Efficacy of Multimodal Facial Rejuvenation Techniques
Efficacy and Histological Evaluation of Combined Muscle Stimulation Technology, Targeted Ultrasound, and Radiofrequency for Complete Facial Rejuvenation
Suneel Chilukuri, M.D., FACMS1*, David E. Kent, M.D., FACMS2
- Suneel Chilukuri, M.D., FACMS Founder & CEO, Refresh Dermatology, Associate Clinical Professor, Baylor College of Medicine, Volunteer Clinical Faculty, University of Texas Health Science Center, Adjunct Clinical Associate Professor, University of North Texas Health Science Center, Cosmetic Fellowship Director, American Society for Dermatologic Surgery
- David E. Kent, M.D., FACMS Clinical Faculty Department of Dermatology, Medical College of Georgia, Clinical Associate Professor of Medicine, Mercer University Medical School, Skin Care Physicians of Georgia, Founding Partner Skin Care Physicians of Georgia,Program Director, ACGME Micrographic Surgery & Dermatologic Oncology, 308 Coliseum Drive #200, Macon, Georgia 31217
OPEN ACCESS
PUBLISHED:31 July 2025
CITATION:CHILUKURI, Suneel; KENT, David E.. Efficacy and Histological Evaluation of Combined Muscle Stimulation Technology, Targeted Ultrasound, and Radiofrequency for Complete Facial Rejuvenation. Medical Research Archives, [S.l.], v. 13, n. 7, july 2025. ISSN 2375-1924. Available at: <https://esmed.org/MRA/mra/article/view/6731>.
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.18103/mra.v13i7.6731
ISSN 2375-1924.
Abstract
Background: Aging is a multifactorial biological process that affects various tissues and structures at the cellular level. Advanced medical technologies that simultaneously target different skin layers may provide synergistic outcomes that surpass those of individual treatments.
Aims: To evaluate the safety and efficacy of a single-session multimodal treatment combining HIFES with Radiofrequency (RF), targeted ultrasound (TUS) with RF, and RF microneedling technologies for facial rejuvenation.
Methods: This prospective study enrolled 52 subjects who received the consecutive application of HIFES+RF, TUS+RF, and RF microneedling in one treatment session. Follow-up visits were conducted at 1 and 3 months post-treatment. Outcome measures included histological analysis of collagen, elastin, and hyaluronic acid (HA) density, 2D photographic evaluation using the Global Aesthetic Improvement Scale (GAIS), and 3D skin analysis with QuantifiCare software focusing on evenness, wrinkle depth, and pore size.
Results: At 3 months, collagen and elastin density increased by 39.53% and 130.04%, respectively, while HA density increased by 230.02%. GAIS scores indicated 100% of subjects showed overall facial improvement. Quantitative skin analysis revealed a 31.20% improvement in skin evenness, a 47.31% reduction in pores, and a 43.36% reduction in wrinkles. Additionally, 84.00% of subjects rated the treatment as comfortable, and 76.90% reported satisfaction with the results.
Conclusion: A single-session multimodal treatment combining HIFES+RF, TUS+RF, and RF microneedling is safe, well-tolerated, and effective in improving key markers of facial aging. The synergistic effects of this approach enhance dermal remodeling through multiple cellular pathways, supporting its clinical relevance in comprehensive facial rejuvenation.
Keywords
facial rejuvenation, HIFES, radiofrequency, targeted ultrasound, microneedling, collagen, elastin, hyaluronic acid
Introduction
The aesthetic medicine market is expanding rapidly, offering diverse procedures to enhance physical appearance and improve self-confidence. This growth is driven by increasing global demand, particularly for non-invasive or minimally invasive treatments. The desire to maintain a youthful appearance has broadened the interest in aesthetic medicine beyond traditional older age groups to include younger generations. Baby Boomers and Generation X typically undergo aesthetic procedures to address visible signs of aging. Meanwhile, Millennials and Generation Z actively seek preventative self-care services to maintain their youthful appearance. The growing demand for aesthetic procedures also extends to men across all age groups. This trend is further supported by shifting societal norms, the influence of social media, and increased visibility of aesthetic treatments among public figures. The increase is likely driven by factors such as the desire to remain competitive in the job market, maintain a youthful look, and the growing normalization and social acceptance of cosmetic treatments.
To address the diverse needs of various patient groups, the use of combined advanced medical technologies has gained popularity in clinical practice, offering synergistic effects that often exceed the results of standalone treatments. Aging results from a complex series of biological processes at the cellular level, making it unrealistic for a single technology to address all the indications a patient may seek to treat. A comprehensive approach that combines multiple technologies to target various tissues, structures, and concerns can achieve superior synergistic effects. By simultaneously targeting superficial and deeper tissue layers, such strategies allow clinicians to tailor treatments to individual patients’ needs and aesthetic goals. This integrated strategy not only enhances the overall efficacy of treatments but also has the potential to deliver better outcomes within a shorter timeframe. By addressing multiple aspects of the aging process through combined aesthetic therapies, patients can experience personalized results, greater effectiveness, and higher levels of satisfaction. However, designing such treatment plans requires careful strategic planning to balance therapeutic efficacy with time investment and cost investment to the patient.
Combining muscle-stimulation technologies, such as high-intensity facial electrical stimulation (HIFES) with skin-tightening technologies, including radiofrequency (RF), targeted ultrasound (TUS), and RF microneedling presents a powerful approach to facial rejuvenation. The combination of RF and HIFES technologies targets both facial skin and muscles, resulting in an overall lifting effect and comprehensive rejuvenation. Thermal and mechanical stress on fibroblasts caused by RF and TUS stimulates the natural synthesis of hyaluronic acid (HA), whose depletion is one of the earliest signs of aging. Meanwhile, RF microneedling uses tiny needles to create microdamage in the skin while inducing controlled thermal damage in the dermis, thereby promoting improved dermal firmness and tightening. When sequenced strategically, these technologies may enhance each other’s effects through tissue priming and amplified cellular responses. A key principle of this approach is treating the deepest tissue layers first. When these technologies are combined in a single session, they work synergistically to address multiple signs of aging while reinforcing a multi-modal approach that aims to achieve comprehensive facial tissue restoration, optimizing rejuvenation outcomes more effectively than standalone treatments.
This clinical study evaluated the safety and efficacy of the consecutive use of HIFES+RF, TUS+RF, and RF microneedling technologies in a single treatment session.
Methods
Study Overview
In this prospective, multi-center, open-label clinical study, fifty-two subjects (n=52, 59 ± 8 years, skin type I-IV) were enrolled. The objective was to evaluate the effects of combining EMFACE (BTL Industries Inc., Boston, MA), EXION Face (BTL Industries Inc., Boston, MA) and EXION Fractional RF (FRF) (BTL Industries Inc., Boston, MA) applicators on overall facial rejuvenation. The study included histological evaluations of the therapy’s effects on collagen, elastin, and HA, as well as photo evaluations focusing on specific indications, such as evenness, wrinkles, and pores. Informed consent was obtained from all participants, and adverse events were monitored throughout the study.
Treatment Protocol
Subjects underwent treatments with HIFES+RF, TUS+RF, and RF microneedling within a single session. Four consecutive treatments were conducted, spaced 7 to 14 days apart. Two follow-up visits were scheduled one month and three months post-treatment (±five days from the last treatment day). Before each session, the treatment areas were carefully prepared by removing any cosmetics, lotions, jewelry, and prominent hairs. The session began with HIFES+RF technology, targeting the cheeks and forehead, with intensity levels adjusted based on patient feedback and comfort. Next, TUS+RF technology was applied to the whole neck, including the submentum, perioral, and periorbital areas. The session concluded with treatment depths customized as follows using fractional RF: 1mm for the forehead, 0.5mm for the periorbital area, 1.5-2.5mm for the cheeks, 1-2mm for the chin, and 1.5-4mm for the submentum (with Extended Mode used as needed). Depending on the treatment area, intensity levels ranged from 30% to 50%.
Histological Assessment
Tissue samples were collected using a punch biopsy (3-mm diameter) from the periauricular area at baseline and three months post-treatment. This region was selected as the optimal site for histological sampling due to consistent skin composition across individuals, minimal aesthetic impact, and well-defined dermal structure. Given the complex cascade of biological processes triggered in the skin, the periauricular area serves as a representative site for observing tissue changes induced by the various applicators used in the multimodal facial restoration approach. The biopsies were preserved in formalin, embedded in paraffin, and sliced into sections, performed according to established protocols to visualize specific tissue components. Hematoxylin and Eosin (H&E) staining was used to examine overall tissue morphology. Masson’s Trichrome staining differentiated collagen fibers from other tissue structures. Hyaluronan-Binding Protein (HABP) staining specifically detected and localized HA within the tissue sections. Orcein staining highlighted elastic fibers, enabling the evaluation of their distribution and structural integrity. The biopsy wounds were carefully closed, disinfected, and closely monitored throughout the study to track the healing process. Participants received detailed instructions for home wound care to ensure adequate and optimal healing. Collagen, elastin, and HA levels were quantified using Image J software with semi-automatic segmentation in the HSB (Hue-Saturation-Brightness) color system. The appropriate threshold differentiating the collagen fibers, elastin fibers, and HA deposits from the background was identified in the selected regions of interest (ROI = 1520×1000 µm). The stained collagen and elastin fibers, as well as HA in the dermis, were quantified and expressed as the occupied area (square micrometers) in the analyzed images.
Dermal Assessment
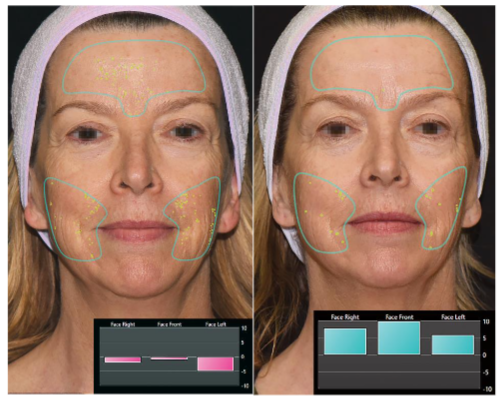
To assess treatment outcomes, two-dimensional (2D) and three-dimensional (3D) digital photographs were taken at baseline, after the last therapy, and at one- and three-month follow-ups. For 2D photographs, a consistent background (black, white, or blue) and uniform lighting were maintained. Images were taken from five standardized angles: front, 45 degrees right, 90 degrees right, 45 degrees left, and 90 degrees left. The Global Aesthetic Improvement Scale (GAIS) was used to evaluate facial skin appearance, providing a standardized assessment of overall aesthetic improvement. Reviewers categorized treatment outcomes into predetermined levels of improvement, ranging from “very much improved” (3) to “worse” (-1). For 3D photographs, the QuantifiCare skin analysis software was utilized to evaluate changes in wrinkle severity using linear waveform analysis, measuring deviations from a mean smooth surface. Evaluations focused on the cheeks and forehead while accounting for the subject’s age, gender, and skin type. Additional metrics included assessments of pores and skin evenness. Improvements, such as pore size reduction or enhanced evenness, were quantified numerically. Each analysis generated a score ranging from -10 to +10, where negative scores indicated greater wrinkle severity compared to an average individual, and positive scores above 0 reflected better results relative to peers of the same age, gender, and skin type.
Subject Satisfaction and Therapy Comfort
Subject satisfaction with the therapy results was evaluated using the 5-point Likert Scale Subject Satisfaction Questionnaire (SSQ), administered after the last treatment, and at 1-month and 3-month follow-ups. Similarly, patient comfort was assessed using the Therapy Comfort Questionnaire (TCQ) after the last treatment session. The TCQ combined a 5-point Likert Scale with a 10-point Visual Analog Scale (VAS) for pain assessment, where 0 indicated no pain and 10 represented maximum bearable pain.
Statistical Analysis
Statistical analysis was conducted using GraphPad Prism software. Descriptive statistics, including the mean and standard error of the mean, were calculated. The Friedman test was employed to assess the statistical significance of the histological and skin analysis evaluations. A one-sample Wilcoxon test was used to compare GAIS scores while the Wilcoxon test was applied to the 3D analysis data to evaluate the differences in improvements between these time points. A significance level of α = 0.05 was adopted.
Results
This study involved 52 subjects (n=52, 48 females, 4 males). All subjects completed the treatments, which were adjusted based on their feedback, and no subjects withdrew from the study. Follow-up visits were conducted at 1 month and 3 months, during which no side effects were observed. Additionally, the punch biopsy sites healed appropriately.
Histological Assessment
Five of the fifty-two patients underwent histological sampling. Histological evaluation revealed improvement in the post-treatment tissue related to collagen, elastin, and HA levels.
Collagen
The average area occupied by collagen in the ROI was 192850.2 µm² at baseline. At a 3-month follow-up visit, the average collagen area had significantly increased to 269083.8 µm² (p-value = 0.0045). Compared to the baseline, the average collagen density was increased by 39.53% at a 3-month follow-up. The observed changes in the examined histology slices are visualized in Figure 1.
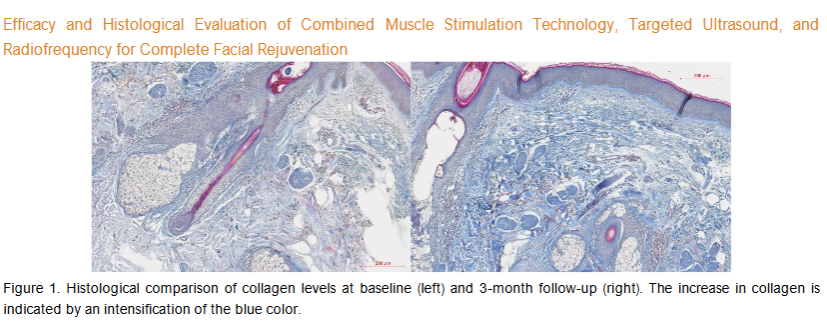
Elastin
The average elastin-occupied area at baseline was 111063.8 µm² in the ROI. At the 3-month follow-up visit, the average elastin amount increased to 255492.5 µm² (p-value = 0.0009). Compared to baseline, the average elastin density was increased by 130.04% at 3-month follow-up. The observed changes in the examined histology slices are visualized in Figure 2.
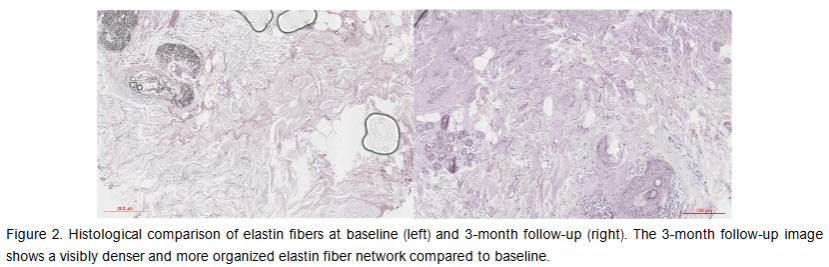
Hyaluronic Acid
The average area occupied by HA at baseline was 310354.8 µm² in the ROI. At a 3-month follow-up visit, the average HA-occupied area significantly increased by 230.02% to 1024219 µm² (p-value = 0.0017) in the ROI. The observed changes in the examined histology slices are visualized in Figure 3.
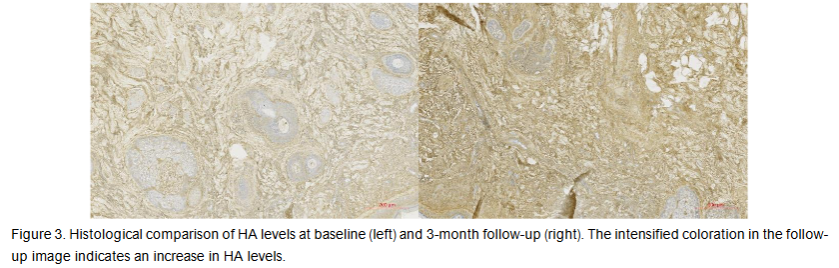
Skin Analysis Evaluation
GAIS Evaluation
The GAIS evaluation was conducted for 43 of the 52 subjects. These subjects showed an average improvement in overall facial appearance of 1.13 ± 0.58 points at 1 month post-treatment. At 3 months post-treatment, the GAIS evaluation showed an average improvement of 1.32 ± 0.55 points. At 3 months post-treatment, 100% of subjects showed improvement (p<0.0001). The distribution of these improvements is detailed in Figure 4.
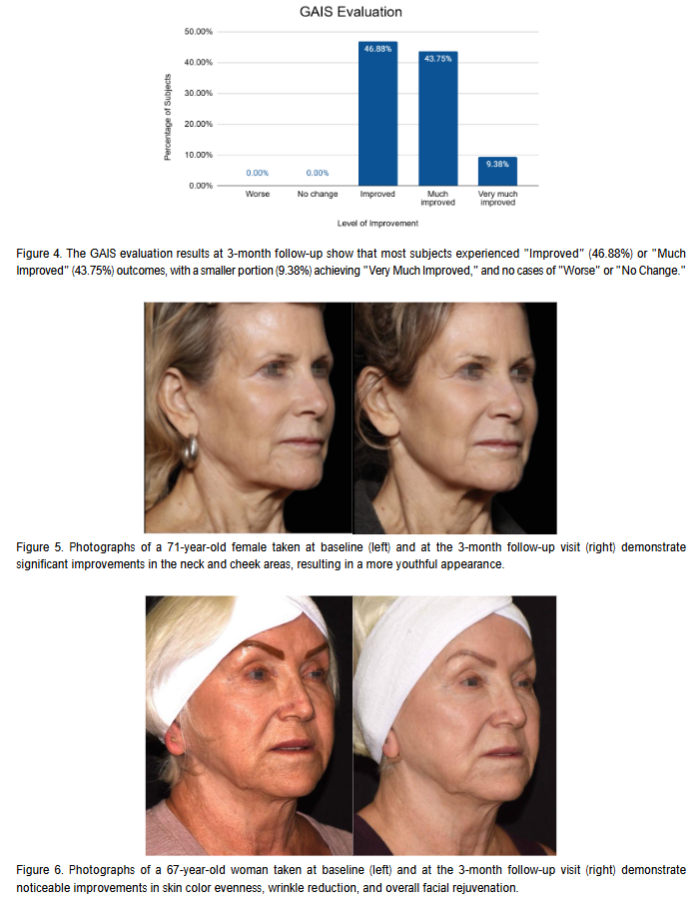
3D Photographs Evaluation
For the evaluation of 3D photographs, 32 of the 52 patients were included. The assessment demonstrated significant improvements in several parameters, including skin evenness, wrinkles, and pores (p<0.0001). On average, patients showed a 23.16% improvement in evenness, a 36.02% reduction in wrinkles, and a 31.27% improvement in pore appearance at the 1-month follow-up. Subjects exhibited a statistically significant improvement of 31.20% in evenness, a 43.36% reduction in wrinkles, and a 47.31% improvement in pore appearance three months after the final treatment (p<0.0001).
Subject Satisfaction and Therapy Comfort
The subjects’ satisfaction with the treatment was 70.42% immediately following the final treatment, increasing to 76.90% at the 3-month follow-up. Based on the Therapy Comfort Questionnaire, 84.00% of participants (n=32) reported the treatment as comfortable, with an average VAS score of 3.58 ± 2.03 points.
Discussion
This study evaluated the effects of combining HIFES+RF, TUS+RF, and RF microneedling. The treatment results demonstrated significant improvements across various parameters. Both collagen and elastin density increased, with hyaluronic acid density showing the most substantial improvement, increasing by 230.02%. At the 3-month follow-up, 100% of subjects demonstrated an improvement in overall appearance, as assessed by the GAIS evaluation. Notable enhancements were observed in skin evenness (31.20%), pore reduction (47.31%), and wrinkle reduction (43.36%). Additionally, the majority of patients expressed satisfaction with the treatment, reporting it as comfortable. The average VAS score of 3.58 aligns with results from previous studies.
These findings demonstrate enhanced outcomes compared to previous studies, emphasizing the benefits of the synergistic interaction across all applied technologies. Previous research has confirmed the efficacy of individual modalities; for instance, Kinney, Bernardy, and Jarosova observed a consistent and significant improvement in all parameters related to muscle tissue quality and function. Additionally, the procedure was shown to enhance the density of collagen and elastin fiber networks, leading to noticeable improvements in wrinkle reduction and skin texture.
Similarly, studies investigating the simultaneous application of RF and TUS have confirmed a direct correlation with increased HA production. This mechanism involves stimulating fibroblasts to enhance the natural synthesis of HA, as evidenced by Boyd, et al., who observed significant improvements in skin hydration levels, along with an overall enhancement in skin quality. Bernardy’s histological study on RF microneedling further supports these findings by demonstrating its efficacy in stimulating collagen production, improving skin texture, and promoting wound healing. Over six weeks, a progressive increase in collagen and elastin content was noted, alongside the reorganization of connective tissue, highlighting its role in skin rejuvenation.
The combination of HIFES technology with synchronized RF, TUS, and RF microneedling leverages the unique mechanisms of each modality to achieve a synergistic effect. By targeting multiple pathways involved in skin aging and tissue regeneration, this multimodal strategy delivers more comprehensive outcomes than standalone therapies. Crucially, the treatment sequence proceeds from the deepest layers to the most superficial, enhancing tissue responsiveness and amplifying the overall rejuvenation effect. HIFES with RF improves muscle tone and skin structure; RF microneedling enhances collagen remodeling and wound healing; and TUS boosts HA production, hydration, and elasticity. Together, these technologies create a multi-layered rejuvenation effect that addresses both superficial and deeper layers of the skin. This approach aligns well with current trends favoring full-face, tissue-depth-oriented treatments that maximize efficacy in fewer sessions.
One challenge often associated with RF microneedling technologies is significant treatment discomfort. Patients frequently report pain associated with needle penetration and RF heating, particularly when deeper needle insertions are required to target subdermal layers. This discomfort arises from the activation of multiple mechanoreceptors in the dermis, with deeper penetrations intensifying the sensation of pain. In contrast, newer RF microneedling systems have been developed to address these challenges by enabling deep tissue penetration without requiring full needle insertion, significantly reducing pain. Furthermore, advanced systems may incorporate an AI pulse control system that provides real-time feedback to ensure optimal energy delivery for each single pulse and eliminates the need for multiple treatment passes. This innovative system ensures precise energy delivery with each pulse from every needle, allowing for a highly effective single-pass procedure that minimizes discomfort while maintaining optimal results. By reducing irritation and overall treatment burden, this advancement makes it feasible to integrate multiple technologies within a single session. This approach would be impractical with conventional microneedling devices due to excessive discomfort and skin sensitivity.
A key strength of this study is its comprehensive evaluation approach, combining histological assessment with advanced skin analysis using 2D and 3D photography. The histological assessment is particularly valuable due to the use of human tissue samples and the application of four distinct staining techniques, enabling the visualization of multiple structures, including collagen, elastin, and HA. This dual approach allows for correlation between cellular-level changes and externally visible improvements, strengthening the validity of the observed aesthetic outcomes. However, the study has several limitations. The three-month study duration may not fully capture the extent of collagen remodeling, which typically occurs over a longer timeframe. Moreover, the study’s demographic representation could be improved by including a larger number of male participants. Male-specific outcomes, such as skin tightening, are increasingly relevant given the rising interest in aesthetic treatments among men. Future research should prioritize extended follow-up periods to assess tissue changes over six months or longer. Additionally, expanding the sample size to include more men would provide valuable insights into gender-specific responses and broaden the applicability of the findings.
Further studies should also explore the sequencing and spacing of technologies more deeply to determine whether certain combinations or intervals result in superior outcomes. Understanding patient subtypes, based on age, skin thickness, or baseline collagen status, could help tailor energy-based protocols to individual needs. While this study highlights the benefits of combining multiple technologies, the concept of synergistic effects, where the cumulative outcome exceeds the sum of individual benefits, remains a subject of ongoing investigation. To substantiate such claims, robust, evidence-based research is essential. Future studies should aim to quantify these potential synergistic effects through histological analyses and other objective measures, providing a solid foundation for validating the efficacy of combined technologies. Randomized controlled trials comparing multimodal regimens to individual treatment arms would be particularly valuable to quantify additive or synergistic effects. By bridging theoretical expectations with reproducible, data-driven results, the scientific basis for employing multimodal approaches can become more credible and widely accepted.
Conclusions
The results of our study demonstrate that combining HIFES+RF, TUS+RF, and RF microneedling technologies in a single treatment session leads to significant improvements in overall facial rejuvenation. Unlike standalone therapies, which address specific aspects of skin aging, this multimodal approach effectively targets multiple pathways of aging. Subjects exhibited remarkable improvements in overall facial appearance, skin texture, facial and neck lifting, and wrinkle reduction. Histological analysis further confirmed these outcomes, showing increases in collagen density by 39.53%, elastin density by 130.04%, and HA by 230.02%. Notably, no adverse effects were observed during the study, underscoring the safety and efficacy of this combined treatment approach.
Conflict of Interest Statement:
None.
Funding Statement:
None.
Acknowledgements:
None.
Citations
- https://www.alliedmarketresearch.com AMR. Aesthetic Medicine Market Size, Share | Forecast Report 2033. Allied Market Research. Accessed December 30, 2024.
- Iqbal SP. The Relationship between Aesthetics and Self-Esteem. J Bahria Univ Med Dent Coll. Published online 2024.
- Woodward JA, Fabi SG, Alster T, Colón-Acevedo B. Safety and efficacy of combining microfocused ultrasound with fractional CO2 laser resurfacing for lifting and tightening the face and neck. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2014;40 Suppl 12:S190-193. doi:10.1097/DSS.0000000000000228
- Beer KR. Combined treatment for skin rejuvenation and soft-tissue augmentation of the aging face. J Drugs Dermatol JDD. 2011;10(2):125-132.
- Alster TS, Doshi SN, Hopping SB. Combination surgical lifting with ablative laser skin resurfacing of facial skin: a retrospective analysis. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2004;30(9):1191-1195. doi:10.1111/j.1524-4725.2004.30370.x
- Carruthers JDA, Glogau RG, Blitzer A, Facial Aesthetics Consensus Group Faculty. Advances in facial rejuvenation: botulinum toxin type a, hyaluronic acid dermal fillers, and combination therapies–consensus recommendations. Plast Reconstr Surg. 2008;121(5 Suppl):5S-30S. doi:10.1097/PRS.0b013e31816de8d0
- Butterwick K, Sadick N. Hand Rejuvenation Using a Combination Approach. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2016;42 Suppl 2:S108-118. doi:10.1097/DSS.0000000000000687
- Friedmann DP, Fabi SG, Goldman MP. Combination of intense pulsed light, Sculptra, and Ultherapy for treatment of the aging face. J Cosmet Dermatol. 2014;13(2):109-118. doi:10.1111/jocd.12093
- Hooper D. Commentary on Maximizing Panfacial Aesthetic Outcomes. Dermatol Surg. 2020;46(6):818. doi:10.1097/DSS.0000000000002287
- Sadick NS. Combination Aesthetic Therapies for Whole-Body Rejuvenation. Clin Surg. 2017;2(14 44). https://www.clinicsinsurgery.com/full-text/cis-v2-id1444.php
- Kim SB, Kim S, Heo Y ran, Kim HJ. Evaluation of a novel device combining RF and HIFES technologies for the non-invasive correction of asymmetric smiles and facial rejuvenation: A case report. Skin Res Technol. 2024;30(8):e13885. doi:10.1111/srt.13885
- Cotofana S, Halaas Y, Kinney B, Goldberg D, Cohen J. Simultaneous Emission of Synchronized Radiofrequency and HIFES for Non-invasive Facial Rejuvenation: The Mechanism of Action.
- Porsch H, Mehić M, Olofsson B, Heldin P, Heldin CH. Platelet-derived growth factor β-receptor, transforming growth factor β type I signaling and stability. J Biol Chem. 2014;289(28):19747-19757. doi:10.1074/jbc.M114.547273
- Duncan D, Bernardy J, Hodkovicova N, Masek J, Prochazkova M, Jarosova R. The Superior Effect of Radiofrequency With Targeted Ultrasound for Facial Rejuvenation by Inducing Hyaluronic Acid Synthesis: A Pilot Preclinical Study. Aesthetic Surg J Open Forum. 2024;6:ojae005. doi:10.1093/asjof/ojae005
- Meyer PF, de Oliveira P, Silva FKBA, et al. Radiofrequency treatment induces fibroblast growth factor 2 expression and subsequently promotes neocollagenesis and neoangiogenesis in the skin tissue. Lasers Med Sci. 2017;32(8):1727-1736. doi:10.1007/s10103-017-2238-2
- El-Domyati M, Barakat M, Awad S, Medhat W, El-Fakahany H, Farag H. Multiple microneedling sessions for minimally invasive facial rejuvenation: an objective assessment. Int J Dermatol. 2015;54(12):1361-1369. doi:10.1111/ijd.12761
- Nguyen L, Blessmann M, Schneider SW, Herberger K. Radiofrequency Microneedling for Skin Tightening of the Lower Face, Jawline, and Neck Region. Dermatol Surg. 2022;48(12):1299. doi:10.1097/DSS.0000000000003607

