthcare-associated-infections/php/data/progress-report.html?CDC_AAref_Val=https://www.cdc.gov/hai/data/portal/progress-report.html
10. Ammerlaan HS, Kluytmans JA, Wertheim HF, Nouwen JL, Bonten MJ. Eradication of methicillin-resistant Staphylococcus aureus carriage: a systematic review. Clin Infect Dis. Apr 1 2009;48 (7):922-30. doi:10.1086/597291
11. Oostdijk EAN, Smet AMGAd, Blok HEM, et al. Ecological Effects of Selective Decontamination on Resistant Gram-negative Bacterial Colonization. American journal of respiratory and critical care medicine. 2010;181(5):452-457. doi:10.1164/rccm. 200908-1210OC
12. D’Amico R, Pifferi S, Torri V, Brazzi L, Parmelli E, Liberati A. Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database of Systematic Reviews. 2009;(4)doi:10.1002/1465185 8.CD000022.pub3
13. Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nature Reviews Immunology. 2013/11/01 2013;13 (11):790-801. doi:10.1038/nri3535
14. Wertheim HF, Melles DC, Vos MC, et al. The role of nasal carriage in Staphylococcus aureus infections. The Lancet Infectious diseases. Dec 2005;5(12):751-62. doi:10.1016/s1473-3099(05)70295-4
15. VandenBergh MF, Yzerman EP, van Belkum A, Boelens HA, Sijmons M, Verbrugh HA. Follow-up of Staphylococcus aureus nasal carriage after 8 years: redefining the persistent carrier state. Journal of clinical microbiology. Oct 1999;37 (10):3133-40.
16. Sim BL, McBryde E, Street AC, Marshall C. Multiple site surveillance cultures as a predictor of methicillin-resistant Staphylococcus aureus infections. Infection control and hospital epidemiology. Aug 2013;34(8):818-24. doi:10.1086/671273
17. Szumowski JD, Wener KM, Gold HS, et al. Methicillin-Resistant Staphylococcus aureus Colonization, Behavioral Risk Factors, and Skin and Soft-Tissue Infection at an Ambulatory Clinic Serving a Large Population of HIV-Infected Men Who Have Sex with Men. Clinical Infectious Diseases. 2009;49(1):118-121. doi:10.1086/599608
18. Russell DL, Flood A, Zaroda TE, et al. Outcomes of colonization with MRSA and VRE among liver transplant candidates and recipients. Am J Transplant. Aug 2008;8(8):1737-43. doi:10. 1111/j.1600-6143.2008.02304.x
19. Montecalvo MA, Shay DK, Gedris C, et al. A Semiquantitative Analysis of the Fecal Flora of Patients with Vancomycin-Resistant Enterococci: Colonized Patients Pose an Infection Control Risk. Clinical Infectious Diseases. 1997;25(4):929-930. doi:10.1086/597643
20. Wang X, Liu J, Li A. Incidence and risk factors for subsequent infections among rectal carriers with carbapenem-resistant Klebsiella pneumoniae: a systematic review and meta-analysis. The Journal of hospital infection. Mar 2024;145:11-21. doi:10. 1016/j.jhin.2023.12.002
21. Almohaya A, Fersovich J, Weyant RB, et al. The impact of colonization by multidrug resistant bacteria on graft survival, risk of infection, and mortality in recipients of solid organ transplant: systematic review and meta-analysis. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. Apr 10 2024;doi:10. 1016/j.cmi.2024.03.036
22. McKinnell JA, Singh RD, Miller LG, et al. The SHIELD Orange County Project: Multidrug-resistant Organism Prevalence in 21 Nursing Homes and Long-term Acute Care Facilities in Southern California. Clinical Infectious Diseases. 2019;69(9):1566-1573. doi:10.1093/cid/ciz119
23. Mody L, Foxman B, Bradley S, et al. Longitudinal Assessment of Multidrug-Resistant Organisms in Newly Admitted Nursing Facility Patients: Implications for an Evolving Population. Clinical Infectious Diseases. 2018;67(6):837-844. doi:10.1093/cid/ciy194
24. Nelson RE, Hatfield KM, Wolford H, et al. National Estimates of Healthcare Costs Associated With Multidrug-Resistant Bacterial Infections Among Hospitalized Patients in the United States. Clinical Infectious Diseases. 2021;72(Supplement_ 1):S17-S26. doi:10.1093/cid/ciaa1581
25. Gussin GM, McKinnell JA, Singh RD, et al. Reducing Hospitalizations and Multidrug-Resistant Organisms via Regional Decolonization in Hospitals and Nursing Homes. Jama. May 14 2024;331 (18):1544-1557. doi:10.1001/jama.2024.2759
26. Kalmeijer MD, Nieuwland-Bollen Ev, Bogaers-Hofman D, Baere GAJd, Kluytmans JAJW. Nasal Carriage of Staphylococcus aureus: Is a Major Risk Factor for Surgical-Site Infections in Orthopedic Surgery. Infection Control & Hospital Epidemiology. 2000;21(5):319-323. doi:10.1086/501763
27. Wenzel RP, Edmond MB. Infection control: the case for horizontal rather than vertical interventional programs. Int J Infect Dis. Oct 2010;14 Suppl 4:S3-5. doi:10.1016/j.ijid.2010.05.002
28. Septimus E, Weinstein RA, Perl TM, Goldmann DA, Yokoe DS. Approaches for preventing healthcare-associated infections: go long or go wide? Infection control and hospital epidemiology. Sep 2014;35 Suppl 2:S10-4.
29. Septimus EJ, Hayden MK, Kleinman K, et al. Does chlorhexidine bathing in adult intensive care units reduce blood culture contamination? A pragmatic cluster-randomized trial. Infection control and hospital epidemiology. Oct 2014;35 Suppl 3:S17-22. doi:10.1086/677822
30. Lee BY, Wiringa AE, Bailey RR, et al. Screening cardiac surgery patients for MRSA: an economic computer model. Am J Manag Care. Jul 1 2010;16(7):e163-73.
31. Immerman I, Ramos NL, Katz GM, Hutzler LH, Phillips MS, Bosco JA, 3rd. The persistence of Staphylococcus aureus decolonization after mupirocin and topical chlorhexidine: implications for patients requiring multiple or delayed procedures. The Journal of arthroplasty. Jun 2012; 27(6):870-6. doi:10.1016/j.arth.2012.01.010
32. Holton DL, Nicolle LE, Diley D, Bernstein K. Efficacy of mupirocin nasal ointment in eradicating Staphylococcus aureus nasal carriage in chronic haemodialysis patients. The Journal of hospital infection. Feb 1991;17(2):133-7. doi:10.1016/0195 -6701(91)90177-a
33. Loeb MB, Main C, Eady A, Walker-Dilks C. Antimicrobial drugs for treating methicillin-resistant Staphylococcus aureus colonization. The Cochrane database of systematic reviews. 2003;(4): Cd003340. doi:10.1002/14651858.Cd003340
34. Bommer J, Vergetis W, Andrassy K, Hingst V, Borneff M, Huber W. Elimination of Staphylococcus aureus in Hemodialysis Patients. ASAIO Journal. 1995;41(1):127-131.
35. Ward A, Campoli-Richards DM. Mupirocin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. Nov 1986; 32(5):425-44. doi:10.2165/00003495-198632050-00002
36. Perl TM, Cullen JJ, Wenzel RP, et al. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med. Jun 13 2002;34 6(24):1871-7. doi:10.1056/NEJMoa003069
37. van Rijen M, Bonten M, Wenzel R, Kluytmans J. Mupirocin ointment for preventing Staphylococcus aureus infections in nasal carriers. The Cochrane database of systematic reviews. Oct 8 2008;(4):Cd 006216. doi:10.1002/14651858.CD006216.pub2
38. Schweizer M, Perencevich E, McDanel J, et al. Effectiveness of a bundled intervention of decolonization and prophylaxis to decrease Gram positive surgical site infections after cardiac or orthopedic surgery: systematic review and meta-analysis. Bmj. Jun 13 2013;346:f2743. doi:10.113 6/bmj.f2743
39. Kallen AJ, Wilson CT, Larson RJ. Perioperative intranasal mupirocin for the prevention of surgical-site infections: systematic review of the literature and meta-analysis. Infection control and hospital epidemiology. Dec 2005;26(12):916-22. doi:10.10 86/505453
40. Perez-Fontan M, Rosales M, Rodriguez-Carmona A, Falcon TG, Valdes F. Mupirocin resistance after long-term use for Staphylococcus aureus colonization in patients undergoing chronic peritoneal dialysis. American journal of kidney diseases: the official journal of the National Kidney Foundation. Feb 2002;39(2):337-41.
41. Vasquez JE, Walker ES, Franzus BW, Overbay BK, Reagan DR, Sarubbi FA. The epidemiology of mupirocin resistance among methicillin-resistant Staphylococcus aureus at a Veterans’ Affairs hospital. Infection control and hospital epidemiology. Jul 2000;21(7):459-64. doi:10.1086/501788
42. Walker ES, Vasquez JE, Dula R, Bullock H, Sarubbi FA. Mupirocin-resistant, methicillin-resistant Staphylococcus aureus: does mupirocin remain effective? Infection control and hospital epidemiology. May 2003;24(5):342-6. doi:10.1086/502218
43. Fawley WN, Parnell P, Hall J, Wilcox MH. Surveillance for mupirocin resistance following introduction of routine peri-operative prophylaxis with nasal mupirocin. The Journal of hospital infection. Mar 2006;62(3):327-32. doi:10.1016/j.jhi n.2005.09.022
44. Hayden MK, Lolans K, Haffenreffer K, et al. Chlorhexidine and Mupirocin Susceptibility of Methicillin-Resistant Staphylococcus aureus Isolates in the REDUCE-MRSA Trial. Journal of clinical microbiology. Nov 2016;54(11):2735-2742. doi:10.1128/jcm.01444-16
45. Dadashi M, Hajikhani B, Darban-Sarokhalil D, van Belkum A, Goudarzi M. Mupirocin resistance in Staphylococcus aureus: A systematic review and meta-analysis. Journal of Global Antimicrobial Resistance. 2020/03/01/ 2020;20:238-247. doi:https://doi.org/10.1016/j.jgar.2019.07.032
46. Semret M, Miller MA. Topical mupirocin for eradication of MRSA colonization with mupirocin-resistant strains. Infection control and hospital epidemiology. Sep 2001;22(9):578-80. doi:10.108 6/501956
47. Huang SS, Septimus EJ, Kleinman K, et al. Nasal Iodophor Antiseptic vs Nasal Mupirocin Antibiotic in the Setting of Chlorhexidine Bathing to Prevent Infections in Adult ICUs: A Randomized Clinical Trial. JAMA. 2023;330(14):1337-1347. doi: 10.1001/jama.2023.17219
48. Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. The New England journal of medicine. Jun 13 2013;368(24):2255-65. doi:10.10 56/NEJMoa1207290
49. Hill RL, Casewell MW. The in-vitro activity of povidone-iodinecream against Staphylococcus aureus and its bioavailability in nasal secretions. The Journal of hospital infection. Jul 2000;45(3) :198-205. doi:10.1053/jhin.2000.0733
50. Rezapoor M, Nicholson T, Tabatabaee RM, Chen AF, Maltenfort MG, Parvizi J. Povidone-Iodine-Based Solutions for Decolonization of Nasal Staphylococcus aureus: A Randomized, Prospective, Placebo-Controlled Study. The Journal of arthroplasty. Sep 2017;32(9):2815-2819. doi:10.10 16/j.arth.2017.04.039
51. Phillips M, Rosenberg A, Shopsin B, et al. Preventing surgical site infections: a randomized, open-label trial of nasal mupirocin ointment and nasal povidone-iodine solution. Infection control and hospital epidemiology. Jul 2014;35(7):826-32. doi:10.1086/676872
52. Saidel-Odes L, Yosipovich R, Benkovich V, et al. Getting the drop on Staphylococcus aureus: Semiquantitative Staphylococcus aureus nasal colony reduction in orthopedic surgery reduces surgical site infection. American journal of infection control. 2024;52(7):785-789. doi:10.1016/j.ajic.2024.02.014
53. Harrison C, Zent R, Schneck E, Flynn CE, Drees M. Infection prevention versus antimicrobial stewardship: Does nasal povidone-iodine interfere with methicillin-resistant Staphylococcus aureus (MRSA) screening? Infection Control & Hospital Epidemiology. 2022;43(7):945-947. doi:10.1017/ ice.2021.152
54. Boyce JM, Pittet D. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HIPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. American journal of infection control. Dec 2002;30(8):S1-46.
55. Steed LL, Costello J, Lohia S, Jones T, Spannhake EW, Nguyen S. Reduction of nasal Staphylococcus aureus carriage in health care professionals by treatment with a nonantibiotic, alcohol-based nasal antiseptic. American journal of infection control. Aug 2014;42(8):841-6. doi:10.1 016/j.ajic.2014.04.008
56. Kanwar A, Kumar JA, Ng-Wong YK, et al. Evaluation of an alcohol-based antiseptic for nasal decolonization of methicillin-resistant Staphylococcus aureus in colonized patients. Infection Control & Hospital Epidemiology. 2019;40(12):1436-1437. doi:10.1017/ice.2019.266
57. Street CN, Pedigo L, Gibbs A, Loebel NG. Antimicrobial photodynamic therapy for the decolonization of methicillin-resistant Staphylococcus aureus from the anterior nares. SPIE; 2009:16.
58. Bryce E, Wong T, Forrester L, et al. Nasal photodisinfection and chlorhexidine wipes decrease surgical site infections: a historical control study and propensity analysis. The Journal of hospital infection. Oct 2014;88(2):89-95. doi:10.1 016/j.jhin.2014.06.017
59. Embleton ML, Nair SP, Cookson BD, Wilson M. Antibody-Directed Photodynamic Therapy of MethicillinResistant Staphylococcus aureus. Microbial Drug Resistance. 2004/06/01 2004;10 (2):92-97. doi:10.1089/1076629041310000
60. Bezerra DT, La Selva A, Cecatto RB, et al. Antimicrobial Photodynamic Therapy in the Nasal Decolonization of Maintenance Hemodialysis Patients: A Pilot Randomized Trial. American journal of kidney diseases : the official journal of the National Kidney Foundation. May 2023;81(5):528-536.e1. doi:10.1053/j.ajkd.2022.09.013
61. Weinstein RA, Milstone AM, Passaretti CL, Perl TM. Chlorhexidine: Expanding the Armamentarium for Infection Control and Prevention. Clinical Infectious Diseases. 2008;46(2):274-281. doi:10.1 086/524736
62. Maki DG, Alvarado CJ, Ringer M. Prospective randomised trial of povidone-iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters. The Lancet. 1991;338(8763):339-343. doi:10.1016/0140-6736(91)90479-9
63. Vernon MO, Hayden MK, Trick WE, et al. Chlorhexidine Gluconate to Cleanse Patients in a Medical Intensive Care Unit: The Effectiveness of Source Control to Reduce the Bioburden of Vancomycin-Resistant Enterococci. Archives of internal medicine. 2006;166(3):306-312. doi:10.10 01/archinte.166.3.306
64. Bleasdale SC, Trick WE, Gonzalez IM, Lyles RD, Hayden MK, Weinstein RA. Effectiveness of Chlorhexidine Bathing to Reduce Catheter-Associated Bloodstream Infections in Medical Intensive Care Unit Patients. Archives of internal medicine. 2007;167(19):2073-2079. doi:10.1001/a rchinte.167.19.2073
65. Borer A, Gilad J, Porat N, et al. Impact of 4% chlorhexidine whole-body washing on multidrug-resistant Acinetobacter baumannii skin colonisation among patients in a medical intensive care unit. Journal of Hospital Infection. 2007;67(2):149-155. doi:10.1016/j.jhin.2007.07.023
66. Climo MW, Yokoe DS, Warren DK, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. The New England journal of medicine. Feb 7 2013;368(6):533-42. doi:10.1056/NEJMoa 1113849
67. Milstone AM, Elward A, Song X, et al. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. Lancet (London, England). 2013;381(9872):1099-1106. doi:10.1016 /S0140-6736(12)61687-0
68. Huang SS, Septimus E, Platt R. Targeted decolonization to prevent ICU infections. The New England journal of medicine. Oct 10 2013;369( 15):1470-1. doi:10.1056/NEJMc1309704
69. Septimus E, Hickok J, Moody J, et al. Closing the Translation Gap: Toolkit-based Implementation of Universal Decolonization in Adult Intensive Care Units Reduces Central Line-associated Bloodstream Infections in 95 Community Hospitals. Clin Infect Dis. Jul 15 2016;63(2):172-7. doi:10.1093/cid/ciw282
70. Derde LPG, Cooper BS, Goossens H, et al. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an interrupted time series study and cluster randomised trial. The Lancet Infectious Diseases. 2014;14(1):31-39. doi:10.101 6/S1473-3099(13)70295-0
71. Noto MJ, Domenico HJ, Byrne DW, et al. Chlorhexidine Bathing and Health Care–Associated Infections: A Randomized Clinical Trial. JAMA. 2015;313(4):369-378. doi:10.1001/jama.20 14.18400
72. Huang SS, Septimus E, Kleinman K, et al. Chlorhexidine versus routine bathing to prevent multidrug-resistant organisms and all-cause bloodstream infections in general medical and surgical units (ABATE Infection trial): a cluster-randomised trial. Lancet (London, England). 2019;393(10177):1205-1215. doi:10.1016/S0140-6736(18)32593-5
73. Tien KL, Sheng WH, Shieh SC, et al. Chlorhexidine Bathing to Prevent Central Line-Associated Bloodstream Infections in Hematology Units: A Prospective, Controlled Cohort Study. Clin Infect Dis. Jul 27 2020;71(3):556-563. doi:10.109 3/cid/ciz874
74. Denkel LA, Schwab F, Clausmeyer J, et al. Effect of antiseptic bathing with chlorhexidine or octenidine on central line–associated bloodstream infections in intensive care patients: a cluster-randomized controlled trial. Clinical Microbiology and Infection. 2022;28(6):825-831. doi:10.1016/ j.cmi.2021.12.023
75. Sturm L, Service N, Bufalino A, et al. Is Hospital-Onset Bloodstream Infection (HOBSI) a Useful Measure to Evaluate Infection Prevention Progress? Infection Control & Hospital Epidemiology. 2020;41(S1):s307-s308. doi:10.101 7/i ce.2020.894
76. Page B, Klompas M, Chan C, et al. Surveillance for Healthcare-Associated Infections: Hospital-Onset Adult Sepsis Events Versus Current Reportable Conditions. Clinical Infectious Diseases. 2021;73(6 ):1013-1019. doi:10.1093/cid/ciab217
77. Yu KC, Jung M, Ai C. Characteristics, costs, and outcomes associated with central-line-associated bloodstream infection and hospital-onset bacteremia and fungemia in US hospitals. Infection control and hospital epidemiology. Dec 2023;44(12):1920-1926. doi:10.1017/ice.2023.132
78. Blauw M, Foxman B, Wu J, Rey J, Kothari N, Malani AN. Risk Factors and Outcomes Associated With Hospital-Onset Peripheral Intravenous Catheter–Associated Staphylococcus aureus Bacteremia. Open forum infectious diseases. 2019; 6(4)doi:10.1093/ofid/ofz111
79. Garcia R, Septimus EJ, LeDonne J, et al. Prevention of Vascular Access Device-Associated Hospital Onset Bacteremia and Fungemia: A Review of Emerging Perspectives and Synthesis of Technical Aspects. Clinical Infectious Diseases. 2024;doi:10.1093/cid/ciae245
80. Buetti N, Marschall J, Drees M, et al. Strategies to prevent central line-associated bloodstream infections in acute-care hospitals: 2022 Update. Infection control and hospital epidemiology. May 2022;43(5):553-569. doi:10.1017/ice.2022.87
81. Block C, Robenshtok E, Simhon A, Shapiro M. Evaluation of chlorhexidine and povidone iodine activity against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecalis using a surface test. Journal of Hospital Infection. 2000;46(2):147-152. doi:10.1053/jhin.2000.0805
82. Smylie HG, Logie JRC, Smith G. From Phisohex to Hibiscrub. British Medical Journal. 1973;4(5892):586-589. doi:10.1136/bmj.4.5892.586
83. null n. Skin Antisepsis before Surgical Fixation of Extremity Fractures. New England Journal of Medicine. 2024/01/31 2024;390(5):409-420. doi:1 0.1056/NEJMoa2307679
84. Widmer AF, Atkinson A, Kuster SP, et al. Povidone Iodine vs Chlorhexidine Gluconate in Alcohol for Preoperative Skin Antisepsis: A Randomized Clinical Trial. JAMA. 2024;doi:10.100 1/jama.2024.8531
85. Calderwood MS, Anderson DJ, Bratzler DW, et al. Strategies to prevent surgical site infections in acute-care hospitals: 2022 Update. Infection control and hospital epidemiology. May 2023;44 (5):695-720. doi:10.1017/ice.2023.67
86. Chan EY, Ruest A, Meade MO, Cook DJ. Oral decontamination for prevention of pneumonia in mechanically ventilated adults: systematic review and meta-analysis. BMJ. 2007;334(7599):889. doi:1 0.1136/bmj.39136.528160.BE
87. Smet AMGAd, Kluytmans JAJW, Cooper BS, et al. Decontamination of the Digestive Tract and Oropharynx in ICU Patients. New England Journal of Medicine. 2009;360(1):20-31. doi:10.1056/NEJ Moa0800394
88. de Smet AMGA, Kluytmans JAJW, Blok HEM, et al. Selective digestive tract decontamination and selective oropharyngeal decontamination and antibiotic resistance in patients in intensive-care units: an open-label, clustered group-randomised, crossover study. The Lancet Infectious Diseases. 2011;11(5):372-380. doi:10.1016/S1473-3099(11)70035-4
89. Oostdijk EAN, Kesecioglu J, Schultz MJ, et al. Effects of Decontamination of the Oropharynx and Intestinal Tract on Antibiotic Resistance in ICUs: A Randomized Clinical Trial. JAMA. 2014;312(14) :1429-1437. doi:10.1001/jama.2014.7247
90. Price R, MacLennan G, Glen J. Selective digestive or oropharyngeal decontamination and topical oropharyngeal chlorhexidine for prevention of death in general intensive care: systematic review and network meta-analysis. BMJ : British Medical Journal. 2014;348:g2197. doi:10.1136/bmj.g2197
91. Klompas M, Branson R, Cawcutt K, et al. Strategies to prevent ventilator-associated pneumonia, ventilator-associated events, and nonventilator hospital-acquired pneumonia in acute-care hospitals: 2022 Update. Infection Control & Hospital Epidemiology. 2022;43(6):687-713. doi:10.1017/ice.2022.88
92. Daneman N, Sarwar S, Fowler RA, Cuthbertson BH. Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta-analysis. The Lancet Infectious Diseases. 2013;13(4):328-341. doi:10.10 16/S1473-3099(12)70322-5
93. Plantinga NL, de Smet A, Oostdijk EAN, et al. Selective digestive and oropharyngeal decontamination in medical and surgical ICU patients: individual patient data meta-analysis. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. May 2018; 24(5):505-513. doi:10.1016/j.cmi.2017.08.019
94. Hammond NE, Myburgh J, Seppelt I, et al. Association Between Selective Decontamination of the Digestive Tract and In-Hospital Mortality in Intensive Care Unit Patients Receiving Mechanical Ventilation: A Systematic Review and Meta-analysis. JAMA. 2022;328(19):1922-1934. doi:10.1 001/jama.2022.19709
95. Huttner B, Haustein T, Uçkay I, et al. Decolonization of intestinal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae with oral colistin and neomycin: a randomized, double-blind, placebo-controlled trial. Journal of Antimicrobial Chemotherapy. 2013;68(10):2375-2382. doi:10.1093/jac/dkt174
96. Saidel-Odes L, Polachek H, Peled N, et al. A Randomized, Double-Blind, Placebo-Controlled Trial of Selective Digestive Decontamination Using Oral Gentamicin and Oral Polymyxin E for Eradication of Carbapenem-Resistant Klebsiella pneumoniae Carriage. Infection Control & Hospital Epidemiology. 2012;33(1):14-19. doi:10.1086/663206
97. Oren I, Sprecher H, Finkelstein R, et al. Eradication of carbapenem-resistant Enterobacteriaceae gastrointestinal colonization with nonabsorbable oral antibiotic treatment: A prospective controlled trial. American journal of infection control. 2013;41(12):1167-1172. doi:10. 1016/j.ajic.2013.04.018
98. Bilsen MP, Lambregts MMC, van Prehn J, Kuijper EJ. Faecal microbiota replacement to eradicate antimicrobial resistant bacteria in the intestinal tract – a systematic review. Current Opinion in Gastroenterology. 2022;38(1):15-25. doi:10.1097/mog.0000000000000792
99. Macareño-Castro J, Solano-Salazar A, Dong LT, Mohiuddin M, Espinoza JL. Fecal microbiota transplantation for Carbapenem-Resistant Enterobacteriaceae: A systematic review. Journal of Infection. 2022;84(6):749-759. doi:10.1016/ j.jinf.2022.04.028
100. Huttner BD, de Lastours V, Wassenberg M, et al. A 5-day course of oral antibiotics followed by faecal transplantation to eradicate carriage of multidrug-resistant Enterobacteriaceae : a randomized clinical trial. Clinical Microbiology and Infection. 2019;25(7):830-838. doi:10.1016/j.cmi.2018.12.009
101. Woodworth MH, Conrad RE, Haldopoulos M, et al. Fecal microbiota transplantation promotes reduction of antimicrobial resistance by strain replacement. Science Translational Medicine. 2023 ;15(720):eabo2750. doi:doi:10.1126/scitranslmed. abo2750
102. Bode LGM, Kluytmans JAJW, Wertheim HFL, et al. Preventing Surgical-Site Infections in Nasal Carriers of Staphylococcus aureus. New England Journal of Medicine. 2010;362(1):9-17. doi:10.10 56/NEJMoa0808939
103. Cimochowski GE, Harostock MD, Brown R, Bernardi M, Alonzo N, Coyle K. Intranasal mupirocin reduces sternal wound infection after open heart surgery in diabetics and nondiabetics. The Annals of thoracic surgery. May 2001;71(5) :1572-8; discussion 1578-9.
104. Schweizer ML, Chiang HY, Septimus E, et al. Association of a bundled intervention with surgical site infections among patients undergoing cardiac, hip, or knee surgery. Jama. Jun 2 2015;313 (21):2162-71. doi:10.1001/jama.2015.5387
105. Bebko SP, Green DM, Awad SS. Effect of a preoperative decontamination protocol on surgical site infections in patients undergoing elective orthopedic surgery with hardware implantation. JAMA surgery. May 2015;150(5):390-5. doi:10.100 1/jamasurg.2014.3480
106. Mullen A, Wieland HJ, Wieser ES, Spannhake EW, Marinos RS. Perioperative participation of orthopedic patients and surgical staff in a nasal decolonization intervention to reduce Staphylococcus spp surgical site infections. American journal of infection control. May 1 2017; 45(5):554-556. doi:10.1016/j.ajic.2016.12.021
107. Bostian PA, Vaida J, Brooks WC, et al. A Novel Protocol for Nasal Decolonization Using Prolonged Application of an Alcohol-Based Nasal Antiseptic Reduces Surgical Site Infections in Total Joint Arthroplasty Patients: A Retrospective Cohort Study. Surg Infect (Larchmt). Sep 2023;24(7):651-656. doi:10.1089/sur.2022.344
108. Sławiński G, Lewicka E, Kempa M, Budrejko S, Raczak G. Infections of cardiac implantable electronic devices: Epidemiology, classification, treatment, and prognosis. Adv Clin Exp Med. Feb 2019;28(2):263-270. doi:10.17219/acem/80665
109. Sandoe JA, Barlow G, Chambers JB, et al. Guidelines for the diagnosis, prevention and management of implantable cardiac electronic device infection. Report of a joint Working Party project on behalf of the British Society for Antimicrobial Chemotherapy (BSAC, host organization), British Heart Rhythm Society (BHRS), British Cardiovascular Society (BCS), British Heart Valve Society (BHVS) and British Society for Echocardiography (BSE). The Journal of antimicrobial chemotherapy. Feb 2015;70(2):325-59. doi:10.1093/jac/dku383

