Diagnostic Accuracy of Phase Contrast MRI in CSF Flow
Diagnostic Accuracy of Phase Contrast MRI Technique in Detecting Cerebrospinal Fluid Flow
Dr. Danish Ansari1, Dr. Sachin Khanduri1, Dr. Kunal Dwari1, Dr. Avani Kanojia1, Dr. Vinima Jaiswal1, Dr. Nida Yasrab1, Dr. K. Prithvi Perumal1, Dr. Sajida Ansari1
- Department of Radiodiagnosis, Era’s Lucknow Medical College & Hospital, Lucknow, India
OPEN ACCESS
PUBLISHED: 31 MaRCH 2025
CITATION:KHANDURI, Dr. Sachin et al. Diagnostic Accuracy of Phase Contrast MRI Technique in Detecting Cerebrospinal Fluid Flow. Medical Research Archives,.Available at: <https://esmed.org/MRA/mra/article/view/6391>.
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ISSN 2375-1924
Abstract
Introduction: Cerebrospinal fluid (CSF) dynamics play a critical role in neurological conditions such as normal pressure hydrocephalus (NPH), aqueductal stenosis, and Chiari malformation and also play a crucial role in maintaining the homeostasis of the central nervous system (CNS). The use of phase-contrast magnetic resonance imaging (PC-MRI) to non-invasively quantify cerebrospinal fluid dynamics (CSF) aid in diagnosis and treating such conditions. The aim of the study is to evaluate the diagnostic accuracy of phase contrast MRI technique in detecting cerebrospinal fluid flow.
Methodology: This study was a cross-sectional study and it included 136 participants and was conducted at the Department of Radiodiagnosis, Era’s Lucknow Medical College and Hospital, Lucknow, India. All subjects under the inclusion criteria, underwent PC-MRI using a 3T MRI scanner to evaluate CSF flow dynamics, with post-processing performed on a Syngovia workstation.
Results: The majority of participants were male (55.88%), with a mean age of 46.84 years. Normal pressure Hydrocephalus (NPH) was the most prevalent condition (35.29%), followed by diffuse cerebral atrophy (23.53%). The study revealed distinct cerebrospinal fluid flow patterns across neurological conditions. Normal pressure Hydrocephalus (NPH) showed the highest peak systolic velocity (PSV) at 9.24 cm/s and a stroke volume of 65 µl. Aqueductal stenosis exhibited a lower PSV of 6.53 cm/s but a higher stroke volume of 70 µl. These findings demonstrate significant variations in CSF dynamics, emphasizing the utility of PC-MRI.
Conclusion: Phase contrast – Magnetic resonance imaging is a valuable tool for assessing cerebro-spinal fluid flow dynamics, providing critical insights into the pathophysiology of neurological conditions. The study highlights the importance of peak velocity as a reliable metric, suggesting its incorporation into routine clinical assessments to enhance diagnostic accuracy and patient outcomes.
Keywords: Cerebrospinal fluid, Phase-contrast MRI, Normal pressure hydrocephalus, Chiari malformation, Aqueductal stenosis, Peak systolic velocity.
Introduction
Cerebrospinal fluid (CSF) flows from the lateral ventricles to the third ventricle via the foramina of Monro, and subsequently enters the fourth ventricle through the aqueduct of Sylvius. From the fourth ventricle, CSF predominantly exits into the subarachnoid space (SAS) through the median foramen of Magendie and the lateral apertures of Luschka, where it circulates around the brain and spinal cord. The spinal SAS is anatomically divided into anterior and posterior sections by dentate ligaments, which provide lateral support to the spinal cord.
Although CSF can potentially circulate through both the central canal and the SAS within the spinal canal, the central canal is typically non-functional in adults over the age of 30. CSF also interacts with brain extracellular spaces and mixes with extracellular fluid within Virchow-Robin spaces. A significant portion of CSF is absorbed through the extracellular matrix and the lymphatic system, including pathways to cervical lymph nodes and the lumbar cistern.
Early MRI techniques visualized CSF flow in the aqueduct by detecting flow voids—artifacts caused by high flow velocities. These flow voids were associated with positive outcomes following shunt procedures. Initial methods for quantifying CSF flow relied on T1 and T2 relaxation times or comparisons of saturation between stationary and flowing tissues. Advances in imaging, such as a modified gradient echo technique using phase shifts from bipolar gradients, improved visualization and facilitated the selection of patients for shunting. Phase-contrast MRI (PC-MRI) operates on the principle that moving spins acquire phase shifts in a magnetic field gradient, while stationary spins remain unaffected. By employing bipolar gradients, static phase effects are neutralized, and motion is indicated by residual phase shifts.
Non-invasive PC-MRI has become a valuable tool for quantifying CSF dynamics, assisting in the diagnosis, prognosis, and treatment of neurological conditions such as syringomyelia, hydrocephalus, and Chiari malformations. Abnormal CSF flow patterns, including increased velocities or flow rates, may signify Chiari malformation and related disorders. Two principal imaging modalities—4D Flow and 2D PC-MRI—are employed to study CSF dynamics, with 2D PC-MRI being the most widely recognized for both qualitative and quantitative analysis. This study aimed to evaluate CSF flow using phase-contrast MRI.
Methodology
STUDY DESIGN AND SETTING
This cross-sectional study was conducted at the Department of Neurology OPD and Ward, and the Department of Radiodiagnosis at Era’s Medical College, Lucknow, India, over a 24-month period. After obtaining ethical clearance and informed consent, 136 subjects were included in the study.
INCLUSION AND EXCLUSION CRITERIA
Participants were recruited based on clinical symptoms, including headache, vomiting, dementia, visual disturbances, or cognitive impairments, along with conventional MRI findings suggestive of conditions such as normal pressure hydrocephalus (NPH), aqueductal stenosis, Chiari malformation, syringomyelia, or arachnoid cysts. Patients were excluded if they had cardiac arrhythmias or absolute contraindications to MRI, such as claustrophobia, pacemakers, or permanent metallic implants. Written informed consent was obtained from all participants after a thorough explanation of the study. The study received approval from the institutional ethics committee, and strict confidentiality of patient data was maintained.
IMAGING PROCEDURE
All participants underwent Phase Contrast Magnetic Resonance Imaging (PC MRI) using a 3T MRI scanner (Magnetom Vida, Siemens Healthineers). Imaging was conducted with standard head coils, with subjects positioned in a neutral supine posture. To minimize motion artifacts, participants were instructed to avoid deep breathing during the procedure. Routine MRI sequences included axial T1, T2, and FLAIR, sagittal T1, and coronal T2. Additionally, a midsagittal steady-state free precession sequence with thin slices (0.5–1 mm) was performed to evaluate the aqueduct of Sylvius. This sequence, referred to as True FISP in Siemens systems and FIESTA in GE systems, provided detailed visualization of CSF flow.
A two-dimensional cine phase-contrast (2D cine-PC) MRI, synchronized with the cardiac cycle, was used to capture CSF flow during systole and diastole. Imaging parameters for this sequence included a repetition time (TR) of 25 ms, echo time (TE) of 4.3 ms, flip angle of 10°, two acquisitions, a field of view (FOV) of 180 mm, matrix dimensions of 128 × 512, slice thickness of 1 mm, phase encoding velocity (VENC) of 5–10 cm/s, and a total acquisition time of approximately 2.25 minutes.
IMAGE ANALYSIS AND POST-PROCESSING
The images were post-processed using a Syngovia workstation, enabling an in-depth analysis of CSF flow dynamics. Experienced radiologists reviewed all the images, and statistical analyses were conducted to formulate the study’s conclusions.
STATISTICAL ANALYSIS
The data were entered into Microsoft Excel and analyzed using SPSS version 26 (SPSS Inc., Chicago, IL, USA). Continuous variables were summarized as mean with standard deviation or as range values, depending on suitability. Dichotomous variables were reported as frequencies and percentages.
Results
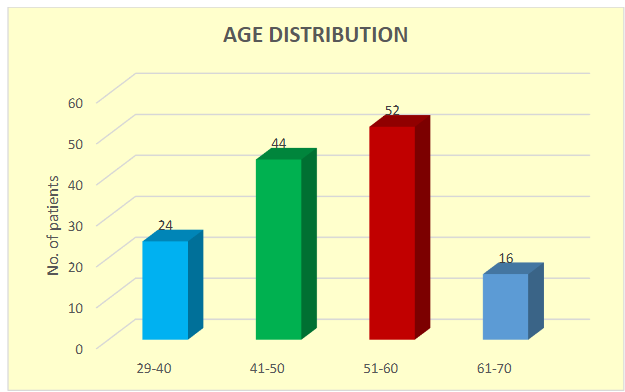
The study found that most participants were between the ages of 51 and 60, comprising 38.24% of the sample, followed by those aged 41 to 50 years (32.35%). The average participant age was 46.84 ± 7.84 years.
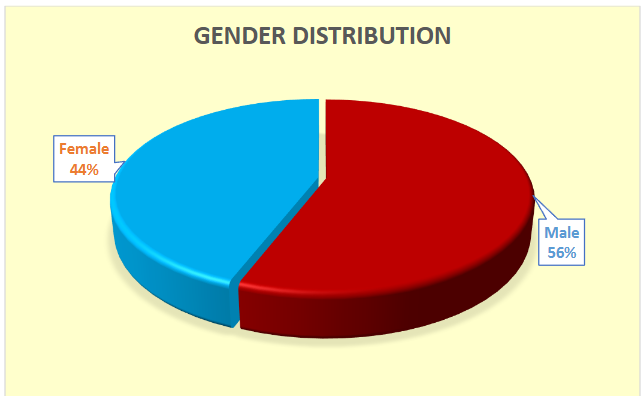
Males constituted the majority, accounting for 55.88% of the cohort.
The most common diagnosis was Normal Pressure Hydrocephalus (NPH), affecting 35.29% of participants, followed by diffuse cerebral atrophy (23.53%). Headache was the most frequently reported symptom, affecting 58.82% of the participants.
| Condition | Number | Percentage |
|---|---|---|
| Diffuse cerebral atrophy | 32 | 23.53% |
| Aqueductal Stenosis | 16 | 11.76% |
| Chiari Malformation | 12 | 8.82% |
| Others (Tumor) | 16 | 11.76% |
| Arachnoid Cysts | 12 | 8.82% |
| NPH (Normal Pressure Hydrocephalus) | 48 | 35.29% |
Distinct CSF flow patterns were identified across various neurological conditions. NPH demonstrated the highest PSV (9.24 ± 4.51 cm/s) and a relatively high stroke volume (65 ± 20 µl). Aqueductal stenosis exhibited lower PSV (6.53 ± 2.25 cm/s) and EDV (2.9 ± 1.5 cm/s) but had a slightly elevated stroke volume (70 ± 25 µl). Chiari malformation showed intermediate values for these parameters. Other conditions presented a PSV of 8.16 ± 4.09 cm/s and a stroke volume of 63 ± 15 µl, representing a range of intermediate measurements.
| Symptoms | Number | Percentage |
|---|---|---|
| Headache | 80 | 58.82% |
| Cognitive Impairment | 48 | 35.29% |
| Gait Disturbance | 40 | 29.41% |
| Visual Disturbances | 32 | 23.53% |
| Nausea/Vomiting | 24 | 17.65% |
| Neck Pain | 20 | 14.71% |
| Dizziness | 20 | 14.71% |
| Numbness/Tingling | 16 | 11.76% |
| Seizures | 12 | 8.82% |
| Urinary Incontinence | 12 | 8.82% |
| Weakness | 12 | 8.82% |
| Sensory Impairment | 8 | 5.88% |
The mean peak systolic velocity (PSV) was 8.58 ± 5.59 cm/s, the mean end-diastolic velocity (EDV) was 3.31 ± 2.21 cm/s, and the mean systolic flow (MSF) was 0.137 ± 0.04 ml/s. The mean systolic duration was 454.63 milliseconds, and the mean stroke volume was 60.47 microliters (µl).
| Parameters | Mean ± SD (cm/s) | Range | Median |
|---|---|---|---|
| Peak Systolic Velocity | 8.58 ± 5.59 | 16.15 (3.92 – 20.07) | 5.18 |
| End Diastolic Velocity | 3.31 ± 2.21 | 7.32 (0.93 – 8.26) | 2.93 |
| Mean Systolic Flow | 0.137 ± 0.04 | 0.20 (0.09 – 0.26) | 0.17 |
| Systolic Duration | 454.63 ± 139.94 | 421 (282 – 701) | 502 |
| Stroke Volume | 60.47 ± 25.96 | 127 (31 – 157) | 56.65 |
| Neurological Conditions | Peak Systolic Velocity (cm/s) | End Diastolic Velocity (cm/s) | Mean Systolic Flow (ml/s) | Systolic Duration (ms) | Stroke Volume (µl) |
|---|---|---|---|---|---|
| Normal Pressure Hydrocephalus [n=48] | 9.24 ± 4.51 | 3.8 ± 1.9 | 0.14 ± 0.03 | 480 ± 120 | 65 ± 20 |
| Aqueductal Stenosis [n=16] | 6.53 ± 2.25 | 2.9 ± 1.5 | 0.11 ± 0.02 | 550 ± 150 | 70 ± 25 |
| Chiari Malformation [n=12] | 7.82 ± 3.67 | 3.2 ± 1.7 | 0.13 ± 0.04 | 510 ± 130 | 60 ± 18 |
| Diffuse cerebral atrophy [n=32] | 2.15 ± 1.81 | 2.5 ± 1.3 | 0.10 ± 0.02 | 600 ± 200 | 75 ± 30 |
| Arachnoid Cysts [n=12] | 6.14 ± 3.17 | 3.0 ± 1.6 | 0.12 ± 0.03 | 490 ± 140 | 68 ± 22 |
| Others (Tumor) [n=16] | 8.16 ± 4.09 | 3.57 ± 1.83 | 0.13 ± 0.03 | 500 ± 110 | 63 ± 15 |
These results underscored significant differences in MRI characteristics among neurological disorders, highlighting the utility of phase-contrast MRI in evaluating CSF flow dynamics. This method proved valuable for the diagnosis and management of various neurological conditions.
Discussion
This study included participants aged 29 to 70, with the largest group (38.24%) in the 51–60 age range. In contrast, Ahmad et al. examined 39 patients aged 1 to 65 years and a control group of nine age- and sex-matched individuals. Sakhare et al. focused on a younger cohort with a mean age of 24.8 ± 3.9 years, significantly younger than our mean age of 46.84 years. Govindarajan et al. reported a broader distribution, with 25% aged 1–20 years, 22.2% aged 21–40, 25% aged 41–60, and 27.8% aged 61–80 years, highlighting variability in age demographics across studies.
Our study recorded a mean End-Diastolic Velocity (EDV) of 3.31 ± 2.21 cm/s, with values ranging from 0.93 to 8.26 cm/s and a median of 2.93 cm/s. Govindarajan et al. found that NPH patients had significantly higher Peak Systolic Velocity (PSV) and EDV compared to controls (9.96 ± 1.73 vs. 4.80 ± 0.39, p < 0.05; 4.72 ± 0.62 vs. 3.21 ± 0.55, p < 0.05). Conversely, age-related brain atrophy and aqueductal stenosis (AS) showed lower PSV and EDV values. While Elsafty et al. reported lower values for NPH, our findings of increased stroke volume (SV) align with reports of hyperdynamic circulation by Kahlon et al.
NPH was the most prevalent condition in our study (35.29%), followed by diffuse cerebral atrophy (23.53%). Aqueductal stenosis and other conditions each accounted for 11.76%, with Chiari malformation and arachnoid cysts at 8.82% each. These findings differ slightly from Govindarajan et al., who examined a similar range of conditions with a focus on age-related variations.
Headache was the most common symptom, reported by 58.82% of participants, followed by cognitive impairment (35.29%) and gait disturbances (29.41%). Visual disturbances were observed in 23.53%, while nausea/vomiting, neck pain, and dizziness were less frequent (17.65% and 14.71%, respectively). Other symptoms, such as numbness/tingling, seizures, urinary incontinence, and weakness, were reported in 8.82%, and sensory impairment was the least common (5.88%).
In our study, the mean systolic flow (MSF) was 0.137 ± 0.04 ml/s, ranging from 0.09 to 0.26 ml/s, with a median of 0.17 ml/s. The mean systolic duration was 454.63 ± 139.94 ms, with values ranging from 282 to 701 ms. These findings align with Sakhare et al., who used 2D cine-PC sequences, while Stankovic et al. highlighted the advancements of 4D flow MRI for volumetric CSF flow analysis.
The mean stroke volume in our study was 60.47 ± 25.96 µl, ranging from 31 to 157 µl, and a median of 56.65 µl. These results align with El Sankari et al., who noted compensatory mechanisms in intracranial pressure regulation, and Stoquart et al. reported lower stroke volumes in older adults. The wide range of stroke volumes in our study suggests varying risks for conditions like hydrocephalus and underscores stroke volume’s diagnostic value.
Specific findings in CSF flow dynamics included higher PSV (9.24 ± 4.51 cm/s) and SV (65 ± 20 µl) in NPH, lower PSV (6.53 ± 2.25 cm/s) and EDV (2.9 ± 1.5 cm/s) but slightly higher SV (70 ± 25 µl) in aqueductal stenosis, and intermediate values for Chiari malformation. Diffuse cerebral atrophy exhibited the lowest PSV (2.15 ± 1.81 cm/s) but the highest SV (75 ± 30 µl). These variations demonstrate the diagnostic utility of phase-contrast MRI in differentiating neurological conditions.
Physiological factors, such as respiration, heart rate, and circadian rhythm, as noted by Schrauben et al., can influence CSF and cerebral blood flow (CBF) dynamics. Variability in venous flow, especially in the internal jugular veins, underscores the need to consider these factors during MRI assessments. Establishing normative flow values by age, gender, and pathology can enhance diagnostic accuracy and enable phase-contrast MRI to serve as a non-invasive biomarker for conditions like hydrocephalus and cognitive impairment.
This study highlights the value of phase-contrast MRI in understanding CSF dynamics and its potential in improving diagnosis and management of neurological disorders. Standardization and further research are needed to fully harness its clinical applications.
Conclusion
The findings of this study highlight the value of phase-contrast MRI in evaluating CSF flow dynamics, demonstrating notable variations across neurological conditions and offering valuable insights into their underlying pathophysiology. While peak velocity was initially underemphasized, the research established it as a more accurate and reliable parameter for assessing cerebral hemodynamics. This shift suggests that incorporating peak velocity measurements into routine clinical practice could improve diagnostic accuracy and patient care. Additionally, the study lays the groundwork for exploring the longitudinal effects of cerebral hemodynamics and encourages further neuroimaging research with flexible and adaptive methodologies.
Despite its strengths, the study has limitations. These include a reliance on subjective self-reported symptoms, the absence of a healthy control group, and unreported measurement reliability, which may affect the accuracy and generalizability of the results. Future research should address these limitations by including larger, more diverse sample populations, incorporating healthy controls, using objective symptom assessments, and investigating how CSF dynamics influence treatment outcomes. Such efforts would yield more robust findings and enhance their applicability to clinical practice.
Conflict of Interest: All authors declare no conflict of interest.
Source of Funding: None
Consent: As per international standards or university standards written participant consent has been collected and preserved by the authors.
Ethical Approval: As per international standards or university standards written ethical permission has been collected and preserved by the author(s).
References
- Anagnostakou V, Epshtein M, Ughi GJ, et al. Transvascular in vivo microscopy of the subarachnoid space. J Neurointerv Surg. 2022;14(5):420-428.
- Ichimura T, Fraser PA, Cserr HF. Distribution of extracellular tracers in perivascular spaces of the rat brain. Brain Res. 1991;545(1-2):103-113.
- Koh L, Zakharov A, Johnston M. Integration of the subarachnoid space and lymphatics: is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal Fluid Res. 2005;2:1.
- Bradley WG Jr, Scalzo D, Queralt J, et al. Normal-pressure hydrocephalus: evaluation with cerebrospinal fluid flow measurements at MR imaging. Radiology. 1996;198(2):523-529.
- Bradley WG Jr, Whittemore AR, Kortman KE, et al. Marked cerebrospinal fluid void: indicator of successful shunt in patients with suspected normal-pressure hydrocephalus. Radiology. 1991;178(2):459-466.
- Lindström EK, Ringstad G, Mardal KA, Eide PK. Cerebrospinal fluid volumetric net flow rate and direction in idiopathic normal pressure hydrocephalus. Neuroimage Clin. 2018;20:731-741.
- Martin BA, Kalata W, Shaffer N, et al. Hydrodynamic and longitudinal impedance analysis of cerebrospinal fluid dynamics at the craniovertebral junction in type I Chiari malformation. PLoS One. 2013;8(10):e75335.
- Heiss JD, Snyder K, Peterson MM, et al. Pathophysiology of primary spinal syringomyelia. J Neurosurg Spine. 2012;17(5):367-380.
- Bapuraj JR, Londy FJ, Delavari N, et al. Cerebrospinal fluid velocity amplitudes within the cerebral aqueduct in healthy children and patients with Chiari I malformation. J Magn Reson Imaging. 2016;44(2):463-470.
- Korbecki A, Zimny A, Podgórski P, et al. Imaging of cerebrospinal fluid flow: fundamentals, techniques, and clinical applications of phase-contrast magnetic resonance imaging. Pol J Radiol. 2019;84:e240.
- Watts R, Steinklein JM, Waldman L, et al. Measuring glymphatic flow in man using quantitative contrast-enhanced MRI. AJNR Am J Neuroradiol. 2019;40(4):648-651.
- Ahmad N, Salama D, Al-Haggar M. MRI CSF flowmetry in evaluation of different neurological diseases. Egypt J Radiol Nucl Med. 2021;52:1-10.
- Sakhare AR, Barisano G, Pa J. Assessing test-retest reliability of phase contrast MRI for measuring cerebrospinal fluid and cerebral blood flow dynamics. Magn Reson Med. 2019;82(2):658-670.
- Govindarajan BR, Sharma PK, Polaka Y, et al. The role of phase-contrast MRI in diagnosing cerebrospinal fluid flow abnormalities. Cureus. 2024;16(3):e57114.
- Elsafty HG, ELAggan AM, Yousef MA, et al. Cerebrospinal fluid flowmetry using phase-contrast MRI technique and its clinical applications. Tanta Med J. 2018;46(2):121-132.
- Kahlon B, Annertz M, Ståhlberg F, Rehncrona S. Is aqueductal stroke volume, measured with cine phase-contrast magnetic resonance imaging scans, useful in predicting outcome of shunt surgery in suspected normal pressure hydrocephalus? Neurosurgery. 2007;60(1):124-130.
- Battal B, Kocaoglu M, Bulakbasi N, et al. Cerebrospinal fluid flow imaging by using phase-contrast MR technique. Br J Radiol. 2011;84(1004):758-765.
- Stankovic Z, Allen BD, Garcia J, et al. 4D flow imaging with MRI. Cardiovasc Diagn Ther. 2014;4(2):173-192.
- ElSankari S, Balédent O, Van Pesch V, et al. Concomitant analysis of arterial, venous, and CSF flows using phase-contrast MRI: a quantitative comparison between MS patients and healthy controls. J Cereb Blood Flow Metab. 2013;33(9):1314-1321.
- Schroth G, Klose U. Cerebrospinal fluid flow. Neuroradiology. 1992;35(1):16-24.
- Kedarasetti RT, Turner KL, Echagarruga C, et al. Functional hyperemia drives fluid exchange in the paravascular space. Fluids Barriers CNS. 2020;17:1-25.
- Stoquart-ElSankari S, Balédent O, Gondry-Jouet C, et al. Aging effects on cerebral blood and cerebrospinal fluid flows. J Cereb Blood Flow Metab. 2007;27(9):1563-1572.
- Scollato A, Tenenbaum R, Bahl G, et al. Changes in aqueductal CSF stroke volume and progression of symptoms in patients with unshunted idiopathic normal pressure hydrocephalus. AJNR Am J Neuroradiol. 2008;29(1):192-197.
- Chen L, Elias G, Yostos MP, et al. Pathways of cerebrospinal fluid outflow: a deeper understanding of resorption. Neuroradiology. 2015;57:139-147.
- Dreha-Kulaczewski S, Joseph AA, Merboldt KD, et al. Inspiration is the major regulator of human CSF flow. J Neurosci. 2015;35(6):2485-2491.
- Addicott MA, Yang LL, Peiffer AM, et al. The effect of daily caffeine use on cerebral blood flow: how much caffeine can we tolerate? Hum Brain Mapp. 2009;30(10):3102-3114.
- Schrauben EM, Johnson KM, Huston J, et al. Reproducibility of cerebrospinal venous blood flow and vessel anatomy with the use of phase contrast–vastly undersampled isotropic projection reconstruction and contrast-enhanced MRA. AJNR Am J Neuroradiol. 2014;35(5):999-1006.
- Mancini M, Lanzillo R, Liuzzi R, et al. Internal jugular vein blood flow in multiple sclerosis patients and matched controls. PLoS One. 2014;9(3):e92730.
- Fushimi Y, Okada T, Okuchi S, et al. Jugular venous reflux on magnetic resonance angiography and radionuclide venography. Acta Radiol Open. 2016;5(12):2058460116681209.

