BCG Vaccination and COVID-19: Impact on Patient Outcomes
Study of BCG Vaccination status of COVID-19 infected patients & its relationship to morbidity, mortality & treatment outcome of the COVID-19 disease
Dr. Anita Sinha1, Dr. Bhoomika Patel2, Dr. Arya B3
OPEN ACCESS
PUBLISHED: 31 January 2025
CITATION: SINHA, Dr. Anita; PATEL, Dr. Bhoomika; B, Dr. Arya. Study of BCG Vaccination status of COVID-19 infected patients & its relationship to morbidity, mortality & treatment outcome of the COVID-19 disease. Medical Research Archives, [S.l.], v. 13, n. 1, feb. 2025. Available at: <https://esmed.org/MRA/mra/article/view/6125>.
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.18103/mra.v13i1.6125
ISSN 2375-1924
ABSTRACT
There have been 760 million cases and 6.9 million recorded deaths worldwide due to COVID-19 infection since December 2019. The actual number is thought to be considerably higher (WHO) than documented worldwide. There was worldwide desperate search for ways to prevent the morbidities and mortalities associated with COVID-19 infection. In this context, BCG vaccination hypothesized to play both preventive as well as protective role against COVID-19 infection. All over the world many trials, ongoing or completed done with different results. According to South African trial conducted amongst 1000 healthcare workers, BCG revaccination stated to have no protective effect. On the other hand, trial in UK stated BCG effect to be dependent on ethnicity or race of infected individual.
Present study aim was to study, compare & analyse the proportion of BCG vaccinated among the COVID-19 patients. Severity of infection, disease progression, mortality, morbidity and duration of recovery or discharge were studied in both prospective & retrospective direction in 549 participants 1keeping in view their BCG vaccination status. The study is an effort to substantiate or refute claims of various studies published regarding BCG vaccination & COVID-19 infections.
Amongst the participants, 27.5% were found to be BCG vaccinated, while 72.5 % were unvaccinated. Majority of patients (50.4%) were with mild COVID-19 infection. The patients affected were mostly of 26-30 years (26.7%) of age. Patients of 36-40 years (2.1%) were from severe disease category.
An association was found between age group (‘p’ value < 0.05) and between genders (‘p’ value < 0.05) and BCG vaccination status. There were no association between gender and severity of COVID-19 infection. Amongst the patients with severe disease, 70% were not vaccinated for BCG. There was an association between the severity of the disease and the BCG vaccination status (‘p’ value<0.05). Respiratory symptoms were common amongst almost all the participants (95.8%). Amongst patients who were BCG vaccinated, none were on invasive ventilation therapy yet amongst the non-BCG vaccinated patients, 2.76% were on invasive ventilation. There was a significant association between BCG status and complications (‘p’ value < 0.05). The association between inflammatory markers with BCG status was also found to be significant (‘p’ value < 0.05).
Introduction
In the era of COVID-19 pandemic where vaccination and treatments were still under research, BCG vaccination was hypothesized to have preventive as well as protective role against COVID-19 infection.
Many trials were underway worldwide with different results stating no protection offering in South African trial where BCG revaccination was done among 1000 health care workers and found to be not effective, to trial in UK confirming BCG effectiveness depending on ethnicity & race of the person.
In humans, BCG vaccination induced acquired immunity has been found to enhance vaccine responsiveness to yellow fever and influenza vaccination.
Small clinical studies have demonstrated reduced neonatal mortality in West African settings, primarily from a reduction in neonatal sepsis and respiratory infections, as well as protection from respiratory syncytial virus infection in African children and respiratory tract infections (RTI) in African adults.
In other studies, BCG vaccine was found to provide protection against leprosy, non-tuberculous mycobacteria lymphadenitis and Buruli Ulcer with an enhanced effect after revaccination. BCG vaccine administered in childhood has been shown to lower mortality from natural causes into adult age like the incidence of lung carcinoma and modifies the course of diabetes mellitus and multiple sclerosis. Most recently, the double-blind, placebo-controlled ACTIVATE trial in 202 elderly European patients demonstrated BCG vaccination resulting in 45% delay in time-to-first all cause infection, and a 42% reduction in all infections, compared to placebo, with major reductions in respiratory infections. Such non-specific beneficial effects have been found not only with BCG, but also with other live vaccines such as smallpox, polio and measles.
A randomized study showed BCG vaccination administered prior to influenza vaccine in healthy individuals resulted in significant higher antibody response against influenza A (H1N1) compared to placebo.
Present study is set among BCG vaccinated and non-BCG vaccinated patients acquiring COVID-19 infection, & aims to study the severity of disease progression, mortality, morbidity and duration of recovery or discharge after admission to a tertiary care Government Medical College & New Civil hospital, Surat, India.
Methodology
-
Study design: Descriptive observational longitudinal cross sectional study
-
Direction of Study: Retrospective & Prospective
-
Study Setting: Government Medical College & New Civil Hospital (GMC & NCH), a tertiary care teaching centre, Surat, Gujarat, India
-
Study Period: A total of 9 months of data collection period including 6 months from 1st wave of COVID-19 (April 2020 to end of September 2020) & 3 months of 2nd wave of COVID-19 infection (April 2021 to June 2021)
Goals & Objectives of the study:
a. To study the B.C.G Vaccination status of patients infected with COVID-19.
b. To study the severity of infection & duration of hospital stay, in relation to BCG Vaccination status of the patient.
Outcome parameters:
Primary Outcome:
-
Morbidity and mortality of the patient infected with COVID-19 with BCG Vaccination.
-
Course of disease (severity) in BCG Vaccinated COVID-19 infected patients.
Secondary Outcome:
-
Duration of hospital stay & thereby its economic impact on COVID-19 infected patients with respect to their BCG Vaccination status.
-
Type of drug treatment requirement of the patients infected with COVID-19 along with their BCG Vaccination status.
Inclusion criteria:
(Section continues beyond what’s visible in the image.)
a. COVID-19 patients with BCG Vaccination scar on them.
b. COVID-19 patients with BCG Vaccination scar &/or having any other co-morbid condition.
c. COVID-19 patients who gave history of childhood BCG Vaccination but did not have proper scar or had absent scar mark on their arms.
Exclusion criteria:
a. COVID-19 patients with HIV as co-infection.
b. Patient with pregnancy & lactation.
Sample Size: A total of 549 cases combined in both 1st wave & 2nd wave of COVID-19 infection.
DATA COLLECTION:
An observational, descriptive, longitudinal cross-sectional study design was employed to explore the effect (if any) of BCG vaccination on COVID-19 infected patients. The study compared the association of BCG vaccinated and unvaccinated participants with respect to the variables such as age, gender, symptoms, and severity of COVID-19 infection.
The study participants consisted of 549 COVID-19 affected patients from different places in the state of Gujarat & also surrounding states of Gujarat, India. After diagnosis, the patients were either sent for home quarantine or were admitted to the tertiary care teaching hospital. The purposive sampling approach guided the selection of the participants.
The primary data was collected through both out-patient records of COVID-19 patients & also from the indoor admitted COVID-19 patients records of the tertiary care centre. The details on their age, gender, symptomatology, and severity of COVID-19 infection were collected.
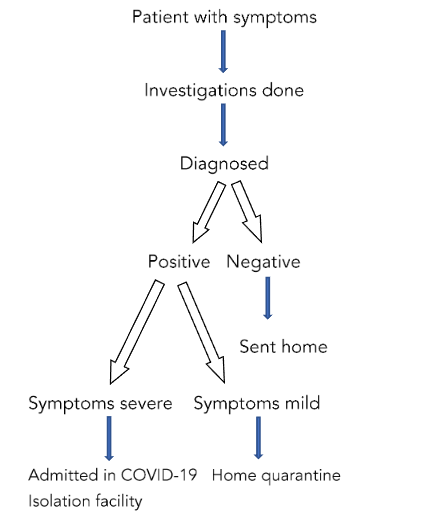
The following normal procedure was followed in the diagnosis & management of a patient with COVID-19 symptoms according to ICMR guideline:
The study had two broad arms of participating patients. One already discharged from the COVID-19 facility for home quarantine & the second group of patients who were moderate to severe in symptoms & were admitted to COVID-19 isolation wards or ICU facilities according to their requirements respectively.
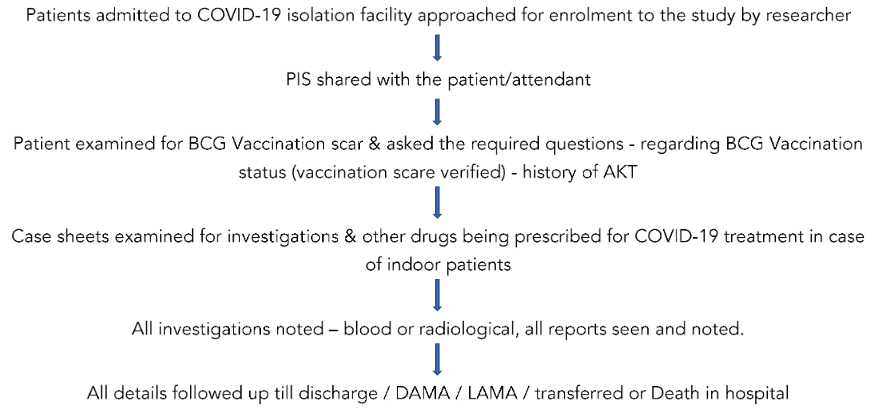
Both groups of patients were enrolled for the study depending upon the pre-specified inclusion & exclusion criteria & also upon the willingness of patients to be part of the study. Informed consents were obtained from the out-patient & indoor facility for patients to be part of the study.
In case of patients with severe symptoms or ICU patients, their attendants were educated regarding the study & informed consents obtained. All details required were noted from case sheets.
In case of non-availability of status regarding BCG vaccination & its scar (if possible), patients were contacted telephonically (home quarantine patients) & if not possible, such cases were excluded from the study. For the study of outcome parameters, patient’s data was obtained after 14 days of COVID-19 infection (for home quarantine) when they came for mandatory follow up & for indoor patients their duration of stay in hospital & mortality outcome was obtained.
The patient’s case sheets were also evaluated for the number & types of investigations undertaken & the drugs used for the management of COVID-19 infection.
Flow chart according to ICMR Guideline:
For data obtained from OPD records (for patients sent for home quarantine), their mobile numbers were obtained & were telephonically contacted. Also, they were followed up in person during their mandatory visit to tertiary centre upon 14th day of home quarantine for routine follow up in COVID OPD (in accordance with ICMR guideline).
The findings from the data were analysed using percentages, Chi Square Test, and Correlation.
Results:
Patients enrolled for the study were a total of 549.
Amongst them, 27.5% were BCG vaccinated, while 72.5% were non-vaccinated.
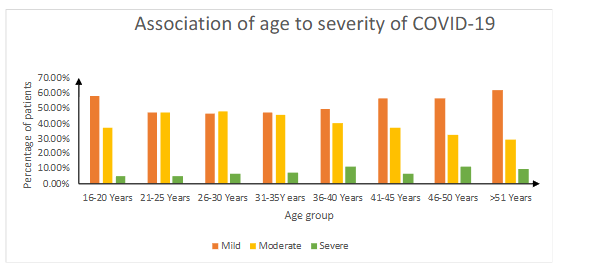
Majority of patients (50.4%) were having mild COVID-19 infections. Only 7.83% were with severe degree of illness. The patients were mostly from age group 26–30 years (26.7%). A greater number were from severe disease of age group 36–40 years (2.1%). No association was found between age and severity of COVID-19 infection with ‘p’ value of > 0.05.
Table 1: Association of age and BCG vaccination status
| Age (in years) | BCG (n, %) | Non-BCG (n, %) | Total (n, %) | Chi Square Value |
|---|---|---|---|---|
| 16–20 | 7 (36.8%) | 12 (63.1%) | 19 (3.4%) | |
| 21–25 | 17 (29.8%) | 40 (70.1%) | 57 (10.3%) | |
| 26–30 | 42 (28.5%) | 105 (71.4%) | 147 (26.7%) | |
| 31–35 | 21 (25.3%) | 62 (74.6%) | 83 (15.1%) | Chi Square = 179.029 |
| 36–40 | 24 (22.2%) | 84 (77.7%) | 108 (19.6%) | df = 7, p < 0.05 |
| 41–45 | 15 (32.6%) | 31 (67.3%) | 46 (8.3%) | |
| 46–50 | 11 (29.7%) | 26 (70.2%) | 37 (6.7%) | |
| >51 | 14 (26.9%) | 38 (73.0%) | 52 (9.5%) | |
| Total | 151 (27.5%) | 398 (72.5%) | 549 |
Highest number of patients were found to be in the age group of 26–30 years (26.7%). Of these, 71.4% were non-BCG vaccinated. Of the total, 72.5% were non-vaccinated. There was an association found between age group and BCG vaccination status as ‘p’ value was < 0.05.
Table 2: Association of Gender to Severity of COVID-19 Infection
| Gender | Mild (n, %) | Moderate (n, %) | Severe (n, %) | Total (n, %) | Chi Square Value |
|---|---|---|---|---|---|
| Female | 122 (49.8%) | 105 (42.8%) | 18 (7.3%) | 245 (44.6%) | Chi Square = 0.31 |
| Male | 155 (50.9%) | 124 (40.7%) | 25 (8.2%) | 304 (55.3%) | df = 2, p > 0.05 |
| Total | 277 | 229 | 43 | 549 |
Most of the patients were males (55.3%). Around 50.99% of the patients were having mild disease. Amongst female patients, 49.80% were with mild disease. There was no association between gender and severity of COVID-19 as ‘p’ value was > 0.05.
Table 3: Association of gender to BCG vaccination status
| Gender | BCG (n, %) | Non-BCG (n, %) | Total | Chi Square Value |
|---|---|---|---|---|
| Male | 83 (27.3%) | 221 (72.6%) | 304 | Chi Square = 5.918 |
| Female | 68 (27.6%) | 177 (72.2%) | 245 | df = 1, p < 0.05 |
Of the total male and female patients, only 27.3% and 27.6% were vaccinated with BCG respectively. Association was found between gender and BCG vaccination status as per ‘p’ value < 0.05.
Table 4: Association of severity of COVID-19 to BCG vaccination status
| Severity | BCG (n, %) | Non-BCG (n, %) | Total | Chi Square Value |
|---|---|---|---|---|
| Mild | 81 (29.2%) | 196 (70.7%) | 277 | Chi Square = 1.32 |
| Moderate | 57 (24.8%) | 172 (75.1%) | 229 | df = 2, p > 0.05 |
| Severe | 13 (30%) |
Amongst patients with severe disease, 70% were non-vaccinated for BCG. An association between severity of disease and BCG vaccination status was not found to be present (‘p’ value > 0.05).
Figure 2: Horizontal Bar chart showing percentage of symptoms
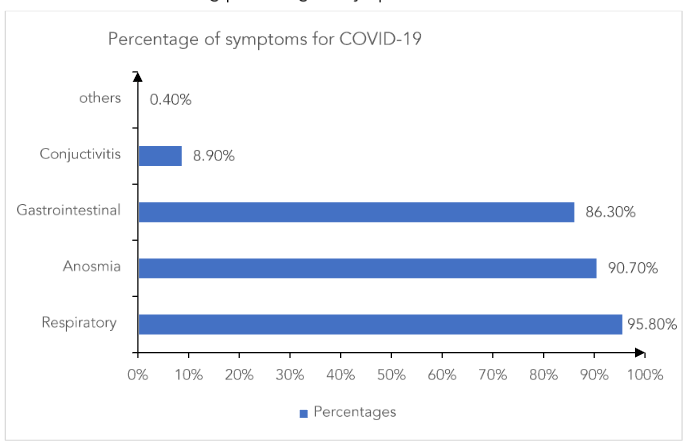
The most common symptom found amongst the study participants was of respiratory system (95.8%). Anosmia and gastrointestinal symptoms were the next common symptoms. Although less, 8.90% had conjunctivitis as a symptom.
| Age (in years) | Non-BCG | BCG | Chi Square Value |
|---|---|---|---|
| 16-20 | 7 (16.20%) | 0 (0%) | Chi-square = 179.02 |
| 21-25 | 17 (39.50%) | 0 (0%) | |
| 26-30 | 42 (26.2%) | 105 (71.4%) | |
| 31-35 | 21 (25.3%) | 74 (74.7%) | |
| 36-40 | 23 (25.3%) | 74 (74.7%) | |
| 41-45 | 22 (25.3%) | 74 (74.7%) | |
| 46-50 | 22 (25.3%) | 74 (74.7%) | |
| >51 Years | 22 (25.3%) | 74 (74.7%) |
Highest number of patients were found to be in the age group of 26-30 years (72.5%).
Amongst BCG vaccinated, 52.98% were on NRBM, while of the non-BCG vaccinated, 44.4% were on O₂. Only one BCG vaccinated patient was found on BIPAP and invasive ventilation. Amongst the non-BCG vaccinated patients, 9.04% were on BIPAP and invasive ventilation.
Thus, association between ventilation status of patients and BCG Vaccination status was found to be positive with p value < 0.05.
Positive RTPCR status was present amongst 21.6% of patients. X-Ray findings were positively present in only 48.2% of patients.
Table 6: BCG Vaccination Status and Complication
| Complications | BCG (n, %) | Non-BCG (n, %) | Total (n, %) | Chi Square Value |
|---|---|---|---|---|
| Complication Present | 12 (28.5%) | 30 (71.4%) | 42 (7.6%) | Chi Square = 393.852 |
| Complication Absent | 139 (27.4%) | 368 (72.5%) | 507 (92.3%) | df = 1, p < 0.05 |
Of the 549 patients enrolled in the present study, only 7.6% had complications; amongst these, 71.4% were non-BCG vaccinated.
The association between BCG vaccination status and complication was significant with p value of < 0.05.
Table 7: Levels of D-DIMER, CRP & IL-6 with BCG Vaccination
| Investigations | BCG (n, %) | Non-BCG (n, %) | Total (n, %) | Chi Square Value |
|---|---|---|---|---|
| D-DIMER Normal | 147 (27.0%) | 396 (72.9%) | 543 (98.9%) | Chi Square = 525.262, df = 1, p < 0.05 |
| D-DIMER Abnormal | 4 (66.6%) | 2 (33.3%) | 6 (1.1%) | |
| CRP Normal | 151 (27.8%) | 392 (72.1%) | 543 (98.9%) | Chi Square = 525.262, df = 1, p < 0.05 |
| CRP Abnormal | 0 | 6 (1.1%) | 6 (1.1%) | |
| IL-6 Not done | 149 (27.3%) | 396 (72.6%) | 545 (99.2%) | Chi Square = 533.117, df = 1, p < 0.05 |
| IL-6 Abnormal | 2 (50%) | 2 (50%) | 4 (0.8%) |
From the total of 549 enrolled study patients:
-
Patients with abnormal D-DIMER were 1.1%. Amongst these, 33.3% were not vaccinated with BCG. The association between BCG vaccination status and levels of D-DIMER was found to be significant with p value of < 0.05.
-
Abnormal CRP levels were present in 1.1% of study participants and all were from non-BCG vaccinated group. The association between BCG vaccination status and CRP levels was found significant with p value of < 0.05.
-
Less than 1% (only 0.8%) had abnormality of IL-6 levels, of which 50% were not vaccinated with BCG. The association between BCG vaccination status and IL-6 levels was found significant with p value of < 0.05.
Table 8: Morbidity status of patients
| Morbidity other than TB | Frequency | Percentage |
|---|---|---|
| Diabetes Mellitus | 92 | 16.75% |
| Hypertension | 56 | 10.20% |
| Ischemic Heart Disease | 78 | 14.20% |
| Liver disease | 32 | 5.82% |
| No Co-morbidity | 291 | 53% |
Majority of patients were without any comorbidity.
Amongst the co-morbid, diabetes mellitus (16.75%) and ischemic heart disease (14.2%) were found in almost similar pattern. Hypertension was found in 10.2% of subjects and only 5.82% had liver disease.
Table 9: Duration of hospital stay (moderate and severe patients)
| Duration of hospital stay (moderate & severe patients) | Frequency | Percentages |
|---|---|---|
| 1 week | 234 | 86.98% |
| 2 weeks | 31 | 11.52% |
| > 2 weeks | 4 | 1.48% |
Amongst patients with moderate & severe disease (269), majority (86.98%) were admitted to the hospital for at least 1 week. Subjects admitted for 2 weeks were 11.52% and only 1.48% stayed for more than 2 weeks in the hospital for treatment.
Table 10: Outcome of severe and moderate disease
| Outcome (moderate & severe patients) | Frequency | Percentages |
|---|---|---|
| Cured | 107 | 39.78% |
| Transfer | 141 | 52.42% |
| Deceased | 2 | 0.74% |
| Complication / ADR | 3 | 1.12% |
| DAMA / LAMA | 16 | 5.95% |
| Total | 269 |
The majority (52.42%) of patients with moderate or severe disease were transferred to another facility. Almost 39.78% of patients were cured of the disease. A minor percentage of patients (1.12%) suffered either from complication or adverse drug reaction. Only two patients lost their lives during the total hospital stay. Some patients (5.95%) were in the category of DAMA (Discharged Against Medical Advice) or LAMA (Left Against Medical Advice).
Table 11: Comparison of Morbidity Status with BCG Vaccination Status
| Morbidity (other than TB) | BCG (n, %) | Non-BCG (n, %) | Total | Chi Square Value |
|---|---|---|---|---|
| Diabetes Mellitus | 35 (38%) | 57 (61%) | 92 | Chi Square = 111.12 |
| Hypertension | 12 (21%) | 44 (78%) | 56 | df = 4, p < 0.05 |
| Ischemic Heart Disease | 16 (20.5%) | 62 (79.48%) | 78 | |
| Liver Disease | 4 (12.5%) | 28 (87.5%) | 32 | |
| No Co-morbidity | 84 (28.86%) | 207 (71.13%) | 291 | |
| Total | 151 | 398 | 549 |
Amongst patients with co-morbid diabetes mellitus, 61% had not taken BCG vaccine and 38% were BCG vaccinated. Majority (78%) of patients with hypertension were also from the non-BCG vaccine group. Similar trend was found for patients of ischemic heart disease (79.48%) and liver disease (87.5%). Also, amongst patients having no comorbid conditions, most had not taken BCG vaccine (71.13%). The result was significant with p value < 0.05.
Table 12: Disease Outcome with BCG status of patients
| Outcome of Disease | BCG (n, %) | Non-BCG (n, %) | Total | Chi Square Value |
|---|---|---|---|---|
| Cured | 74 (27.8%) | 192 (72.18%) | 266 | |
| Transfer | 64 (28.07%) | 164 (71.92%) | 228 | |
| Deceased | 2 (33.33%) | 4 (66.66%) | 6 | Chi Square = 584.48, df = 4, p < 0.05 |
| Complication / ADR | 1 (11.11%) | 8 (88.88%) | 9 | |
| DAMA / LAMA | 10 (25%) | 30 (75%) | 40 | |
| Total | 151 | 398 | 549 |
Among cured patients, 72.18% were from non-BCG vaccine group. Also, amongst transferred patients, 71.92% were non-vaccinated.
Majority (66.66%) of patients who lost their lives had not taken BCG vaccine. Maximum complication or adverse drug reactions were also recorded in patients without BCG vaccination.
Among the patients who claimed DAMA / LAMA, 75% were not vaccinated with BCG.
The result was significant with p value < 0.05.
Discussion:
At the time of the COVID-19 epidemic & before COVID vaccines came into picture (and were still under research), BCG vaccine was hypothesised to play a protective role in many viral, bacterial infections, cancer etc., as well as to have potential to be part of treatment in the epidemic of COVID-19 infection. Conflicting effects of BCG vaccination are found in different researches & trials published so far.
BCG revaccination was found to have no protective role in decreasing either the duration or severity among healthcare workers in an observational randomized control double-blind phase III trial done in South Africa among 1000 health care workers. They advised non-specific effects of BCG revaccination might be population, age & disease specific.
Many studies showing cross-protective effects of BCG vaccine toward non-tuberculosis related diseases have been thought to be explained in part by trained immunity, which is a recently discovered program of innate immune memory, characterized by non-permanent epigenetic.
Reprogramming of macrophages leading to increased inflammatory cytokine production and consequently potent immune responses. These recent work highlighted potential use of BCG for treating respiratory infectious diseases and then ongoing SARS-CoV-2 clinical trials.
Bacille Calmette-Guérin (BCG) vaccination thought to have off-target (non-specific) effects associated with protection against unrelated infections and decrease all-cause mortality in infants. In contrast to the beneficial off-target effects reported following neonatal BCG in infants, a small increased risk of symptomatic febrile or respiratory illness was observed in the 12 months following BCG vaccination in adults. There was no evidence of difference in the risk of severe disease.
In anticipation of development of COVID-19 specific vaccine, several randomized controlled trials (RCTs) also explored the potential of BCG vaccination protection against COVID-19. Available results from 12 RCTs suggest BCG vaccination to be not an effective intervention against COVID-19. Study emphasized the importance of rigorous clinical trials validating hypotheses, even in urgent situations such as a pandemic.
Ecology study evaluating association of BCG vaccination on morbidity and mortality of SARS-CoV-2, adjusted for country-specific responses to the epidemic, demographics and health was undertaken with SARS-CoV-2 cases and deaths as reported by WHO including countries with policy of current BCG vaccination, having previously had BCG vaccination, and never had BCG vaccination.
In a log-linear regression model, no effect of country-level BCG status on SARS-CoV-2 cases or deaths was found. Univariable log-linear regression models showed a trend towards a weakening of the association over time. No statistical evidence for an association between BCG vaccination policy and either SARS-CoV-2 morbidity or mortality was found. The study urged countries to rather consider alternative tools with evidence supporting their effectiveness for controlling SARS-CoV-2 morbidity and mortality.
The ACTIVATE II trial also did not meet the primary endpoint of the reduction of risk for COVID-19, 3 months after BCG vaccination; however, the secondary endpoint of the reduction of the risk for COVID-19, 6 months after BCG vaccination was met. This study stated BCG vaccination might have a promising approach against the COVID-19 pandemic.
Present study had 549 total enrolled patients. Amongst them, 27.5% were BCG vaccinated, while 72.5% were non-vaccinated.
The highest number of patients fell in the age group of 26–30 years (26.7%), amongst whom 71.4% were non-vaccinated with BCG. An association was found between age group and BCG vaccination status with ‘p’ value of < 0.05.
Amongst the patients with severe disease, 70% were non-vaccinated for BCG. An association was found between severity of disease and the BCG vaccination status of the patients (‘p’ value < 0.05). Association was also found between the ventilation status and BCG vaccination status of patients as ‘p’ value came to < 0.05.
The present study shows that those who were BCG vaccinated had less severity of disease presentation as compared to non-BCG vaccinated patients who acquired COVID-19 infection (Table 3), though no significant association was found statistically. Among the 549 patients enrolled in the study, only 7.6% were having complications, of which 71.4% were not vaccinated with BCG. The association between BCG status and complications is significant with ‘p’ value < 0.01 (Tables 4, 5, 6). Here the co-morbidities and inflammatory markers were significantly less as compared to non-BCG vaccinated patients.
All presumptive cases of COVID-19 suspect presenting with mild, moderate, and severe illness, timely implementation of early supportive therapy and monitoring in terms of supportive oxygen therapy with different oxygen devices starting from nasal cannula, oxygen mask to non-invasive and invasive ventilation as per ventilation perfusion demand along with conservative fluids, empirical antibiotics, and judicious use of systemic glucocorticoids as per indication was started simultaneously along with appropriate laboratory investigations. Management of all were in accordance with revised guidelines on clinical management of COVID-19, Government of India, Ministry of Health & Family Welfare, Directorate General of Health Services (EMR Division).
COVID-19 has made patients, states, countries, and the whole world poorer in terms of health and economics. Patients lost their working days (thereby wages) due to prolonged lockdown which led to prolonged standstill in economic activities worldwide. COVID-19 patients lost their health along with economic loss, in terms of cost of admission, investigation, and treatment of the disease condition, as hospital admissions with mandatory isolation and home quarantine (for mild and moderate patients) was enforced according to international and ICMR India guidelines.
The economic cost was both at personal as well as state/country level in accordance with wages lost, work shutdown, and medical cost of investigation and treatment of COVID-19 at individual and government levels. Cost of managing the disease itself included both mandatory investigations along with drugs and mandatory gears like PPE kits, masks, gloves, etc. As cost analysis was not taken up as the primary objective of the study and only economic impact was to be seen as secondary objective, further study along those lines was not taken up. Approximate cost could be estimated by the expense incurred as treatment cost, investigation cost, and government expenditures.
Even after extensive literature review, any study (comparative or any other) that had both mortality and morbidity as well as severity of COVID-19 infection data in its study among patients who are BCG vaccinated at birth and non-BCG vaccinated patients, was not found, more so especially in developing countries like India. In view of the same, the present study is thought to be novel in that respect.
Conclusion:
With the background of conflicting study reports as shown in various cited references, the present study at Government Medical College & New Civil Hospital, Surat, Gujarat, India, leaned more towards positive results confirming its utilization towards other conditions (COVID-19) in addition to present world-wide usage in prevention and containment of tuberculosis while using history of BCG Vaccination in COVID-19 patients.
In conclusion, the study of BCG Vaccine along with its proposed utilization for conditions other than tuberculosis in any capacity, warrants further research to reach towards a concrete conclusion regarding its multiple usage.
As the vaccine is being used for containment of tuberculosis, it needs further extensive research with substantial sample size and validation to reach towards conclusion regarding its usage in COVID-19 or the likes of such epidemic lest its original usage gets compromised.
Conflict of Interest:
None.
Source of Fund:
No funding from any source. The study was done in a government set-up with no external source of any added fund in any capacity.
Acknowledgements & Contribution:
We acknowledge the contribution of our patients, nursing and other staff members, our colleagues and administration of Government Medical College & New Civil Hospital, Surat, Gujarat, India for providing invaluable support.
References
1. Upton CM, van Wijk RC, Mockeliunas L, Simonsson USH, McHarry K, van den Hoogen G, et al; BCG CORONA Consortium. Safety and efficacy of BCG re-vaccination in relation to COVID-19 morbidity in healthcare workers: A double-blind, randomised, controlled, phase 3 trial. EClinical Medicine. 2022 Jun; 48:101414. doi: 10.1016/j.ecli nm.2022.101414. E pub 2022 May 12. PMID: 35582122; PMCID: PMC9098089.
2. Gong W, Mao Y, Li Y, Qi Y. BCG Vaccination: A potential tool against COVID-19 and COVID-19-like Black Swan incidents. Int Immunopharmacol. 2022 Jul;108:108870. doi: 10.1016/j.intimp.2022.1 08870. Epub 2022 May 17. PMID: 35597119; PMCID: PMC9113676.
3. Singh AK, Netea MG, Bishai WR. BCG turns 100: its nontraditional uses against viruses, cancer, and immunologic diseases. J Clin Invest. 2021 Jun 1;131(11):e148291. doi: 10.1172/JCI148291. PMID : 34060492; PMCID: PMC8159679.
4. Evangelos J. Giamarellos-Bourboulis, Maria Tsilika, Simone Moorlag, Nikolaos Antonakos, Antigone Kotsaki, et al. Activate: Randomized Clinical Trial of BCG Vaccination against Infection in the Elderly, Cell, Volume 183, Issue 2, 2020, Pages 315-323.e9, ISSN 0092-8674, https://doi.org/10.1016/j.cell.2020.08.051. (https://www.sciencedirect.com/science/article/pii/S0092867420311399)
5. Benn CS, Martins CL, Andersen A, Fisker AB, Whittle HC, Aaby P. Measles Vaccination in Presence of Measles Antibody May Enhance Child Survival. Front Pediatr. 2020 Feb 7; 8:20. doi: 10.3389/fped.2020.00020. PMID: 32117827; PMCID: PMC7020693.
6. Sørup S, Stensballe LG, Krause TG, Aaby P, Benn CS, Ravn H. Oral Polio Vaccination and Hospital Admissions With Non-Polio Infections in Denmark: Nationwide Retrospective Cohort Study. Open Forum Infect Dis. 2015 Dec 17;3(1):ofv204. doi: 10.1093/ofid/ofv204. PMID: 26885538; PMCID: PMC4751340.
7. P. Aaby, C.S. Benn, Developing the concept of beneficial non-specific effect of live vaccines with epidemiological studies, Clinical Microbiology and Infection, Volume 25, Issue 12, 2019, Pages 1459-1467, ISSN 1198-743X, https://doi.org/10.1016/j.cmi.2019.08.011. (https://www.sciencedirect.com/science/article/pii/S1198743X19304525)
8. Leentjens J, Kox M, Stokman R, Gerretsen J, Diavatopoulos DA, van Crevel R, et al. BCG Vaccination Enhances the Immunogenicity of Subsequent Influenza Vaccination in Healthy Volunteers: A Randomized, Placebo-Controlled Pilot Study. J Infect Dis. 2015 Dec 15;212(12):1930-8. doi: 10.1093/infdis/jiv332. Epub 2015 Jun 12. PMID: 26071565.
9. Gonzalez-Perez M, Sanchez-Tarjuelo R, Shor B, Nistal-Villan E, Ochando J. The BCG Vaccine for COVID-19: First Verdict and Future Directions. Front Immunol. 2021 Mar 8;12:632478. doi: 10.3389/fimmu.2021.632478. PMID: 33763077; PMCID: PMC7982405.
10. Laure F. Pittet et al. Bacille Calmette-Guérin vaccination to prevent febrile and respiratory illness in adults (BRACE): secondary outcomes of a randomised controlled phase 3 trial, e Clinical Medicine Volume 72, 2024, 102616, ISSN 2589-5370, https://doi.org/10.1016/j.eclinm.2024.1-2615.102616. (https://www.sciencedirect.com/science/article/pii/S258953702401950) https://www.mcri.edu.au/research/projects/brace
11. Chimoyi L, Velen K, Churchyard GJ, Wallis R, Lewis JJ, Charalambous S. An ecological study to evaluate the association of Bacillus Calmette-Guerin (BCG) vaccination on cases of SARS-CoV2 infection and mortality from COVID-19. PLoS One. 2020 Dec 17;15(12):e0243707. doi: 10.1371/journa l.pone.0243707. PMID: 33332418; PMCID: PMC77 46266.
12. Noble CCA, Messina NL, Pittet LF, Curtis N. Interpreting the Results of Trials of BCG Vaccination for Protection Against COVID-19. J Infect Dis. 2023 Nov 11;228(10):1467-1478. doi: 10.1093/infdis/jiad316. PMID: 37558650; PMCID: PMC10640778.
13. Specht, A. G., Ginese, M., Kurtz, S. L., Elkins, K. L., et al. (2024). Host Genetic Background Influences BCG-Induced Antibodies Cross-Reactive to SARS-CoV-2 Spike Protein. Vaccines (Basel), 12(3), 242-. https://doi.org/10.3390/vaccines12030242
14. Pittet, L. F., Noble, C. C. A., Messina, N. L., & Curtis, N. (2024). Using BCG vaccination to protect against COVID-19: when reality fails to meet expectation. Nature Reviews. Immunology, 24(2), 83–84. https://doi.org/10.1038/s41577- 024-00992-z
15. Tsilika M, Taks E, Dolianitis K, Kotsaki A, Leventogiannis K, Damoulari C, et al. ACTIVATE-2: A Double-Blind Randomized Trial of BCG Vaccination Against COVID-19 in Individuals at Risk. Front Immunol. 2022 Jul 5; 13:873067. doi: 10.3389/fimmu.2022.873067. Erratum in: Front Immunol. 2022 Aug 31; 13:1018384. doi: 10.3389/fimmu.2022.1018384. PMID: 35865520; PMCID: PMC9294453.
16. Government of India Ministry of Health & Family Welfare Directorate General of Health Services (EMR Division) Revised Guidelines on Clinical Management of COVID – 19, 31 March, 2020 (ICMR)