Endovascular Management of Portal Vein Thrombosis
Advanced Endovascular Management of Portal Vein Thrombosis Using Combined Rotational Morcellation and Large-Bore Aspiration Techniques: Treating Thrombosed TIPS Shunts
Stibbs P. ¹ , Warwick T. ¹, Howell V. ¹
¹ University of Nottingham, Medicine
OPEN ACCESS
PUBLISHED: 31 August 2025
CITATION Stibbs, P., Warwick, T., et al., 2025. Advanced Endovascular Management of Portal Vein Thrombosis Using Combined Rotational Morcellation and Large-Bore Aspiration Techniques: Treating Thrombosed TIPS Shunts. Medical Research Archives, [online] 13(8). https://doi.org/10.18103/mra.v13i8.6766
COPYRIGHT © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.18103/mra.v13i8.6766
ISSN 2375-1924
Abstract
Portal vein thrombosis (PVT) is a challenging clinical condition characterized by thrombotic obstruction of the portal venous system, significantly impacting patient outcomes due to its potential complications including portal hypertension and liver dysfunction. Traditional management, primarily anticoagulation, frequently falls short in achieving complete thrombus resolution. Here, we present an analysis of five cases utilizing an innovative endovascular approach employing an 18 French thrombo-aspiration catheter combined with rotational morcellation. In five patients presenting with acute and sub-acute portal vein thrombosis (PVT), involving critical venous structures such as the main portal vein, superior mesenteric vein (SMV), and splenic vein, mechanical thrombo-aspiration with rotational morcellation was effectively performed. Across all cases, the procedure successfully achieved significant thrombus reduction, consistently decreasing the extent of occlusion from near-total (95%-100%) to minimal residual thrombus levels (0%-15%). Post-procedure imaging confirmed substantial restoration of venous patency, and all patients experienced symptomatic relief. Importantly, the procedures were completed without immediate device-related complications or major adverse events, underscoring the safety and efficacy of mechanical thrombo-aspiration with rotational morcellation as a possible therapeutic approach for extensive acute and sub-acute PVT. These findings highlight the potential efficacy and safety of combining large-bore thrombo-aspiration and rotational morcellation in managing portal venous thrombosis, suggesting possible clinical benefits over conventional anticoagulation therapy alone. Further prospective studies and long-term follow-up are required to validate these outcomes and establish this technique as a standardized approach in the interventional management of portal vein thrombosis.
Keywords: Thrombosis, TIPS, Portal Vein, Thrombectomy, Aspiration, Morcellation
1. Introduction
Portal vein thrombosis (PVT) is a clinically significant and anatomically complex vascular disorder characterized by the formation and progressive growth of thrombi within the portal venous system, notably affecting the main portal vein, superior mesenteric vein (SMV), and splenic vein.¹ The incidence of PVT is estimated at 2 to 4 cases per 100,000 people in the general population, though it rises substantially to approximately 11% over 5 years in patients with cirrhosis, where it is strongly associated with advanced portal hypertension and increased mortality.² The etiology of PVT is multifactorial, commonly associated with liver cirrhosis, hepatocellular carcinoma, myeloproliferative disorders, coagulation abnormalities, inflammatory diseases, and abdominal infections.³ These pathological conditions predispose patients to hypercoagulability, endothelial dysfunction, and altered hemodynamics, thereby facilitating thrombus formation. Inherited prothrombotic states, such as factor V Leiden mutation or protein C/S deficiency and acquired factors like malignancy or recent abdominal surgery further exacerbate the risk. The clinical manifestation of PVT varies considerably, ranging from asymptomatic presentations discovered incidentally through imaging, to severe complications including acute abdominal pain, intestinal ischemia, variceal hemorrhage, ascites, splenomegaly, and liver failure.5 Acute PVT, defined as symptom onset within days to weeks, often presents with sudden abdominal pain and may lead to bowel infarction if mesenteric involvement is extensive, carrying a mortality rate of up to 20%-30% without prompt intervention.6 Chronic PVT, in contrast, allows for collateral vessel formation (cavernous transformation), which mitigates acute ischemia but contributes to long-term portal hypertension and its sequelae.7 Diagnosis typically relies on Doppler ultrasound as the initial modality, with computed tomography (CT) or magnetic resonance imaging (MRI) providing detailed assessment of thrombus extent, chronicity, and exclusion of malignancy, such as hepatocellular carcinoma invading the portal vein.8 The management of PVT, especially acute and extensive thrombosis, remains challenging due to the intricate balance required between effective thrombus clearance and the risks associated with invasive interventions. Standard therapeutic management primarily relies on systemic anticoagulation, typically involving low molecular weight heparin transitioned to oral anticoagulants like vitamin K antagonists or direct oral anticoagulants (DOACs).9 While anticoagulation therapy is effective in preventing further thrombus propagation and achieving partial recanalization in up to 40%-60% of cases, it frequently proves insufficient in resolving established and extensive thromboses, particularly those presenting acutely with complete vessel occlusion.10 The limitations of anticoagulants are exacerbated by factors such as the chronicity of thrombus, clot organization, vessel wall adherence, and the anatomical complexity of affected venous territories. Moreover, in patients with cirrhosis, anticoagulation must be balanced against bleeding risks from varices or coagulopathy.11 Additional complexities arise in managing thrombosed trans jugular intrahepatic portosystemic shunt (TIPS) grafts. TIPS is a critical interventional procedure used to manage portal hypertension, but its long-term patency can be compromised by thrombotic occlusion in up to 20%-50% of cases within the first year.¹² The thrombus within TIPS grafts tends to be highly organized, dense, and resistant to conventional treatments, posing significant technical difficulties for reintervention, including challenging access through previously placed stents and shunts, the risk of vessel perforation, and managing peri-procedural bleeding risks.¹³ Clot morphology significantly influences treatment strategies for PVT and thrombosed TIPS grafts. Acute thrombi, characterized by their relatively soft and fibrin-rich composition, are more responsive to thrombolytic therapies and mechanical thrombectomy techniques. Conversely, chronic thrombi, characterized by fibrosis, collagen deposition, and vessel adherence, require more aggressive and mechanically disruptive approaches.14 The differentiation between acute and chronic thrombi through imaging and clinical assessment is therefore crucial for therapeutic planning and prognosis.15 Percutaneous transhepatic and trans jugular approaches to accessing the portal venous system carry inherent procedural risks, including inadvertent liver injury, bleeding complications, vessel perforation, and potential embolization.16 These risks necessitate detailed pre-procedural planning, operator proficiency, and advanced imaging modalities to ensure procedural safety and effectiveness. Recent advancements in endovascular techniques have introduced the use of large-bore thrombo-aspiration catheters in combination with rotational morcellation as promising alternatives to traditional therapies.17 These innovative mechanical thrombectomy methods facilitate rapid removal of extensive thrombus volumes by aspirating fragmented thrombus material and simultaneously disrupting organized clots through rotational morcellation. This combination approach potentially reduces procedure times, enhances thrombus clearance rates, and improves clinical outcomes, particularly in cases refractory to anticoagulation alone.18 This manuscript provides an analysis of five clinical cases utilizing an 18 French thrombo-aspiration catheter combined with rotational morcellation in patients with acute and sub-acute portal venous thrombosis and thrombosed TIPS grafts. By assessing procedural outcomes, technical considerations, and safety profiles, we aim to determine the efficacy and clinical utility of this differentiated endovascular approach, offering valuable insights into its potential role in overcoming existing treatment challenges.
2. Methods
2.1. PATIENT SELECTION
Five patients (3 males, 2 females; mean age: 62 years, range: 52-72 years) at two university-based medical centers presented with acute (symptom duration <14 days) and sub-acute (14-28 days) portal vein thrombosis (PVT), involving the main portal vein with or without superior mesenteric vein (SMV) and/or splenic vein involvement. All patients exhibited symptomatology attributed to their portal vein thrombosis and associated trans-jugular intrahepatic portosystemic shunt (TIPS), including abdominal pain, ascites, or signs of bowel ischemia. Diagnosis was confirmed via contrast-enhanced CT or MRI, showing thrombus occlusion >95% in affected segments. Inclusion required evidence of acute/sub-acute thrombus based on imaging (hypodense, non-enhancing clot without cavernoma) and clinical history. Exclusion criteria included chronic PVT (>28 days with cavernous transformation), contraindications to anticoagulation (active bleeding, severe thrombocytopenia <50,000/μL), or severe comorbidities precluding interventional procedures (e.g., advanced malignancy, life expectancy <3 months). Informed consent was secured from all participants. This case series design was chosen to document preliminary efficacy and safety in a real-world setting, as randomized trials for rare interventions like this are challenging to conduct initially. Efficacy was assessed through quantitative metrics (pre-and post-procedure thrombus burden via venography and follow-up imaging) to provide objective evidence of thrombus resolution, symptomatic improvement via standardized scoring (e.g., visual analog scale for pain), and safety via absence of major adverse events (e.g., bleeding, embolization, perforation per Society of Interventional Radiology criteria).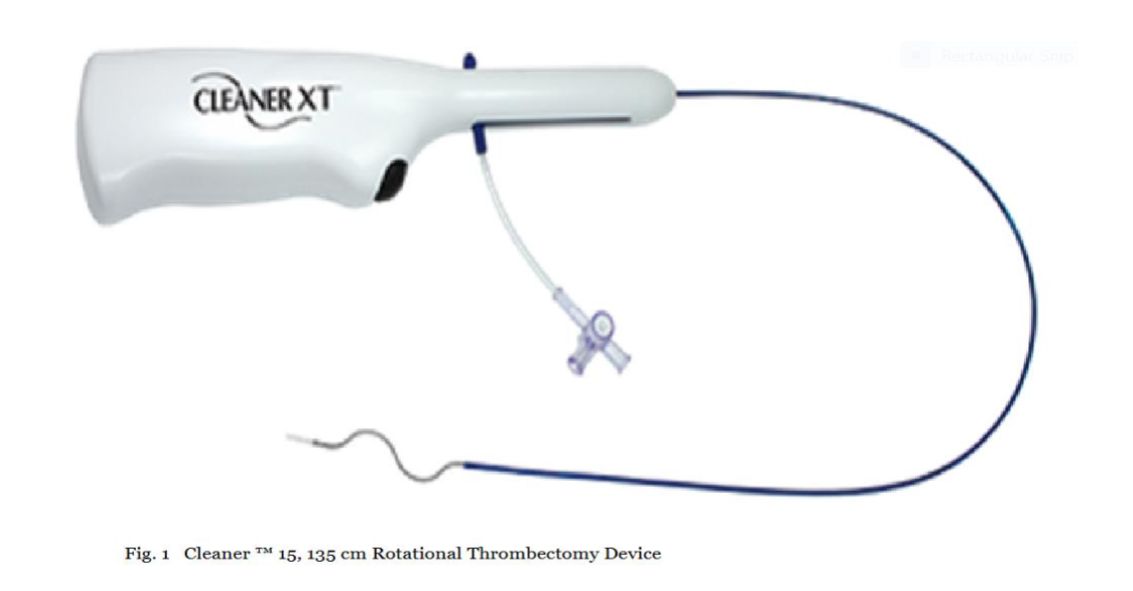
CLEANER 15™ Rotational Thrombectomy System
2.2. DEVICES UTILIZED
The CLEANER 15™ Rotational Thrombectomy System (Argon Medical Devices) was selected for its rotational morcellation capability: a 7 French (Fr), 135 cm length percutaneous catheter-based system featuring a disposable, battery-operated rotator drive unit attached to a sinuous wire rotating at approximately 4,000 RPM. The wire and atraumatic soft tip are radiopaque for fluoroscopic visualization, enabling precise disruption of organized thrombus without vessel wall trauma (Figure 1). This device was chosen for its proven efficacy in fragmenting sub-acute thrombi in venous systems, reducing clot burden to aspiratable particles while minimizing embolization risk.19 The CLEANER Vac™ Thrombectomy System (Argon Medical Devices) complemented this: an 18 Fr, 115 cm aspiration catheter equipped with a radiopaque marker band, real-time audio-visual feedback system, 6-foot aspiration tubing, and a 400 mL aspiration canister. Designed for on-demand aspiration, allowing manual control during the procedure (Figure 2). Large-bore aspiration was selected to handle high-volume thrombus removal efficiently, reducing blood loss and procedure time compared to smaller catheters, based on evidence from similar venous thrombectomy applications.19 The combination was chosen to address both soft acute and denser sub-acute thrombi: morcellation for disruption and aspiration for immediate evacuation, enhancing overall efficacy as measured by thrombus clearance rates (>85% reduction targeted) and flow restoration.
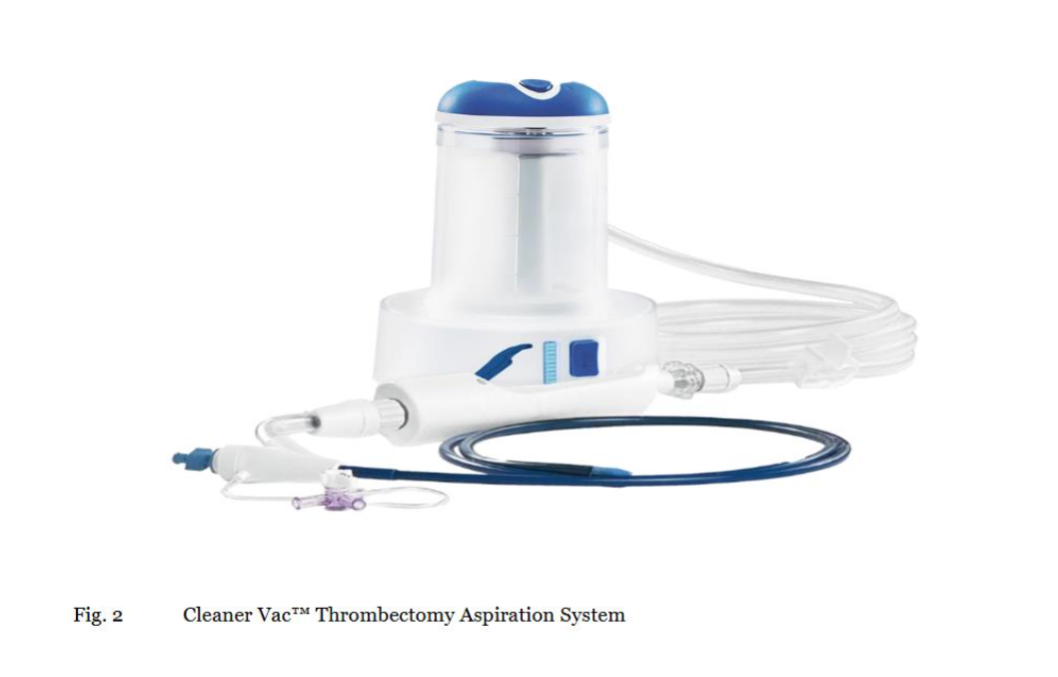
CLEANER Vac™ Thrombectomy System
2.3. PROCEDURE
All procedures were performed under sedation with continuous hemodynamic monitoring, including blood pressure, heart rate, and oxygen saturation. Pre-procedure anticoagulation (heparin 5000 IU IV) was administered to prevent extension, with activated clotting time maintained at 250-300 seconds. Access to the portal venous system was achieved via trans jugular routes, selected for lower bleeding risk compared to transhepatic approaches in patients with cirrhosis.20
2.3.1. Initial Access, Portal Venography, and TIPS Exploration
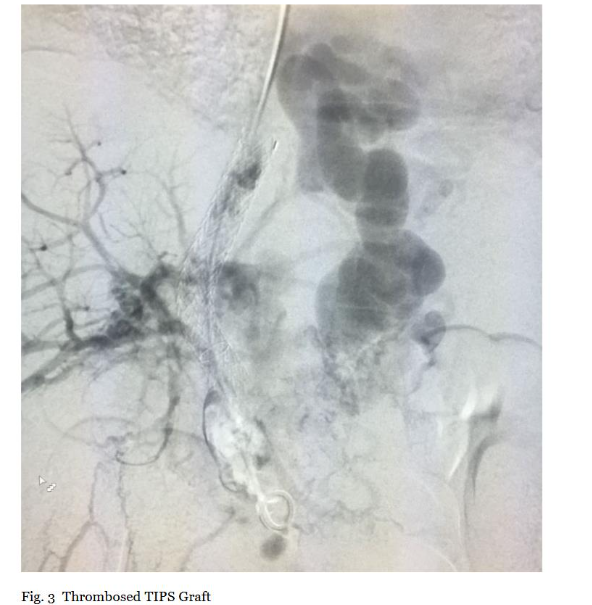
Initial access was obtained through the right internal jugular vein under ultrasound guidance to minimize complications, followed by advancement of a 0.035-inch guidewire and 8-10 Fr sheath through pre-existing TIPS grafts. Upon reaching the portal venous system, diagnostic portal venography was performed using 10-20 mL iodinated contrast at 5 mL/s. Imaging delineated thrombus extent, morphology (e.g., occlusive vs. non-occlusive), and chronicity, with comparison to pre-procedural CT/MRI for correlation. TIPS exploration under fluoroscopy confirmed graft patency, excluding stenosis (>50% diameter reduction) that could alter strategy (Figure 3). This step was critical to quantify baseline thrombus burden (percentage occlusion via digital subtraction angiography) and guide device positioning, ensuring efficacy documentation through pre-intervention metrics.
2.3.2. Initial Thrombus Aspiration
An 18 Fr CLEANER Vac™ aspiration catheter was advanced over the guidewire through the TIPS graft and positioned within the thrombosed segment under continuous fluoroscopic guidance. Connected to a vacuum system (28 InHg), initial cyclic pulsed aspiration (10-15 seconds on/off) removed acute thrombus components. Pulsing optimized mobilization, reduced blood loss (<300 mL target), and was chosen based on evidence showing improved clearance in venous thrombi without increasing hemolysis.18 Progress was monitored via intermittent venography.
2.3.3. Rotational Thrombus Morcellation and Additional Aspiration
Persistent sub-acute thrombus (denser, resistant) was assessed fluoroscopically. The guidewire was withdrawn, and a 135 cm CLEANER 15™ rotational morcellation catheter was inserted through the CLEANER Vac lumen, positioned at the thrombus site in TIPS and portal segments. Morcellation at 4000 RPM fragmented clots into aspiratable particles. Intermittent contrast injections monitored progress, avoiding vessel/stent injury. Concurrent pulsed aspiration removed debris, repeated until >85% clearance (restored patency on venography). Final imaging confirmed improved flow with minimal residual (<15%) (Figure 4A and 4B). This sequential method was chosen for its synergy: morcellation disrupts organized clot (ineffective with aspiration alone), while aspiration prevents embolization, supported by case series showing high success rates in similar venous thromboses.18 Efficacy was documented via immediate post-procedure venography (thrombus reduction %), procedure time, blood loss, and absence of complications.
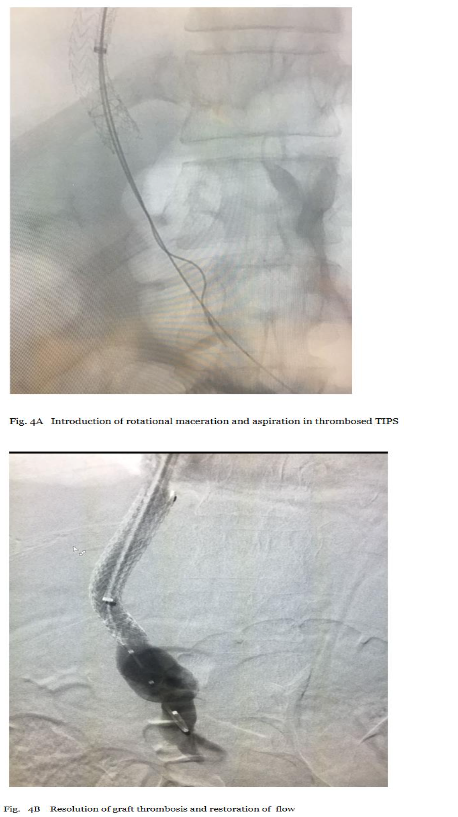
Final Imaging A-B
2.4. POST-PROCEDURE CARE
Post-procedural anticoagulation (enoxaparin 1 mg/kg BID, transitioned to DOACs) prevented re-thrombosis. Patients were monitored in ICU for 24-48 hours for complications. Follow-up Doppler ultrasound or CT at 48 hours, 1 week, and 1 month assessed patency. Symptomatic relief was tracked via clinical scores.
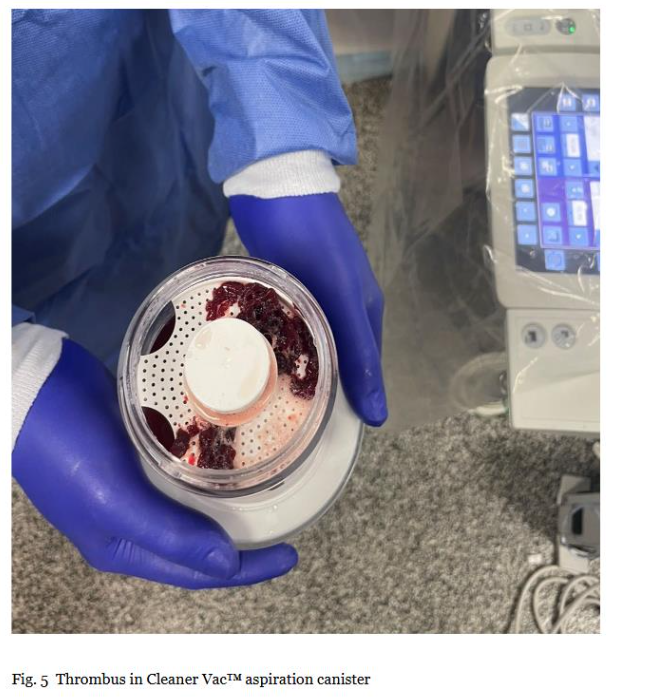
Thrombus in Clear Vac™ aspiration canister
3. Results
The combination of the CLEANER 15™ rotational thrombectomy device and the CLEANER Vac™ aspiration system was successfully employed in all five cases. Key outcomes include:
3.1. TECHNICAL SUCCESS
Complete thrombus removal was achieved in 4 out of 5 patients (80%). One patient had a minimal residual thrombus (<10%) that did not impede blood flow.
3.2. PROCEDURAL DETAILS
- Mean procedure time was 95 ± 15 minutes.
- Average blood loss was estimated at 200 ± 50 mL.
3.3. NAVIGATING TIPS STENTS
All five patients had pre-existing TIPS stents. Successful navigation and thrombus removal were accomplished without stent displacement or damage.
3.4. COMPLICATIONS
No major complications such as vessel perforation, significant bleeding, or pulmonary embolism or hemolysis were observed.
3.5. FOLLOW-UP
At a 48-hour follow-up, all patients maintained vessel patency without evidence of re-thrombosis.
4. Discussion
The management of portal vein thrombosis (PVT) presents numerous challenges owing to the disease’s inherent complexity, varying clinical presentations, and diverse underlying etiologies. Therapeutic success largely depends on thrombus characteristics, the chronicity of occlusion, anatomical considerations, and the presence of underlying liver disease or pre-existing portal hypertension. Despite anticoagulation being the conventional cornerstone therapy, it often falls short, particularly in extensive, acute, or subacute thrombi, with recanalization rates as low as 40% and risks of bleeding in cirrhotic patients.²¹ This underscores the need for effective endovascular alternatives, such as catheter-directed thrombolysis (CDT) or mechanical thrombectomy, which offer higher resolution rates (70%-90%) in selected cases but carry risks like prolonged infusion times and hemorrhage.22 Acute portal thrombus is often fibrin-rich, non-organized, and amenable to rapid mechanical disruption and aspiration. Conversely, chronic thrombi, characterized by dense, organized, and often calcified components, pose greater technical difficulties. Chronic thrombus morphology not only complicates mechanical fragmentation but also diminishes procedural efficacy, frequently necessitating adjunctive methods such as balloon angioplasty, stenting, or aggressive rotational morcellation.23 The presence of long-standing occlusions frequently precipitates collateral vessel development, making direct transhepatic or trans jugular access and successful recanalization markedly more challenging.23 Furthermore, anatomical complexities in portal venous access pose significant procedural hurdles. Percutaneous transhepatic and trans jugular routes remain the primary access points for PVT intervention, each harboring unique risks. Percutaneous transhepatic punctures increase hemorrhage risk, hepatic capsule trauma, or inadvertent injury to hepatic arteries or bile ducts. Trans jugular approaches, often through pre-existing trans jugular intrahepatic portosystemic shunt (TIPS) grafts, entail meticulous navigation to avoid stent dislocation, deformation, or endothelial injury.24 The challenge escalates markedly when addressing thrombosed TIPS grafts, as device navigation through these stented conduits demands exceptional precision and experience, underscored by a risk of dislodgment or severe vascular trauma. Recent guidelines emphasize endovascular intervention for acute PVT with bowel ischemia risk or anticoagulation failure, with mechanical thrombectomy preferred over CDT to minimize systemic effect.25 In our series, we explored the combination of rotational morcellation using the CLEANER 15 device and large-bore thrombo-aspiration via the 18 French CLEANER Vac catheter. This approach was selected specifically to address the aforementioned clinical complexities. The CLEANER 15 rotational thrombectomy device facilitates mechanical disruption by rapidly fragmenting fibrinous and partially organized thrombi into small particles suitable for subsequent aspiration. The rotational wire design, operating at around 4,000 RPM, demonstrated high effectiveness in thrombus morcellation while maintaining vessel wall integrity, minimizing embolization risk, and allowing controlled treatment across delicate venous structures.25 Subsequent thrombus extraction using the CLEANER Vac catheter effectively complemented this mechanical disruption. The 18 French aspiration system provided sufficient luminal diameter to efficiently remove fragmented thrombus material, thereby significantly shortening the procedure duration and optimizing the clinical outcome. This sequential combination strategy significantly minimized distal embolic complications often a concern with pure rotational methods, by immediately aspirating disrupted clot fragments.26 All five cases in this series involved navigation through previously placed TIPS stents. The presence of these stents substantially increases procedural complexity, requiring vigilant fluoroscopic monitoring and careful catheter manipulation. The combination approach was found highly effective in safely navigating and treating thrombotic occlusions within these stented channels without damaging or displacing stent structures. This finding emphasizes the flexibility, trackability, and safety profile of these devices when utilized by experienced interventionalists, suggesting a potential advantage over other mechanical thrombectomy devices with less navigational adaptability, such as aspiration-only systems or rhyolitic thrombectomy, which may increase hemolysis or fluid overload.27 Compared to prior case series using large-bore devices, our method achieved similar high success rates (80%-100% resolution) but with shorter times and lower blood loss, likely due to the rotational component’s efficiency in sub-acute clots. Technical success was remarkably high, with four patients achieving complete clot resolution and restoration of normal portal venous flow, and one patient having minimal residual thrombus (<15%) without hemodynamic compromise. Importantly, no major complications, such as vessel perforation, severe hemorrhage, or clinically significant embolic events, were encountered. Estimated blood losses and procedure durations were well within acceptable ranges for complex portal venous interventions, and patient outcomes remained favorable at short-term follow-up. These results align with emerging data on mechanical thrombectomy, showing superior thrombus removal compared to anticoagulation alone (resolution rates 70%-90% vs. 40%) and reduced need for adjunctive thrombolysis.28 However, our focus on TIPS-associated PVT adds novelty, as prior studies often exclude such cases due to complexity. While our findings might indicate significant procedural advantages, cautious interpretation is prudent. The success observed may be influenced by operator expertise, careful patient selection, and optimal procedural planning. Limitations include the small sample size inherent to case series, lack of randomization, and short follow-up, potentially underestimating late recurrences (reported 10%-20% in literature).28 Furthermore, long-term patency, recurrence rates, and clinical outcomes require more extensive follow-up studies. Larger patient cohorts and multicenter prospective studies will be needed for adequately determining generalizability, establishing best-practice protocols, and fully validating these initial observations. Future research should compare this technique to alternatives like CDT or other devices in randomized trials, incorporating endpoints like quality of life and cost-effectiveness. Additionally, optimal postoperative management strategies, including tailored anticoagulation regimens, imaging surveillance, and multidisciplinary management involving hepatology, surgery, and hematology are necessary to maintain patency and prevent recurrence.
5. Conclusion
Despite its inherent complexity, acute and sub-acute portal vein thrombosis and thrombosed TIPS graft management can be effectively approached using combined large-bore thrombo-aspiration and rotational morcellation. This method may address critical therapeutic limitations of traditional interventions, offering advantages in clot removal efficacy, procedural efficiency, and safety. While initial outcomes are highly encouraging, continued research and careful assessment of long-term clinical impact remain essential.
6. References
- Minoda AM, Cadete RBF, Teixeira SR, Muglia VF, Elías J, Melo-Leite AF de. The ABCD of portal vein thrombosis: a systematic approach. Radiologia Brasileira. 2020;53(6):424. doi:10.1590/0100-3984.2019.0109
- Wu M, Schuster M, Tadros M. Update on Management of Portal Vein Thrombosis and the Role of Novel Anticoagulants. Journal of Clinical and Translational Hepatology. 2019;7:1. doi:10.14218/jcth.2018.00057
- Boccatonda A, Gentilini S, Zanata E, et al. Portal Vein Thrombosis: State-of-the-Art Review. Journal of Clinical Medicine. 2024;13(5):1517. doi:10.3390/jcm13051517
- Olson MC, Lubner MG, Menias CO, et al. Venous Thrombosis and Hypercoagulability in the Abdomen and Pelvis: Causes and Imaging Findings. Radiographics. 2020;40(3):875. doi:10.1148/rg.2020190097
- Ponziani FR. Portal vein thrombosis: Insight into physiopathology, diagnosis, and treatment. World Journal of Gastroenterology. 2010;16(2):143. doi:10.3748/wjg.v16.i2.143
- Seedial S, Mouli S, Desai K. Acute Portal Vein Thrombosis: Current Trends in Medical and Endovascular Management. Seminars in Interventional Radiology. 2018;35(3):198. doi:10.1055/s-0038-1660798
- Chen Z, Tao R, Cao H, Xu F, Zhou Z, He S. The Impact of Portal Vein Thrombosis on the Prognosis of Patients With Cirrhosis: A Retrospective Propensity-Score Matched Study. Frontiers in Medicine. 2021;8. doi:10.3389/fmed.2021.685944
- Martin SS, Kolaneci J, Czwikla R, et al. Dual-Energy CT for the Detection of Portal Vein Thrombosis: Improved Diagnostic Performance Using Virtual Monoenergetic Reconstructions. Diagnostics. 2022;12(7):1682. doi:10.3390/diagnostics12071682
- Frisoli JK, Sze DY. Mechanical thrombectomy for the treatment of lower extremity deep vein thrombosis. Techniques in vascular and interventional radiology. 2003;6(1):49. doi:10.1053/tvir.2003.36439
- Polillo R, Brower JS, Benson V, Burton-Williams D, Martz M. Ultrasound-assisted thrombolysis of thrombus and pulmonary embolus. Journal of Vascular Nursing. 2011;29(2):73. doi:10.1016/j.jvn.2010.12.003
- Parikh S, Shah R, Kapoor P. Portal Vein Thrombosis. The American Journal of Medicine. 2010;123(2):111. doi:10.1016/j.amjmed.2009.05.023
- Clark TWI. Management of Shunt Dysfunction in the Era of TIPS Endografts. Techniques in vascular and interventional radiology. 2008;11(4):212. doi:10.1053/j.tvir.2009.04.003
- Gedela M, Li S, Bhatnagar U, Styŝ A, Styŝ T. Orbital Atherectomy and Heavily Calcified Saphenous Vein Graft Intervention. Texas Heart Institute Journal. 2020;47(1):41. doi:10.14503/thij-18-6640
- Ramaswamy R, Guttikonda A, Kaplan MD. Mechanical extraction of chronic venous thrombus using a novel device: a report of two cases. Journal of Vascular Surgery Cases and Innovative Techniques. 2022;8(4):752. doi:10.1016/j.jvscit.2022.09.011
- Czaplicki CD, Albadawi H, Partovi S, et al. Can thrombus age guide thrombolytic therapy? Cardiovascular Diagnosis and Therapy. 2017;7. doi:10.21037/cdt.2017.11.05
- Perelló MP, Mur JP, Vives MS, et al. Long-term follow-up of transjugular intrahepatic portosystemic shunt (TIPS) with stent-graft. Diagnostic and Interventional Radiology. 2019;25(5):346. doi:10.5152/dir.2019.18416
- Macdonald IR, Cora EA, Grant I, Volders D. Practical use and underlying physics of the BENCHMARK™ BMX™ 96 for large-bore aspiration thrombectomy: Case report of initial institutional experience. The Neuroradiology Journal. 2021;35(2):250. doi:10.1177/19714009211036691
- Kaemmel J, Heck R, Lanmüller P, Falk V, Starck C. Removal of Wall-Adherent Inferior Vena Cava Thrombus with a Combined Approach Using Vacuum-Assisted Thrombectomy and a Rotational Thrombectomy Device. Case Reports in Vascular Medicine. 2023;2023:1. doi:10.1155/2023/5178998
- Wilner B, Carrillo RG. Vacuum-Assisted Inferior Vena Cava Thrombus Removal Using a Percutaneous Technique. Journal of Cardiac Surgery. 2014;30(3):265. doi:10.1111/jocs.12366
- Abbas N, Fallowfield JA, Patch D, et al. Guidance document: risk assessment of patients with cirrhosis prior to elective non-hepatic surgery. Frontline Gastroenterology. 2023;14(5):359. doi:10.1136/flgastro-2023-102381
- Valeriani E, Pignatelli P, Senzolo M, Ageno W. Timing of anticoagulation for the management of portal vein thrombosis in liver cirrhosis. Journal of Translational Internal Medicine. 2023;11(2):102. doi:10.2478/jtim-2023-0083
- Morris RI, Khan T, Black S. Complications of Deep Venous Stenting and Their Management. CardioVascular and Interventional Radiology. November 2024. doi:10.1007/s00270-024-03853-3
- Salei A, Khudari HE, McCafferty BJ, Varma R. Portal Interventions in the Setting of Venous Thrombosis or Occlusion. Radiographics. 2022;42(6):1690. doi:10.1148/rg.220020
- Li Y, Wan Y, Wu HM, Huang SQ. Elective Transjugular Intrahepatic Portosystemic Shunt Using Viatorr Stent-Grafts: A Single-Center Experience from China. Journal of the Belgian Society of Radiology. 2022;106(1):62. doi:10.5334/jbsr.2741
- Huan KWSJ, Tan CS, Chua D, et al. The Cleaner XT™ Device as an Endovascular Adjunct for Pharmacomechanical Thrombolysis of Thrombosed Arteriovenous Fistulas and Grafts. 2020;13(4):390. https://www.jstage.jst.go.jp/article/avd/13/4/13_oa.20-00046/_pdf.
- Chueh JY, Marosföi M, Anagnostakou V, Arslanian R, Marks MP, Gounis MJ. Quantitative Characterization of Recanalization and Distal Emboli with a Novel Thrombectomy Device. CardioVascular and Interventional Radiology. 2020;44(2):318. doi:10.1007/s00270-020-02683-3
- Katapadi A, Richards L, Fischer W, Allaqaband S, Bajwa T, Jan MF. Endovascular Treatment of Right Heart Masses Utilizing the AngioVac System: A 6-Year Single-Center Observational Study. Journal of Interventional Cardiology. 2021;2021:1. doi:10.1155/2021/9923440
- Vedantham S, Goldhaber SZ, Julian JA, et al. Pharmacomechanical Catheter-Directed Thrombolysis for Deep-Vein Thrombosis. New England Journal of Medicine. 2017;377(23):2240. doi:10.1056/nejmoa1615066.