Enhancing Rehab in Spinal Cord Injury with Behavioral Activity
Enforcing Extra Behavioral Activity in Spinal Cord-Injured Rats Can Be a Rehabilitation Process to Enhance the Effects of Pentoxifylline and Tacrolimus Treatment: A Nursing Care Perspective
Hamood Alharbi ¹, Ahmed Alsadoun ¹, Abdualrahman Alshehry ¹, Mohammad Ahmad ¹
¹ Department of Medical-Surgical Nursing, College of Nursing, King Saud University, Riyadh, Saudi Arabia
OPEN ACCESS
PUBLISHED: 31 August 2025
CITATION: Alharbi, H., Alsadoun, A ., et al., 2025. Enforcing Extra Behavioral Activity in Spinal Cord-Injured Rats Can Be a Rehabilitation Process to Enhance the Effects of Pentoxifylline and Tacrolimus Treatment: A Nursing Care Perspective. Medical Research Archives [online] 13(8). https://doi.org/10.18103/mra.v13i8.6803
COPYRIGHT © 2025 European Society of Medicine. This is an open -access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
DOI: https://doi.org/10.18103/mra.v13i8.6803
Abstract
Background: Pentoxifylline and Tacrolimus are known to improve the functional outcome of spinal cord injury (SCI). The present study aims to develop suitable rehabilitative interventions in conjunction with therapeutic agents to enhance functional recovery. Furthermore, this research aims to raise awareness among nurses about caring for SCI patients and utilize their passion for developing their caregiving skills through nursing research.
Methods: Young adult male rats were subjected to spinal trauma by the compression method of the exposed spinal cord. Animals were allocated to seven groups, each consisting of eight animals, namely Group 1 was standard uninjured control; Group 2 was sham control with laminectomy but no spinal injury; Group 3 was SCI group with laminectomy and spinal injury; Group 4 and 5 as SCI treated groups A and C, respectively, were the same as Group 3 but were treated with a daily oral administration of pentoxifylline (10 mg/kg) and tacrolimus (1 mg/kg), respectively, for 29 days and subjected to Basso-Beatie-Breshnahan behavioral test in which the hind limb function was scored from 0 (complete paralysis or paraplegia) to 21 (complete mobility), every alternate day in a “Gait Performance Tunnel”; groups 6 and 7 were as SCI treated groups B and D respectively, that was same as groups 4 and 5 except that the animals were further subjected to an enforced extra five walks as exercise in gait performance tunnel test.
Results: In both drugs, all treated groups A, B, C, and D significantly recovered in Groups B and D (p<0.001) than in Groups A and C. Furthermore, it was observed that tacrolimus was comparatively more effective than pentoxifylline. Conclusion: It is concluded that if SCI animals are subjected to extra-enforced daily behavioral exercises in addition to drug treatments, functional recovery can be improved more quickly, making it a rehabilitative activity that enhances treatments. Such studies can be innovations for nursing research.
Keywords:
- Spinal cord injury
- pentoxifylline
- tacrolimus
- Basso-Beatie-Breshnahan scoring
- rehabilitation
- nursing care
Introduction
Spinal cord injury (SCI) is a devastating trauma that changes the lives of most patients, leaving them paralyzed for their lifetime. SCI, most often caused by accidents, is characterized by partial or total loss of motor and sensory function below the level of the injury, resulting in individual disabilities that depend on the level and severity of the lesion. However, it has been demonstrated that SCI can be recovered with proper intervention. Locomotor rehabilitation is a well-established intervention that benefits individuals affected by the SCI condition. Rehabilitation’s primary aim is to facilitate functional recovery toward daily independence, particularly regaining stepping ability or hand function. Rehabilitation is the only strategy that has proven effective and beneficial for patients over the last decades.
Suitable animal models of SCI for experimental studies simulating clinical conditions observed in humans are essential sources for understanding the disease’s pathophysiology and developing research modalities for effective rehabilitation and treatment. The present study aims to understand the behavioral episode that often follows the initial primary injury and to create suitable rehabilitative interventions, along with effective pharmacological agents, that may enhance sensory and motor functions in an improved manner.
Tacrolimus (FK506), a macrolide lactane antibiotic, was introduced as an immunosuppressive agent with no side effects. It is a potent calcineurin inhibitor, exhibits neuroprotection actions in several experimental models of central nervous system trauma, including stroke, and improved neurological recovery following peripheral and spinal cord injuries. Tacrolimus improves the functional outcome of spinal cord injury and has an in vivo neurotrophic action whereby it enhances the rate of axon regeneration, leading to more rapid neurological recovery. It is reported that FK506 has beneficial effects on SCI recovery, involving various mechanisms such as neuroregeneration and neuroprotection, promotion of axonal outgrowth, and suppression of oxidative stress. Rat models have reported significant functional recovery from SCI due to FK506 treatment.
Pentoxifylline (PTX), a methylxanthine derivative, is a potent antioxidant and modulator of various transmitters and penetrates the blood-brain barrier rapidly and efficiently after systemic administration. This drug was initially introduced for treating peripheral circulatory disorders, primarily because of its hemorheological effects. Recent studies have demonstrated the neuroprotective effect of PTX against various neurobehavioral disorders, including ischemic brain injury, neurotrauma, dementia, and stroke. Pentoxifylline may exert its pharmacological effects by several mechanisms, including inhibition of phosphodiesterase enzymes (PDEs), leading to increases in cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP), blockade of adenosine receptors, translocation of extracellular calcium, an inhibitory effect on inflammatory mechanism, and free radical scavenging activity. Pentoxifylline (PTX) has also been shown to have neuroprotective effects against a host of neurobehavioral disorders, including ischemic brain injury, neurotrauma, dementia, and stroke, and it exhibits a protective role in spinal cord injury following ischemia.
In light of the above information, the present study was designed to investigate the comparative neuroprotective effects of FK506 and PTX on behavioral recovery from SCI and to assess if the enforcement of SCI animals to extra behavioral activities may assist as a rehabilitation process to accentuate the functional recovery from SCI with better protective effect of the therapeutic interventions. Furthermore, the present research has been designed to raise awareness among nurse communities working in occupational health settings about the importance of demonstrating passion for SCI patient care through exploring and learning standardized and innovative rehabilitation methods for managing and practicing practical nursing care skills.
Materials and Methods
ANIMALS:
Young adult male Sprague-Dawley rats, weighing 250-280g, bred, reared, and housed under controlled conditions (diurnal 12-hour light-dark cycle, temperature 22±1°C, humidity 50-60%, free access to food and water), were used in the present study. Every effort was made to minimize animal stress and suffering. Moreover, all animal practices and study protocols were approved by the Research and Ethics Committee of King Saud University, Riyadh, Saudi Arabia.
SPINAL CORD INJURY (SCI):
Rats were anesthetized with chloral hydrate (450mg/kg) and were subjected to spinal trauma by the modified method. After shaving the back of the animal, a longitudinal incision was made on the midline of the back, exposing the paravertebral muscles. Laminectomy was performed under a surgical operating binocular microscope at T7-8 level, leaving the dura intact. Spinal injury was produced by placing a metallic rectangular plate (2.5 × 5.0mm) loaded with a total weight of 35g for 5 minutes over the exposed extradural area of the spinal cord for compression. The wound was closed in layers through aseptic surgical stitching, and the animals were allowed to recover from anesthesia by placing them on a warm heating pad maintained at 37 °C ± 1 °C. All animals received intramuscular gentamycin injections at a dose of 3 mg/kg for 3 days post-surgery. The animals’ bladders were manually pressed twice daily to avoid urinary complications until the rats regained normal bladder function. Sham-injured animals were only subjected to laminectomy with the same surgical procedures without any compression.
EXPERIMENTAL GROUPS:
Rats were randomly allocated to the following five groups, with eight animals in each group:
- Group 1: Normal control group, without any laminectomy or compression injury.
- Group 2: Sham group with laminectomy only, but no spinal compression injury.
- Group 3: SCI control group with laminectomy surgery and spinal compression injury.
- Groups 4 and 5: SCI-treated groups A and B, respectively.
- Groups 6 and 7: SCI-treated groups C and D, respectively.
Groups A, B, C, and D were the same as SCI control groups except that SCI-treated Groups A and B were used to study the effect of FK506 (1 mg/kg), and Groups C and D were used to study the effect of PTX (10 mg/kg) treatment for the recovery from SCI using the behavioral parameter of BBB scoring every alternate day as described below. The doses of these drugs were selected based on our pilot screening at various doses. The drugs were administered orally in the morning session. The first dose of the medicines was administered one hour after SCI and thereafter, daily for three weeks.
BEHAVIORAL ANALYSIS:
In the form of Basso-Beatie-Breshnahan (BBB) scoring, behavioral motor functions were observed in the evening session and assessed blindly. The scores of each test were evaluated the next day after injury and every alternate day for 29 days after SCI for each animal. Hind limb motor function (including hind limb reflexes and coordinated use of hind limbs) was assessed using the BBB locomotor rating scores. The method for scoring this BBB rating was modified in that, instead of placing the individual rats in an open field to evaluate hind limb motor behavior, the animals were allowed to travel through a “Gait Performance Tunnel” (GPT). This innovative GPT consisted of a narrow tunnel constructed from a wooden block of size (180 X 10 X 5 cm) with side walls made of clear perspex glass (180 X 18 cm2) so that the animal movement was clearly visible from the side walls to the blinded observer and the score was assessed carefully for the rehabilitative coordinated movement of the hind limbs and placement of the hind paws. The GPT was placed at a height of 30 cm on the working table. The animal was allowed to enter at one end and travel up to the other. Soft bedding was placed under the other end of the GPT to avoid any injury in case of a fall from the GPT after completing the walk. No time was fixed for the walk in the GPT. The observer could quickly assess the movements of the hind limbs and the placement of the hind paws of the animals through the Perspex glass sheet of the GPT. Hind limb function was scored from 0 (complete paralysis or paraplegia) to 21 (complete mobility). SCI-treated Groups A, B, C, and D were subjected to a similar experimental process to observe the behavioral parameter of BBB scoring every alternate day, except with a difference in that the animals of Groups B and D were also repeatedly subjected to a daily additional 5 times enforced walking on the GPT with an interval of 5 min rest between the walks. After completing the BBB test on alternate days, the animals in Groups A and C were generally left in their home cages with no further disturbance. Whereas, after BBB scoring on every alternate day, Groups B and D animals were subjected to an additional daily enforced walking in the GPT. For an enforced walk, the animals were allowed to enter at one end of the GPT and travel through the GPT to reach the other end freely without any fixed timing, and this was considered one walk. Similarly, the animals were subjected to five enforced walks on the GPT, with a 5-minute rest interval between each walk. After completing five walks, the animals of Groups B and D were left in their home cages, as were the animals in Groups A and C.
Statistical analysis
The data from the experimental SCI group, which passed the normality test (p > 0.10), were compared to the SCI uninjured control group. In contrast, the data from the drug-treated groups were compared with those from the experimental SCI group using ANOVA with post-hoc testing using either the Tukey-Kramer Multiple Comparison Test or the Student-Newman-Keuls Multiple Comparison Test. All results were expressed as means ± SEM, and significance was defined as p<0.05 for the test.
Results
The results indicated that treatment with FK506 and PTX induced functional recovery from SCI over time. However, the drugs had an attenuating effect on SCI in all the treated groups A, B, C, and D, as compared to the SCI-only control group. The effectiveness of FK506 on the behavioral recovery from SCI in treated Group B was significantly (p<0.001) greater than in treated Group A.
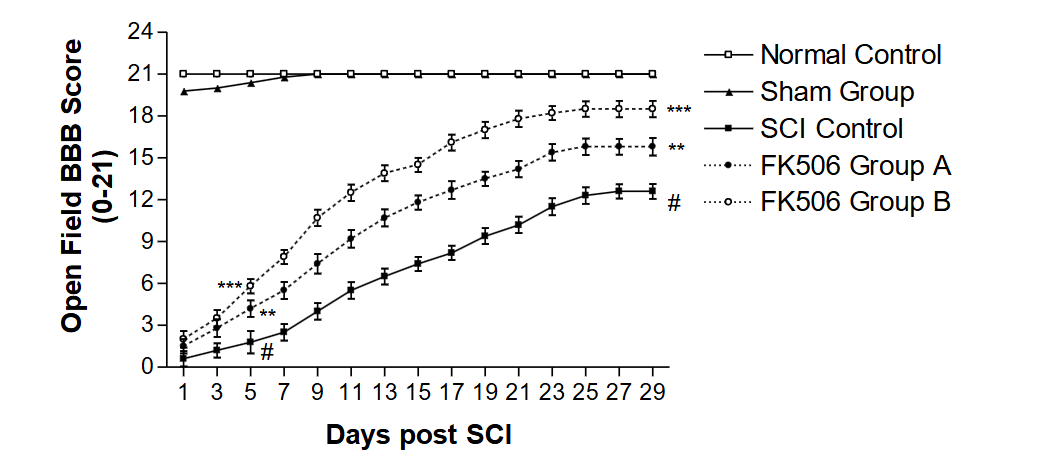
The effectiveness of PTX on behavioral recovery from SCI in treated Group D was significantly (p<0.001) higher than that of treated Group C.
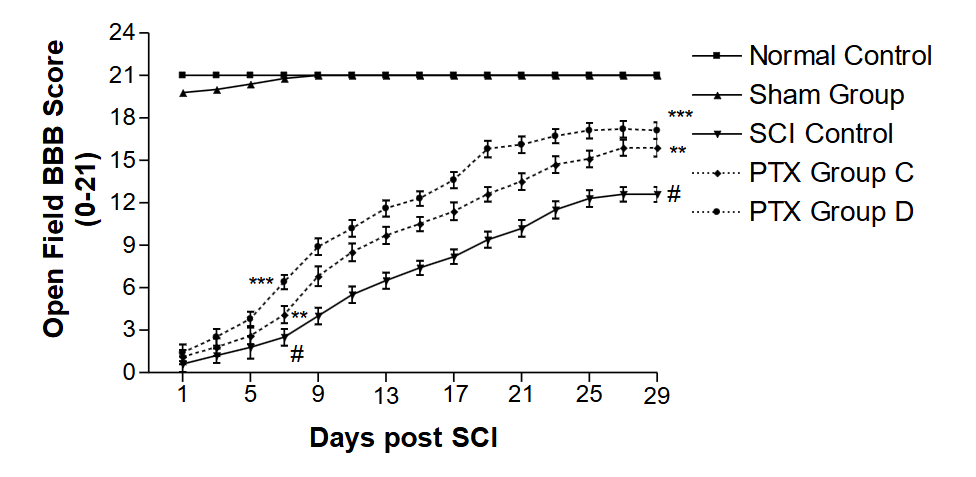
The comparative rehabilitation of the SCI Groups B and D, following enforced walking and treatment with FK506 (Group B) and PTX (Group D), demonstrated that FK506 was significantly (p < 0.001) more effective than PTX.
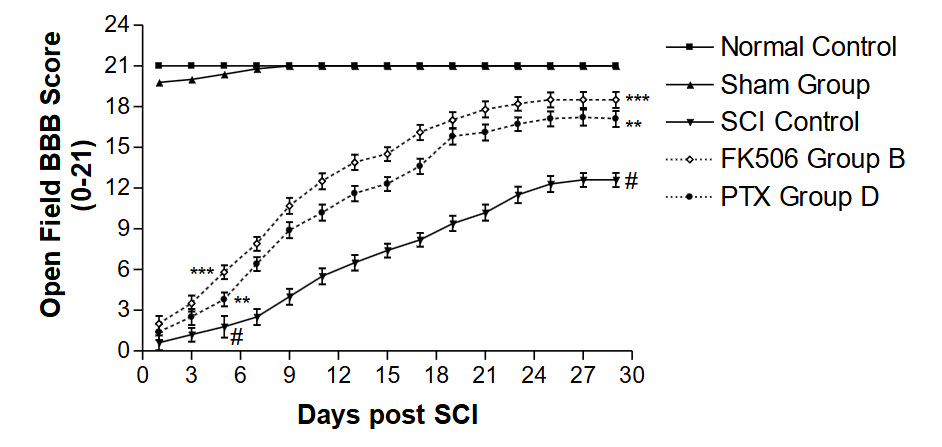
The sham group showed minimal alterations in behavioral activities. It achieved similar scoring levels to the naïve control groups within a few days, indicating no damage to the spinal cord. However, the behavioral recovery from SCI (in the SCI-only group) at 29 days was less significant (p < 0.01) compared to the naïve control.
Discussion
Our study aimed to observe recovery from spinal cord injury (SCI) in all control, sham, and treatment groups through overall comparisons of behavioral parameters, as measured by BBB scores. All groups exhibited severe deficits in hind limb function and moved using only the forelimbs, dragging the paraplegic hind limbs. Although, the functional recovery in rats treated with both drugs showed significant recovery compared to the SCI controls from day 3 after injury, the mechanism of the drugs under study is likely neuroprotective rather than tissue regenerative, as suggested earlier. Bearing in mind that FK506 exhibits neuroprotection actions in several experimental models, including improved neurological recovery following peripheral and spinal cord injuries, and improves the functional outcome of spinal cord injury and has an in vivo neurotrophic action whereby it enhances the rate of axon regeneration, leading to more rapid neurological recovery. It is reported that FK506 has beneficial effects on SCI recovery, involving various mechanisms such as neuroregeneration and neuroprotection, promotion of axonal outgrowth, and suppression of oxidative stress. Rat models have reported significant functional recovery from SCI due to FK506 treatment.
On the other hand, PTX has also been shown to have neuroprotective effects against a host of neurobehavioral disorders, including ischemic brain injury, neurotrauma, dementia, and stroke, and it exhibits a protective role in spinal cord injury following ischemia. The present results demonstrate that treatment with FK506 (groups A and B) and PTX (groups C and D) resulted in significant recovery from functional deficits starting at 1 week post-SCI injury. The treated groups exhibited recovery in hind limb reflexes more rapidly, with higher recovery responses in coordinated hind limb use for maintaining their body position from days 14 to 28 compared to the injured, untreated SCI control groups. Group B in FK506-treated groups and group D in PTX-treated groups recovered with a better, more significant functional recovery than groups A and C, possibly because the animals were additionally subjected to daily enforced five walks on the GPT. Thus, FK506-treated groups A and B were effective in FK506 group B > FK506 group A. Similarly, in PTX-treated groups C and D, both groups were effective, with the order of effectiveness being PTX group D > PTX group C. In addition to the therapeutic effects of FK506 and PTX, the daily walk-in groups B and D appear to be a potential factor in assisting the animals, as daily rehabilitative exercises helped them recover significantly faster than the animals in groups A and C from SCI. Furthermore, comparing the effective functional recovery due to the therapeutic effects of FK506 and PTX, along with enforced walks in the GPT, in groups B and D, showed that FK506 is more effective than PTX.
Earlier studies have supported the view that the chronically injured spinal cord is capable of Plasticity, and locomotor rehabilitation is the only known intervention that benefits affected individuals. From the perspective of nursing care, the present study aims to serve as a model research activity for the general nurses and/or the specialized nurses working in the field of rehabilitative services to SCI patients in particular, and in general to all other fields as well, working for improved mobilization of patients for better healthcare and wellbeing. Furthermore, emphasis is placed on increasing clinical research, which leads to a need for innovative, improved, and standardized education on research opportunities provided to nurses. Thus, the present study can highlight the inextricable link between changing the way healthcare nurses deliver compassionate care and demonstrating their compassionate abilities in clinical nursing rehabilitation practices.
Conclusion
It is concluded from this study that if SCI animals are subjected to enforced daily repetitive behavioral exercises (in the present study, as enforced walking in the GPT), it can improve functional restoration at a faster rate following SCI. It can be considered a rehabilitative process to accentuate intervention treatments (FK506 and PTX in the present study). Such interventions with a physiotherapeutically related rehabilitation process can also help repair the traumatically injured spinal cord. However, more detailed studies are required to understand this presumption.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding statement
This research had no funding.
Acknowledgment
The authors are thankful to the Deanship of Scientific Research, College of Nursing Research Center at King Saud University for supporting this research.
References
- Armour BS, Courtney-Long EA, Fox MH, Fredine H, Cahill A. Prevalence and causes of paralysis-United States, 2013. Am J Public Health. 2016;106:1855–57.
- Kakulas BA. Neuropathology: the foundation for new treatments in spinal cord injury. Spinal Cord. 2004;42:549–63.
- Cote MP, Murray M, Lemay MA. Rehabilitation strategies after spinal cord injury: inquiry into the mechanisms of success and failure. J Neurotrauma. 2017;34:1841–57.
- Loy K, Bareyre FM. Rehabilitation following spinal cord injury: how animal models can help our understanding of exercise-induced neuroplasticity. Neural Regen Res. 2019; 14:405-12.
- Starzl TE, Todo S, Fung J, Demetris AJ, Venkataraman R, Jain A. FK506 for liver, kidney and pancreas transplantation. Lancet. 1989;2:1000-4.
- Sosa I, Reyes O, Kuffler DP. Immunosuppressants: Neuroprotection and promoting neurological recovery following peripheral nerve and spinal cord lesions. Exp Neurol. 2005; 195: 7-15.
- Bochelen DMR, Sauter A. Calcineurin inhibitors FK506 and SDZASM981 alleviate the outcome of focal cerebral ischemic/reperfusion injury. J Pharmacol Experiment Thera. 1999;288:653-59.
- Butcher SP, Henshall DC, Teramura Y, Iwasaki K, Sharkey J. Neuroprotective actions of FK506 in experimental stroke: In vivo evidence against an antiexcitotoxic mechanism. J Neuroscience. 1997;17:6939-46.
- Madsen JR, McDonald P, Irwin N, Goldberg DE, Yao GL, Meiri KF. Tacrolimus (FK506) increases neuronal expression of GAP-43 and improves functional recovery after spinal cord injury in rats. Exp Neurol. 1998;154:673-83.
- Saganova K, Galik J, Blasko J, Korimova A, Racekova E, Vanicky I. Immunosuppressant FK506: Focusing on neuroprotective effects following brain and spinal cord injury. Life Sciences. 2012;91:77-82.
- Yamaji T, Yamazaki S, Li J, Price RD, Matsuoka N, Mutoh S. FK1706, a novel non-immunosuppressant neurophilin ligand, ameliorates motor dysfunction following spinal cord injury through its neurodegenerative action. Europ J Pharmacol. 2008;591:147-52.
- Zhang N, Fang M, Chen H, Gou F, Ding M. Evaluation of spinal cord injury animal models. Neural Regen Res. 2014;9:2008-12.
- Gordon T, Sulaiman O, Boyd JG. Experimental strategies to promote functional recovery after peripheral nerve injuries. J Peripher Nerv Syst. 2003;8:236-50.
- Navarro X, Udina E, Ceballos D, Gold BG. Effects of FK506 on nerve regeneration and reinnervation after graft or tube repair of long nerve gaps. Muscle & Nerve. 2001;24:905-15.
- Sulaiman OA, Voda J, Gold BG, Gordon T. FK506 increases peripheral nerve regeneration after chronic axotomy but not after chronic Schwann cell denervation. Exp Neurol. 2002;175:127-37.
- Udina E, Rodrigues FJ, Verdu E, Espejo M, Gold BG, Navarro X. FK506 enhances regeneration of axons across long peripheral nerve gaps repaired with collagen guides seeded with allogenic Schwann cells. Glia. 2004;47:120-9.
- Pan F, Chen A, Guo F, Zhu C, Tao F. Effect of FK506 on expression of hepatocyte growth factor in murine spinal cord following peripheral nerve injury. Journal of Huazhong University of Science and Technology. Medical Sciences. 2008;28:159-62.
- Yousuf S, Atif F, Kesharwani V, Agrawal SK. Neuroprotective effects of Tacrolimus (FK-506) and Cyclosporin (CsA) in oxidative injury. Brain and Behavior: A Cognitive Neuroscience Perspective. 2011;1:87-94.
- Ahmad M, Zakaria A, Almutairi KM. Effectiveness of minocycline and FK506 alone and in combination on enhanced behavioral and biochemical recovery from spinal cord injury in rats. Pharmacol Biochem Behav. 2016;145:45-54.
- Cai J, Sun Y, Yin Z, Wang D, Shi K, Fu Y. Analysis of FK506-mediated functional recovery and neuroprotection in a rat model of spinal cord injury indicates that EGF is modulated in astrocytes. Exp Thera Med. 2018;16:501-10.
- Eun BL, Liu XH, Barks JD. Pentoxifylline attenuates hypoxic–ischemic brain injury in immature rats. Paediatr Res. 2000;47:73–8.
- Creager MA. Medical management of peripheral arterial disease. Cardiol Rev. 2001;9:238–45.
- Windmeier C, Gressner AM. Pharmacological aspects of pentoxifylline with emphasis on its inhibitory actions on hepatic fibrogenesis. Gen Pharmacol. 1997;29:18196.
- Savas S, Delibas N, Savas C, Sutcu R, Cindas A. Pentoxifylline reduces biochemical markers of ischemia–reperfusion induced spinal cord injury in rabbits. Spinal Cord. 2002;40:224–9.
- Banfi C, Sironi L, De Simoni G. Pentoxifylline prevents spontaneous brain ischemia in stroke-prone rats. J Pharmacol Exp Ther. 2004;310:890–5.
- Nadkarni, Vinay; Fraser, Justin; Tice, Lisa; Barone, Carol; Corddry, David. Pentoxifylline effect on functional, biochemical and histological ratings after experimental spinal cord impact. Critical Care Med. 1999;27:p 51A.
- Zhu D, Xia B, Bi Q, Zhang S, Qiu B, Zhao C. Functional protection of pentoxifylline against spinal cord ischemia/reperfusion injury in rabbits: necrosis and apoptosis effects. Chinese Med J. 2008;121:2444-9.
- Nystrom B, Berglund JE. Spinal cord restitution following compression injuries in rats. Acta Neurologica Scandinavica. 1998;78:467-72.
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1-21.
- Wells JE, Hurlbert RJ, Fehlings MG, Yong VW. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 2003:1628-37.
- Lynskey JV, Belanger A, Jung R. Activity-dependent plasticity in spinal cord injury. J Rehabil Res Dev. 2008;45:229-40.

