Diabetes, Atrial Fibrillation, and Cardiovascular Risks
CARDIOVASCULAR OUTCOME IN TYPE 2 DIABETES AND ATRIAL FIBRILLATION- How to modify the increased cardiovascular risk?
Thomas Meinertz1, Angelika Costard-Jäckle2, Karl-Heinz Kuck3
- German Heart Foundation, Frankfurt a. M. and University Hospital Hamburg-Eppendorf, Germany
- Heart and Diabetes Center NRW Bad Oeynhausen, University Hospital (Ruhr-University, Bochum), Germany
- University Hospital Schleswig-Holstein – Campus Lübeck, Lübeck, Germany
OPEN ACCESS
PUBLISHED: 31 December 2024
CITATION: Meinertz, T., Costard-Jäckle, A., et al., 2024. ARTICLETITLE. Medical Research Archives, [online] 12(12). https://doi.org/10.18103/mra.v12i12.6025
COPYRIGHT: © 2024 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI https://doi.org/10.18103/mra.v12i12.6025
ISSN 2375-1924
ABSTRACT
The increased cardiovascular risk in patients with type 2 diabetes mellitus (DM) is further augmented by the presence of atrial fibrillation (AF). Therefore, the treatment strategy should be directed at improving the metabolic situation – e.g. avoiding marked fluctuations of blood glucose levels – and eliminating AF. Some of the life-style modifications recommended for patients with type 2 DM – such as weight reduction, engagement in regular physical activity, and cessation of tobacco use – can decrease the susceptibility to AF. The first-line pharmacological therapy of type 2 DM is metformin which also decreases the susceptibility to AF by improving insulin sensitivity. The most effective add-on therapy, especially in diabetes with atrial fibrillation, are sodium-glucose cotransporter 2 (SGLT2) inhibitors, dapagliflozin and empagliflozin. These drugs have been shown to reduce hospitalization for heart failure, cardiovascular mortality and the risk of incident atrial fibrillation. A promising new agent is the non-steroidal mineralocorticoid receptor antagonist finerenone. It reduced cardiovascular death, and the risks of hospitalization and new-onset AF. Since the risk of stroke and thromboembolism is especially high in DM with AF, nearly all affected patients need anticoagulation. Direct oral anticoagulants are more effective and safer than vitamin K antagonists. Atrial fibrillation occurs in type 2 DM despite optimal treatment of metabolic and clinical risk factors. In the past, antiarrhythmic drug treatment was used with limited success. In contrast to antiarrhythmic drug therapy, catheter ablation is an effective and safe treatment modality for the restoration of sinus rhythm. Since the long-term arrhythmia-free survival after catheter ablation is lower among patients with type 2 DM, individual ablation strategies are required in some of these patients. In summary, DM patients with AF require optimal treatment of metabolic risk factors, concomitant diseases, and specific strategies to prevent AF.
Keywords: Type 2 Diabetes, Atrial Fibrillation, Antidiabetic Medication, Catheter Ablation
Introduction
Contrary to patients with type 2 diabetes mellitus (DM) but no atrial fibrillation (AF), DM patients with AF have an increased risk of major coronary events, stroke, heart failure, and cardiovascular death. Therefore, the prevention and treatment of AF in these patients seems mandatory. The aim of this review is to discuss which antidiabetic strategy is most helpful to prevent AF and which treatment of AF is most effective to eliminate this arrhythmia.
Association between atrial fibrillation and diabetes mellitus
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia. It was once thought to be benign so long as the ventricular rate was controlled. However, AF is associated with significant cardiac morbidity and mortality. The development of AF is multifactorial in aetiology, with age, sex, genetics, race, and comorbid conditions such as smoking, obesity, hypertension, and type 2 diabetes mellitus (DM) playing a role. Like AF, the prevalence of DM is steadily increasing worldwide.
Based on epidemiologic studies, a potential association between DM and the incidence of AF has long been postulated. The Framingham Heart Study, a long-term prospective cohort study, was one of the first to demonstrate an increased risk of AF in men and women with DM. In a meta-analysis of several cohort and case-control studies, patients with DM had a 34% greater risk of developing AF. In another meta-analysis conducted in 2018, patients with DM showed a 49% higher prevalence of AF compared with the general population and, with adjusting for the risk factors of hypertension, obesity and heart disease, a relative greater risk of 23%. These studies examining the potential association between DM and AF incidence share important limitations. Most publications are secondary reports from large cohorts assembled for another purpose, without detailed characterization of the type, duration, or severity of diabetes, or standardized AF detection protocols. Furthermore, there has been diverging adjustment for confounding by other risk factors such as obesity, obstructive apnoea, and heart failure. This may in part explain the contradictory results. Notwithstanding these limitations, DM appears to be an independent but modest risk factor for the development of AF. Even more important is the observation that the increased cardiovascular risk in patients with DM is further augmented by the presence of AF. Specifically, Atrial fibrillation in diabetic patients is associated with a 61% greater risk of all-cause mortality and comparable higher risk of cardiovascular death, stroke, and heart failure. Similar results were presented in the ORBIT AF study, as high symptom burden, low life quality, cardiovascular and overall mortality was higher for AF patients with DM compared to AF patients without DM. In addition, it has been shown recently in a nationwide cohort study with more than 60,000 patients that diabetics with concurrent AF have an increased risk for macrovascular complications such as nephropathy and diabetic foot.
PATHOGENESIS OF ATRIAL FIBRILLATION IN DIABETES MELLITUS
The pathogenesis of AF in DM is complex, involving an interplay between several electrical and mechanical factors (Figure 1). The basic mechanisms are glucose intolerance, oxidative stress, and a proinflammatory environment. These mechanisms finally lead to atrial and structural remodeling and to autonomic dysfunction. Structural remodeling results in subendocardial interstitial fibrosis, atrial enlargement, and left ventricular hypertrophy. These alterations adversely impact electrical function and enhance the risk of AF.
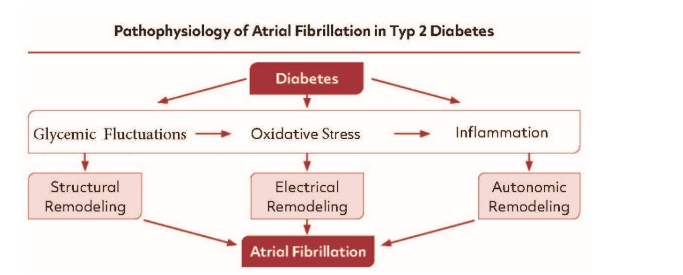
Electrical remodeling in DM may involve alterations in gap junction function that effect atrial conduction velocity due to changes in the expression of localization of connexins. Electrical remodeling can also occur due to change in atrial action potential morphology; in association, these changes in ionic currents can affect conduction velocity and evoke triggered activity. Structural remodeling in DM results in atrial fibrosis which can alter conduction patterns and susceptibility to re-entry; in addition, increases in atrial adipose tissue, especially in DM, can lead to disturbances in atrial conduction velocity and conduction patterns that may affect arrhythmogenesis. Moreover, data from experimental and clinical studies provide evidence that proinflammatory cytokines secreted from the epicardial adipose tissue may also mediate the proinflammatory response.
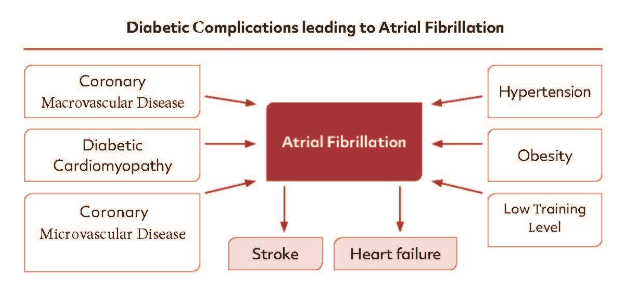
From the clinical point of view many DM patients are characterized by overweight, hypertension and a low training level, all known to favour the induction of AF (Figure 2).
Antidiabetic Treatment
Non-pharmacological lifestyle modification is recommended as part of the first-line treatment for patients with DM. This includes going on a healthy diet, engaging in regular physical activity, maintaining healthy bodyweight, getting regular and sufficient sleep and cessation of tobacco use. Some of these lifestyle modifications have been shown to also decrease the susceptibility to AF: weight reduction, engaging in regular physical activity, and tobacco cessation. Obesity, characterized by a high body mass index, is particularly concerning as it directly elevates the risk of AF and other cardiovascular morbidities. The long-term effect of weight management was investigated in the LEGACY study in an atrial fibrillation cohort. Overweight patients with an average body mass index of 27 were successful in losing approximately 36 pounds. Even this modest loss showed a marked AF reduction. Nearly half of patients went into complete remission with no further need of antiarrhythmic agents or catheter ablation. At the same time DM went into remission in 88% of the patients.
Physical inactivity contributes to AF burden and represent an independent risk factor for AF. In addition, physical activity may partially offset the elevated risk of AF associated with obesity.
With respect to drug treatment of DM, medication should aim to lower blood glucose levels and prevent glycaemic fluctuations. In this clinical setting, antidiabetic medication may differently influence the development of AF (Table 1). Metformin is the most prescribed medication. It inhibits hepatic gluconeogenesis and increases insulin sensitively. Moreover, its use has been shown to lower the risk of new-onset AF after adjusting for comorbidities and medication.
| Medication | Effect on Atrial Fibrillation |
|---|---|
| Metformin | Reduces risk of new-onset AF |
| SGLT2 Inhibitors | Lower risk of incident AF |
| GLP1 Agonists | Uncertain effect |
| Thiazolidinediones | May lower risk of AF |
Second-line add-on therapy should be considered for patients in whom HbA1c remains abnormal despite metformin treatment and lifestyle modifications. The selection of an add-on-therapy is largely directed by comorbidities and co-existing risk factors.
For patients with atherosclerotic vascular risk, heart failure or chronic kidney diseases Sodium-Glucose Cotransporter 2 (SGLT2) inhibitors or glucagon-like-peptide-1 receptor (GLP1R) agonists are the preferred option.
SODIUM-GLUCOSE COTRANSPORTER 2 (SGLT2) INHIBITORS
These drugs have well documented effects on reducing cardiovascular mortality and hospitalization for heart failure, although the effect on AF has not been comprehensively investigated. Therefore, a systematic analysis was performed to study the association of AF and treatment with SGLT2 inhibitors in patients with and without DM. More than 60,000 patients from 20 randomized trials were included. The follow-up ranged from 24 to 202 weeks. Among the SGLT2 inhibitors studied were dapagliflozin and empagliflozin. This meta-analysis indicated that SGLT2 inhibitors are associated with a lower risk of incident AF but without a significant effect on the stroke risk in either patient population.
The effect of dapagliflozin on AF has been studied in patients with DM in detail. In that retrospective analysis, dapagliflozin reduced the risk of AF relapse by 19% during a 4-year observation period. The analysis of the EMPA-REG-OUTCOME trial explored cardiovascular and renal outcomes in patients with type 2 DM without AF. Analyses were conducted in patients distinguished by the presence or absence of AF at baseline. Outcome events were more frequent in patients with AF than those without. Empagliflozin compared to placebo reduced cardiovascular events, death, or heart failure hospitalization consistently in both groups of patients. The absolute number of prevented events was higher in patients with AF, resulting in larger absolute treatment effects of empagliflozin. Empagliflozin and dapagliflozin initiators had no differences in 6-year cardiovascular outcomes in adults with treated type 2 DM with or without co-existing atherosclerotic cardiovascular disease or heart failure. Recently, however, their comparative effectiveness on AF become under debate. A real-world, population-based study demonstrated that patients with DM using dapagliflozin may have a lower risk (-11.5%) of developing nonvalvular AF than those using empagliflozin. That analysis has one major limitation: The database does not provide information on the type or burden of AF, such as whether it was paroxysmal, persistent, or permanent. Therefore, the clinical consequences of this study result remain completely unclear.
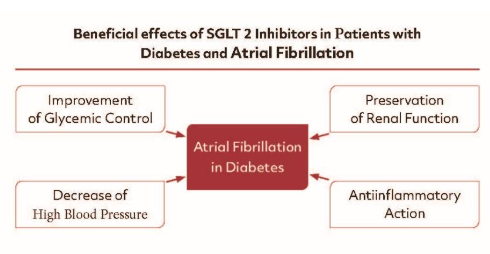
The beneficial cardiovascular effects of SGLT2 inhibitors can be explained by several mechanisms (Figure 3). However, which of these mechanisms proven at the bench play a major role in the clinical setting is still under investigation. Yet, it seems clear that the beneficial cardiovascular effects of the SGLT2 inhibitors are the consequences of several of these mechanisms acting “in cooperation”. Since the clinical benefits occur also in patients without DM and without AF, the antidiabetic and antiarrhythmic mechanisms are unlikely to play the key role for the beneficial effects of these agents.
GLUCAGON-LIKE-PEPTIDE 1 RECEPTOR (GLP1R) AGONISTS
Glucagon-Like-Peptide 1 receptor agonists are used to treat DM but the effects on AF remain unclear. In a DM animal model (mice) which is highly susceptible to AF GLP1 receptor agonists as well as long acting GLP1 receptor agonists liraglutide reduce the atrial electrical and structural remodeling and thereby prevent AF. The effect of GLP1-R agonists on AF was analysed in a systematic review and meta-analysis of randomized controlled trials. The authors concluded that the treatment with GLP1-receptor agonists is not associated with AF. A recently updated meta-analysis of randomized controlled trials demonstrated that the treatment with GLP1-receptor agonists is associated with a reduction of major cardiovascular events, cardiovascular and all-cause mortality, and stroke. Conversely, the effects on heart failure and AF remained uncertain.
THIAZOLIDINEDIONES (TZDs)
Animal studies have shown that TZDs limit inflammatory atrial fibrosis, which may lower the risk for DM-associated AF. In a meta-analysis, patients treated with TZDs had a 27% lower risk of AF compared with controls. Subgroup analyses revealed that pioglitazone, but not rosiglitazone, was associated with a lower risk of AF.
FINERENONE
The non-steroidal mineralocorticoid receptor antagonist finerenone has been studied in three prospective randomized trials in patients with “cardio-kidney-metabolic syndrome”. The results were summarized in a prespecified participant level analysis. The three trials included about 19,000 participants. During a median follow up of 2.9 years the primary outcome of cardiovascular death occurred in 4.4% assigned to finerenone and 5.0% assigned to placebo (p=0.076). Finerenone reduced the risk of hospitalizations and new-onset AF. The treatment effects were constant across all clinically relevant subgroups. These beneficial effects of finerenone on AF might be due to an improved structural remodeling at the atrial and ventricular level and an increase in serum potassium levels.
ANTICOAGULANT TREATMENT
The major feature of DM associated with AF is the markedly increased risk of cerebral and systemic embolic events. Several studies have reported the interaction between DM, AF, and the thromboembolic risk. For example, the ATRIA study showed that the duration of DM ≥ 3 years compared to that of < 3 years was associated with an increased risk of ischemic stroke (adjusted HR 1.74). The risk of thromboembolic events was also increased in patients requiring insulin and in those with a clearly elevated HbA1c. Overall, the annual incidence of stroke in AF patients with DM is between 3.6% and 8.6%. One reason for the markedly increased risk of thromboembolic complications is a state of hypercoagulability due to platelet activation, high inflammatory status, oxidative stress, and insulin treatment.
Management of AF in patients with DM is not generally different from that recommended for subjects without DM. Anticoagulant treatment is the top priority in the management of AF associated with DM to prevent stroke or systemic embolism. It should be kept in mind that patients with paroxysmal, persistent, or permanent AF have the same risk of stroke. Although the risk of stroke is high in diabetic patients with AF, it varies among patients. Thus, the need of anticoagulant therapy should rely on risk evaluation for stroke compared to the risk of bleeding complications caused by anticoagulant therapy. The European Society of Cardiology guidelines for the management of AF (ESC 2010) recommended the use of the so called CHA2DS2-VASc Score. Using this scoring system, most DM patients with AF should be treated with anticoagulant therapy. Warfarin and phenprocoumon have been the anticoagulants of choice for many decades. However, only a low percentage of patients reach treatment goals (<40%) and close monitoring of patients is required. Currently, these anticoagulants have been replaced by direct thrombin inhibitors (dabigatran) or direct factor Xa inhibitors (apixaban, rivaroxaban, edoxaban). The effects of these agents have been studied in large clinical trials and meta-analyses versus vitamin K antagonists. The analyses referred to the RE-LAY (dabigatran), ROCKET-AT-(rivaroxaban), ARISTOTLE (apixaban), ENGATIKI 48 (edoxaban) studies. The studies mentioned did not specifically investigate the efficacy of direct oral anticoagulants (DOACs) vs. warfarin on nonvalvular AF in diabetic patients. However, DM accounted for 30-35% in the total analysed population. No differences were reported in the sub-analysis for atrial fibrillation in DM compared to all other cases in the studies.
A first meta-analysis from 2014 comprising the four core studies and including more than 70,000 patients yielded conclusive results. Compared with warfarin, DOACs reduced stroke and systemic embolism in nonvalvular AF (by 19%) all-cause mortality (by about 10%) intracerebral haemorrhage (by about 50%). The results of the studies in subgroups of diabetic patients suggest that patients with longer duration of diabetes, increased HbA1c and insulin-dependent diabetes may benefit more from oral anticoagulation – even in the absence of other major risk factors for thromboembolism.
ANTIARRHYTHMIC TREATMENT
Atrial fibrillation occurs in patients with DM despite optimal treatment of metabolic and clinical risk factors. In the past, antiarrhythmic drug treatment was the only way to achieve a suppression of AF. Previous animal studies suggested that antiarrhythmic drugs may be less effective in patients with DM. Currently, no clinical study has evaluated the effectiveness of antiarrhythmic drugs in patients with AF and DM. However, DM patients may be at an increased risk of adverse effects from antiarrhythmic agents, due to the large prevalence of silent coronary artery disease, heart failure, and chronic kidney disease. Furthermore, the high incidence of QTc prolongation among patients with DM may further increase the proarrhythmic risk. Since many of the patients with DM have also contraindications against antiarrhythmic agents like flecainide and propafenone, low-dose amiodaron treatment remains as a therapy of choice. However, long term amiodaron therapy is limited by the well-known side effects of this drug – thyroid disorder, neuropathy, liver toxicity.
By contrast, catheter ablation therapy is an effective treatment modality for the restoration and maintenance of sinus rhythm in this patient population. Prior clinical studies have examined the effectiveness and safety of catheter ablation for AF in patients with DM. It could be shown that catheter ablation can significantly improve quality of life, with data supporting its superiority relative to antiarrhythmic agents for long-term prognostic outcome. Furthermore, catheter ablation of AF in diabetic patients reduced the risk of stroke and mortality in a propensity score-matched analysis from a large patient population. Interestingly the risk was predominantly decreased in patients with high CHA2DS2-VASc score.
CATHETER ABLATION
Previous studies on the long-term efficacy of catheter ablation of AF in DM have resulted in conflicting results. In a multicenter cohort study from Germany which enrolled 8,175 patients with AF for catheter ablation, 944 patients with DM were included. During a one-year follow-up there was no difference in AF recurrence between the 2 groups. Similarly, a systematic review and meta-analysis found no difference in the efficacy and safety of catheter ablation in patients with and without DM. These meta-analyses examining the recurrence of AF following catheter ablation in diabetic and non-diabetic patients have important limitations. Most publications are secondary reports from large cohorts assembled for another purpose. There is a lack of detail characterization of the type, duration, and the variety of DM and of an external standardized protocol for the detection of AF. Furthermore, there have been variable adjustments for other confounding risk factors. This may at least in part explain the contradictory results.
In a retrospective analysis, 351 patients who underwent first-time AF ablation were followed for 29.5 months after the intervention. Arrhythmia recurrence was significantly higher in the DM group than in the no-DM group after adjustment for baseline differences (56.9% in the diabetic and 33.1% in the non-diabetic group). There was a non-significant trend toward higher AF recurrence with worse glycaemic control. AF ablation was not associated with increased procedural risk, despite the higher burden of comorbidities in this population. Creta and coworkers performed a nonrandomized, observational study in 7 high-volume centers in Europe. A total of 2,504 patients who underwent catheter ablation of AF were included. Patients with DM were older, had a higher BMI and suffered more frequently from persistent AF. Arrhythmia recurred more frequently in the DM group (32.0% vs. 25.3%). After adjusting for the type of AF (paroxysmal vs. persistent) during a median follow up of 17 months, atrial arrhythmia-free survival was lower in the diabetic group with persistent AF and comparable for paroxysmal AF. Diabetes was also an independent predictor of AF recurrence in the multivariate analysis. There were no significant differences in the periprocedural complication rate between non-diabetics and diabetics. The impact of DM on AF recurrence and major adverse cardiac and cerebrovascular events following catheter ablation was studied recently in a large Chinese patient population. Patients with and without DM were followed prospectively for an average period of about 4 years: A higher incidence of AF recurrence (34.6% vs 24.6%) and a higher frequency of major adverse cardiac events (9.3% vs 6.5%) was observed among diabetic patients. Interestingly, new-onset DM was also found to be an independent predictor of AF recurrence.
Taken together, these studies indicate that recurrences of AF in diabetics seems to be higher than in non-diabetics. Observations in the electrophysiological laboratory may explain this phenomenon. Patients with diabetes and AF have larger scar areas in the left atrium than AF patients without DM. These areas can be localized as left atrial low-voltage areas which were especially often found in diabetics with impaired glycaemic control. It is well known that these areas are associated with an increased susceptibility to AF. From these observations it can be deduced that AF in the DM patient may be related to large scar areas which can be treated not only by pulmonary vein isolation but also, on an individual basis, with additional interventions within the left atrium. In patients with DM and AF, SGLT2-inhibitors seem to reduce the susceptibility to AF. In line with these findings, SGLT2-inhibitors lowered the risk of AF recurrence, the need for new antiarrhythmic drug therapy and repeat AF ablation during a 12-month period following the ablation procedure. However, the problem of AF recurrence following catheter ablation in the diabetic patient cannot be solved by drug treatment. Most patients need a repeat procedure.
Conclusions
Since AF impairs the life expectancy of the diabetic patient the therapeutic strategy must be directed at preventing and/or eliminating this arrhythmia. “Non-pharmacological” life style modifications such as weight reduction and physical activity may help to decrease the susceptibility to AF. Additionally, some antidiabetic medications may lower the risk of this arrhythmia. Despite these measures AF may develop in many of the diabetic patients. In most of them, anticoagulation with direct oral anticoagulants is necessary and complete elimination of AF can only be achieved by catheter ablation.
References
- Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840-844.
- Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. AmJ Cardiol. 2011;108:56-62. Doi:10.1016/j.amjcard.2011.03.004.
- Xiang H, Xue Y, Chen Z, Yu Y, Peng Y, Wang J, et al. The Association Between Left Ventricular Hypertrophy and the Occurrence and Prognosis of Atrial Fibrillation: A Meta-Analysis. Front Cardiovasc Med. 2021;8:639993. Doi: 10.3389/fcvm.2021.639993.
- Bohne LJ, Johnson D, Rose RA, Wilton SB, Gillis AM. The Association Between Diabetes Mellitus and Atrial Fibrillation: Clinical and Mechanistic Insights. Front Physiol. 2019;10:135. Doi:10.3389/fphys.2019.00135.
- Costard-Jäckle A, Tschöpe D, Meinertz T. Cardiovascular outcome in type 2 diabetes and atrial fibrillation. Herz 2019;44:522–525. Doi: 10.1007/s00059-018-4704-4.
- Lorenzo-Almorós A, Casado Cerrada J, Álvarez-Sala Walther LA, Méndez Bailón M, Lorenzo González Ó. Atrial Fibrillation and Diabetes Mellitus: Dangerous Liaisons or Innocent Bystanders? J Clin Med. 2023;12(8):2868. Doi: 10.3390/jcm12082868.
- Du X, Ninomiya T, de Galan B, Abadir E, Chalmers J, Pillai A, et al.; ADVANCE Collaborative Group. Risks of cardiovascular events and effects of routine blood pressure lowering among patients with type 2 diabetes and atrial fibrillation: results of the ADVANCE study. Eur Heart J. 2009;30:1128-1135. Doi: 10.1093/eurheartj/ehp055.
- Echouffo-Tcheugui JB, Shrader P, Thomas L, Gersh BJ, Kowey PR, Mahaffey KW, et al. Care Patterns and Outcomes in Atrial Fibrillation Patients With and Without Diabetes: ORBIT-AF Registry. J Am Coll Cardiol. 2017;70):1325-1335. Doi: 10.1016/j.jacc.2017.07.755.
- Kwon S, Lee SR, Choi EK, Ahn HJ, Lee SW, Jung JH et al. Association Between Atrial Fibrillation and Diabetes-Related Complications: A Nationwide Cohort Study. Diabetes Care. 2023;46:2240-2248. Doi: 10.2337/dc23-0931.
- Wang A, Green JB, Halperin JL, Piccini JP Sr. Atrial Fibrillation and Diabetes Mellitus: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;74:1107-1115. Doi: 10.1016/j.jacc.2019.07.020.
- Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, et al. Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study (LEGACY). J Am Coll Cardiol. 2015;65:2159-2169. Doi: 10.1016/j.jacc.2015.03.002.
- Chung MK, Eckhardt LL, Chen LY, Ahmed HM, Gopinathannair R, Joglar JA, et al.; American Heart Association Electrocardiography and Arrhythmias Committee and Exercise, Cardiac Rehabilitation, and Secondary Prevention Committee of the Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and Council on Lifestyle and Cardiometabolic Health. Lifestyle and Risk Factor Modification for Reduction of Atrial Fibrillation: A Scientific Statement from the American Heart Association. Circulation. 2020;141:e750-e772. Doi: 10.1161/CIR.0000000000000748.
- Chang SH, Wu LS, Chiou MJ, Liu JR, Yu KH, Kuo CF, et al. Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: a population-based dynamic cohort and in vitro studies. Cardiovasc Diabetol. 2014;13:123. Doi: 10.1186/s12933-014-0123-x.
- Zheng RJ, Wang Y, Tang JN, Duan JY, Yuan MY, Zhang JY. Association of SGLT2 Inhibitors With Risk of Atrial Fibrillation and Stroke in Patients With and Without Type 2 Diabetes: A Systemic Review and Meta-Analysis of Randomized Controlled Trials. J Cardiovasc Pharmacol. 2022;79:e145-e152. Doi: 10.1097/FJC.0000000000001183.
- Zelniker TA, Bonaca MP, Furtado RHM, Mosenzon O, Kuder JF, Murphy SA, et al. Effect of Dapagliflozin on Atrial Fibrillation in Patients With Type 2 Diabetes Mellitus: Insights From the DECLARE-TIMI 58 Trial. Circulation. 2020;141:1227-1234. Doi:10.1161/CIRCULATIONAHA.119.044183.
- Böhm M, Slawik J, Brueckmann M, Mattheus M, George JT, Ofstad AP, et al. Efficacy of empagliflozin on heart failure and renal outcomes in patients with atrial fibrillation: data from the EMPA-REG OUTCOME trial. Eur J Heart Fail. 2020;22:126-135. Doi: 10.1002/ejhf.1663.
- Bonnesen K, Heide-Jørgensen U, Christensen DH, Lash TL, Hennessy S, Matthews A, et al. Comparative Cardiovascular Effectiveness of Empagliflozin Versus Dapagliflozin in Adults with Treated Type 2 Diabetes: A Target Trial Emulation. Circulation. 2024 Aug 29. Doi:10.1161/circulationaha.124.068613.
- Lim J, Kwak S, Choi YJ, Rhee TM, Park CS, Kim B, et al. Differing Efficacy of Dapagliflozin Versus Empagliflozin on the Risk of Incident Atrial Fibrillation in Patients With Type 2 Diabetes: A Real-World Observation Using a Nationwide, Population-Based Cohort. J Am Heart Assoc. 2024;13:e030552. Doi:10.1161/JAHA.123.030552.
- Bohne LJ, Jansen HJ, Dorey TW, Daniel IM, Jamieson KL, Belke DD, et al. Glucagon-Like Peptide-1 Protects Against Atrial Fibrillation and Atrial Remodeling in Type 2 Diabetic Mice. JACC Basic Transl Sci. 2023;8:922-936. Doi:10.1016/j.jacbts.2023.01.005.
- Wei J, Wang R, Ye H, Wang Y, Wang L, Zhang X. Effects of GLP-1 receptor agonists on arrhythmias and its subtypes in patients with type 2 diabetes: A systematic review and meta-analysis. Front Endocrinol (Lausanne). 2022;13:910256. Doi:10.3389/fendo.2022.910256.
- Stoll L, Lo JC. GLP-1 Receptor Agonists, the Holy Grail Preventing Atrial Fibrillation in Patients With T2D? JACC Basic Transl Sci. 2023;8:937-938. Doi:10.1016/j.jacbts.2023.03.022.
- Nreu B, Dicembrini I, Tinti F, Sesti G, Mannucci E, Monami M. Major cardiovascular events, heart failure, and atrial fibrillation in patients treated with glucagon-like peptide-1 receptor agonists: An updated meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2020;30:1106-1114. Doi: 10.1016/j.numecd.2020.03.013.
- Kume O, Takahashi N, Wakisaka O, Nagano-Torigoe Y, Teshima Y, Nakagawa M, et al. Pioglitazone attenuates inflammatory atrial fibrosis and vulnerability to atrial fibrillation induced by pressure overload in rats. Heart Rhythm. 2011;8:278-285. Doi:10.1016/j.hrthm.2010.10.029.
- Zhang Z, Zhang X, Korantzopoulos P, Letsas KP, Tse G, Gong M, et al. Thiazolidinedione use and atrial fibrillation in diabetic patients: a meta-analysis. BMC Cardiovasc Disord. 2017;17:96. Doi:10.1186/s12872-017-0531-4.
- Filippatos G, Bakris GL, Pitt B, Agarwal R, Rossing P, Ruilope LM, et al.; FIDELIO-DKD Investigators. Finerenone Reduces New-Onset Atrial Fibrillation in Patients With Chronic Kidney Disease and Type 2 Diabetes. J Am Coll Cardiol. 2021;78:142-152. Doi:10.1016/j.jacc.2021.04.079.
- Ashburner JM, Go AS, Chang Y, Fang MC, Fredman L, Applebaum KM, et al. Effect of Diabetes and Glycemic Control on Ischemic Stroke Risk in AF Patients: ATRIA Study. J Am Coll Cardiol. 2016;67(3):239-47. Doi:10.1016/j.jacc.2015.10.080.
- Patti G, Lucerna M, Cavallari I, Ricottini E, Renda G, Pecen L, et al. Insulin-Requiring Versus Noninsulin-Requiring Diabetes and Thromboembolic Risk in Patients with Atrial Fibrillation: PREFER in AF. J Am Coll Cardiol. 2017;69:409-419. Doi:10.1016/j.jacc.2016.10.069.
- Saliba W, Barnett-Griness O, Elias M, Rennert G. Glycated hemoglobin and risk of first episode stroke in diabetic patients with atrial fibrillation: A cohort study. Heart Rhythm. 2015;12:886-92. Doi:10.1016/j.hrthm.2015.01.025.
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al.; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373-498. Doi:10.1093/eurheartj/ehaa612.
- Itzhaki Ben Zadok O, Eisen A. Use of non-vitamin K oral anticoagulants in people with atrial fibrillation and diabetes mellitus. Diabet Med. 2018;35:548-556. Doi: 10.1111/dme.13600.
- Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962. Doi:10.1016/S0140-6736(13)62343-0.
- Veglio M, Bruno G, Borra M, Macchia G, Bargero G, D’Errico N, et al. Prevalence of increased QT interval duration and dispersion in type 2 diabetic patients and its relationship with coronary heart disease: a population-based cohort. J Intern Med. 2002;251:317-524. Doi:10.1046/j.1365-2796.2002.00955.x.
- Blomström-Lundqvist C, Gizurarson S, Schwieler J, Jensen SM, Bergfeldt L, Kennebäck G, et al. Effect of Catheter Ablation vs Antiarrhythmic Medication on Quality of Life in Patients With Atrial Fibrillation: The CAPTAF Randomized Clinical Trial. JAMA. 2019;321:1059-1068. Doi:10.1001/jama.2019.0335.
- Asad ZUA, Yousif A, Khan MS, Al-Khatib SM, Stavrakis S. Catheter Ablation Versus Medical Therapy for Atrial Fibrillation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Circ Arrhythm Electrophysiol. 2019;12:e007414. Doi:10.1161/CIRCEP.119.007414.
- Saliba W, Schliamser JE, Lavi I, Barnett-Griness O, Gronich N, Rennert G. Catheter ablation of atrial fibrillation is associated with reduced risk of stroke and mortality: A propensity score-matched analysis. Heart Rhythm. 2017;14:635-642. Doi:10.1016/j.hrthm.2017.02.001.
- Bogossian H, Frommeyer G, Brachmann J, Lewalter T, Hoffmann E, Kuck KH, et al. Catheter ablation of atrial fibrillation and atrial flutter in patients with diabetes mellitus: Who benefits and who does not? Data from the German ablation registry. Int J Cardiol. 2016;214:25-30. Doi:10.1016/j.ijcard.2016.03.069.
- Anselmino M, Matta M, D’ascenzo F, Pappone C, Santinelli V, Bunch TJ, et al. Catheter ablation of atrial fibrillation in patients with diabetes mellitus: a systematic review and meta-analysis. Europace. 2015;17:1518-1525. Doi:10.1093/europace/euv214.
- Wang A, Truong T, Black-Maier E, Green C, Campbell KB, Barnett AS, et al. Catheter ablation of atrial fibrillation in patients with diabetes mellitus. Heart Rhythm O2. 2020;1:180-188. Doi:10.1016/j.hroo.2020.04.006.
- Creta A, Providência R, Adragão P, de Asmundis C, Chun J, Chierchia G, et al. Impact of Type-2 Diabetes Mellitus on the Outcomes of Catheter Ablation of Atrial Fibrillation (European Observational Multicentre Study). Am J Cardiol. 2020;125:901-906. Doi: 10.1016/j.amjcard.2019.12.037.
- Lin M, Wang J, Rong B, Zhang K, Chen T, Han W, et al. Impact of Diabetes Mellitus on Atrial Fibrillation Recurrence and Major Adverse Cardiac and Cerebrovascular Events following Catheter Ablation. Int J Clin Practice 2024;1087623. Doi:10.1155/2024/1087623.
- Guckel D, Isgandarova K, Bergau L, Piran M, El Hamriti M, Imnadze G, et al. The Effect of Diabetes Mellitus on the Recurrence of Atrial Fibrillation after Ablation. J Clin Med. 2021;10:4863. Doi:10.3390/jcm10214863.
- Williams TL, Ma JK, Martin Ginis KA. Participant experiences and perceptions of physical activity-enhancing interventions for people with physical impairments and mobility limitations: a meta-synthesis of qualitative research evidence. Health Psychol Rev. 2017;11:179-196. Doi:10.1080/17437199.2017.1299027.
- Ouyang F, Ernst S, Chun J, Bänsch D, Li Y, Schaumann A, et al. Electrophysiological findings during ablation of persistent atrial fibrillation with electroanatomic mapping and double Lasso catheter technique. Circulation. 2005;112:3038-3048. Doi:10.1161/CIRCULATIONAHA.105.561183.
- Rottner L, Metzner A, Ouyang F, Heeger C, Hayashi K, Fink T, et al. Direct Comparison of Point-by-Point and Rapid Ultra-High-Resolution Electroanatomical Mapping in Patients Scheduled for Ablation of Atrial Fibrillation. J Cardiovasc Electrophysiol. 2017;28:289-297. Doi:10.1111/jce.13160.
- Guckel D, Sohns C. Diabetes mellitus und Vorhofflimmern: Erhöhtes kardiales Risiko, fokussierte Therapiekonzepte. Dtsch Arztebl 2024;121:[6]; DOI: 10.3238/PersKardio.2024.09.20.01. German
- Matsuda Y, Masuda M, Uematsu H, Sugino A, Ooka H, Kudo S, et al. Impact of diabetes mellitus and poor glycaemic control on the prevalence of left atrial low-voltage areas and rhythm outcome in patients with atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2024;35:775-784. Doi:10.1111/jce.16219.
- Abu-Qaoud MR, Kumar A, Tarun T, Abraham S, Ahmad J, Khadke S, et al. Impact of SGLT2 Inhibitors on AF Recurrence After Catheter Ablation in Patients With Type 2 Diabetes. JACC Clin Electrophysiol. 2023;9:2109-2118. Doi:10.1016/j.jacep.2023.06.008.
- Kishima H, Mine T, Fukuhara E, Kitagaki R, Asakura M, Ishihara M. Efficacy of Sodium-Glucose Cotransporter 2 Inhibitors on Outcomes After Catheter Ablation for Atrial Fibrillation. JACC Clin Electrophysiol. 2022;8:1393-1404. Doi:10.1016/j.jacep.2022.08.004.

