GHR106: Novel Antibody-Based GnRH Antagonist Insights
GHR106, the First in Class Antibody-based GnRH Antagonist for Broad Clinical Applications
Gregory Lee1, Bixia Ge1, Samuel Mao1, Andrew Lee1
- UBC Center for Reproductive Health, University of British Columbia, Vancouver, Canada
OPEN ACCESS
PUBLISHED: 30 April 2025
CITATION: Lee, G., Ge, B., et al., 2025. GHR106, the First in Class Antibody-based GnRH Antagonist for Broad Clinical Applications. Medical Research Archives, [online] 13(4). https://doi.org/10.18103/mra.v13i4.6346
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI https://doi.org/10.18103/mra.v13i4.6346
ISSN 2375-1924
ABSTRACT
During the last two decades, our laboratory has identified and studied a GHR106 monoclonal antibody which was generated against N1-29 oligopeptide located in the extracellular domains of human GnRH receptor. GHR106 was shown to exhibit bioactivities as GnRH antagonist, similar to those of small molecules such as Cetrorelix and Elagolix, except that former has a much longer half-life (days vs. hours). Previous studies have indicated that GnRH receptor is localized mainly in anterior pituitary and placenta during pregnancy as well as other reproduction-related tissues in minor amount. It was also known that GnRH receptor is highly expressed among different cancer cells. By using GHR106 and other GnRH antagonists as the target probe, the biological functions of GnRH receptor were found to be tissue-dependent. When acting on the pituitary receptor, GHR106 can cause reversible suppressions of reproductive hormones, such as gonadotropins, E2 and progesterone, whereas HCG and E2, progesterone were suppressed upon targeting to placental GnRH receptor to cause pregnancy terminations. Therefore, we believe that GHR106 is a suitable long acting GnRH antagonist for control of fertility regulations and terminations of ectopic pregnancy and can be used to treat numerous related gynecological diseases. On the other hand, when targeting the same receptor on cancer cells, GHR106 will induce cellular apoptosis to almost all cancer cells. Therefore, GHR106 is also beneficial to immunotherapeutic applications of many human cancers, irrespective of their hormone dependence. Additional modifications of this unique antibody are likely in the future, including CAR (chimeric antigen receptor)-T cell constructs, bispecific antibody formulations and/or antibody drug conjugates. Based on our comprehensive studies, GHR106 can be developed into antibody drugs of multi-indications and could be more beneficial to the existing small molecular GnRH antagonists.
Keywords: GHR106, Antibody-based GnRH Antagonist, Fertility Control, Cancer Immunotherapy
I. Introduction
Gonadotropin releasing hormone (GnRH) is a decapeptide hormone from hypothalamus which was discovered initially to act on GnRH receptor located in the anterior pituitary. The main function of GnRH receptor is to stimulate the release of gonadotropins (LH and FSH) and subsequently the productions of E2 and progesterone in reproductive tissues. In view of the short half-life (minutes), the structure of native GnRH receptor was modified into analogs of longer half-life (hours), or even higher affinity. They were in general classified into agonist or antagonist, depending on their relative bioactivities to stimulate or inhibit the hormone releases upon specific interactions. Several small organic molecules were also created to serve as GnRH antagonists, comparably to those of decapeptide GnRH analogs. Besides anterior pituitary, GnRH receptor is also expressed in placental tissue to promote HCG productions as well as the subsequent hormone release of E2 and progesterone during early stages of pregnancy. The mechanisms of actions of placental GnRH and GnRH receptor were known decades ago. In addition, widespread expressions of GnRH receptor on many cancer cells were also well known. Based on these previous observations, it is apparent that GnRH analogs may play differential roles or biological actions upon interactions to the same GnRH receptor distributed in different tissues. Therefore, GnRH antagonists of different formulations may be beneficial to various disease treatments.
Soon after the structural elucidation of human GnRH receptor, numerous monoclonal antibodies have been generated against the extracellular domains of this membrane-bound receptor for binding and specificity studies. Since the last decade, our effort has been focused on the biological activities associated with the receptor interactions and their correlations with other types of known GnRH antagonists. Through years of efforts, GHR106 monoclonal antibody was among the one selected for further investigations in terms of its biological activities and potential clinical applications. In this review, GnRH antagonistic activities of GHR106 are described and compared with known ones for future broad clinical applications.
II. GHR106 as Antibody-based GnRH Antagonist
II-1. Binding specificity studies.
Upon generations of monoclonal antibodies by employing the established hybridoma technology, binding studies were performed initially with cancer cell lines including methods of immunohistochemical staining (IHC), Western blot, RT-PCR as well as indirect immunofluorescence. Almost all of the thirty cancer cell lines including those of the placental origin were positively stained (> 95%) by either of the above methods. This observation indicated the universal expressions of GnRH receptor among all cancer cells. Due to the lack of suitable immunological probes, GnRH antagonists of small molecules such as Cetrorelix or Elagolix are not used for comparative studies.
II-2. Tissue Distributions of GnRH Receptor as probed by GHR106.
Besides the high positive expressions of GnRH receptor among many permanent cancer cell lines, as detected by GHR106, the same methods were also performed with cancerous tissues or sections. It was generally observed that positive incidence of GnRH receptor expressions varied with cases as well as the methods (43-100%). However, they are not associated with any tissue origins and listed as follows: breast (52-60%), ovary (78-80%), endometrium (77-100%), prostate (86-100%), brain (43%) and pancreas (57-100%).
II-3. Comparative Induced Cellular Apoptosis Studies with Culturing Cancer Cells.
Comparative apoptosis studies were performed with GHR106 antibody (1-10 µg/ml) and a decapeptide antagonist, Antide (1µg/ml) in the presence of more than 10 different cell lines in culture, including those of ovary, prostate, breast and lung origins. Typical results of such analysis by the TUNEL assays are presented in many of previous studies for comparisons. GHR106 monoclonal antibodies in murine or humanized forms are included for such comparative studies, and found to have little difference in terms of the degree of apoptosis. The sensitivity of such an apoptosis assay can be as low as 1 µg/ml (about 10 nM for the antibody-based GHR106. The binding affinity or Kd of GHR106 to GnRH receptor was estimated to be around 2-4 nM through typical binding assays. In conclusion, the ability of GHR106 to induce apoptosis in vitro is comparable to that of the decapeptide analogs based on the molar ratios.
III. Functional Studies of GHR106 Interactions with GnRH Receptor expressed by different Normal and Cancer Cells for potential Clinical Applications
During the past decade, GHR106 as a long acting antibody-based GnRH antagonist was established to have comparable biological activities to those of antagonists of small molecular size. GHR106 is known to react with N1-29 extracellular domain peptide of GnRH receptor with a similar affinity (Kd 2-4 nM) to that of the whole receptor from well-coated cancer cells. Many biological and immunological studies were performed and highlighted in the following sections.
III-1. Nude mouse experiments were demonstrated to reveal the anti-tumor efficacy of GHR106.
Upon implantations of HepG2 liver cancer cells to nude mice, dose-dependent reduction of tumor volume were observed, upon two injections of GHR106 at dose of 1-2mg/Kg. No significant body weight changes during the 20-day study period were observed as compared to those of the negative control.
III-2. Proof of Concept rabbit experiments were performed to demonstrate the targeting actions of GHR106 on pituitary GnRH receptor.
Upon a single injection of GHR106 (1-3 mg/Kg) to rabbit, suppressions of gonadotropins (LH/FSH) together with related reproductive hormones such as estradiol (E2) in the female and testosterone in the male were observed over 1-2 weeks period.
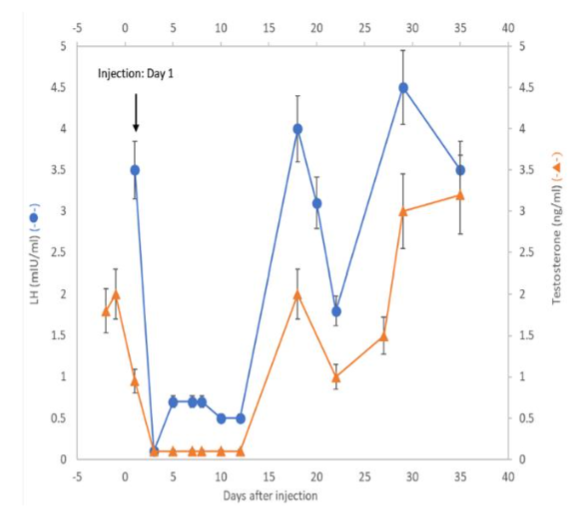
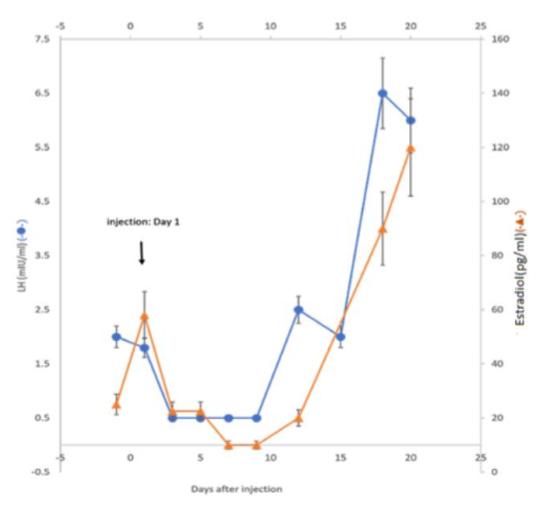
The results of these studies revealed that GHR106 can serve as antagonist similar to those of small size analogs. However, we believe that GHR106 with its long half-life may be beneficial to the treatments of certain gynecological and fertility related diseases, as compared to the current ones.
III-3. Cellular apoptosis was induced by GHR106 and other known GnRH antagonists (e.g. Antide), suggesting that similarity in gene regulations was observed.
By using semi-quantitative RT-PCR methods, as many as ten genes were selected for expression level changes upon the ligand bindings. GHR106 and a peptide analog, Antide, were used for such comparative studies. The results of such studies suggest that gene regulations change upon the binding of these two ligands are highly correlated (90-95%), indicating almost identical mechanisms of actions between GHR106 and Antide.
III-4. Our studies also suggest that GHR106 reacts to placental GnRH receptor, similar to those of other analogs to suppress the release of HCG and other related reproductive hormone, leading to the pregnancy loss.
Further studies are currently in progress. This observation could have important implications for terminations of ectopic pregnancy in future clinical applications of long acting GHR106.
III-5. Cancer Immunotherapy with Applications of GHR106-CAR-T Cell Construct.
CAR-T (Chimeric antigen receptor-transfected T Cell) technology has been evolved for new applications in cancer immunotherapy. Following immunizations, GHR106 in humanized forms are produced and ScFv (single chain variable fragments) formulated into GHR106 (ScFv)-CAR-T cell construct. The CAR-transfected T cells can be perfused back to the autologous individual to induce cytotoxic killings of cancer cells in vitro or in vivo which will achieve the therapeutic objectives by an in vitro model study (with C33A, the cell lysis is validated by the cytokine release assays of IL-2, IL-7 and INF-gama which could be dose- and incubation time-dependent).
IV. Final Conclusion
In this short review, our efforts to generate a new class of a long acting antibody-based GnRH antagonists are highlighted. Our extensive studies have revealed that GHR106 monoclonal antibody is bioequivalent to those of small decapeptide or organic compounds in terms of their respective biological activities in vitro and in vivo. Therefore, GHR106 can be developed into alternative GnRH antagonist for diversified clinical applications. Basically, the biological functions of GnRH receptor in response to its binding to GnRH ligands or analogs are highly dependent on the nature of tissues. If GnRH receptor is located at the anterior pituitary, upon interactions with GHR106 as GnRH antagonist, reversible suppressions of reproductive hormones can be observed for as long as one to two weeks under physiological doses (1-3 mg/Kg bodyweight). When acting on placental GnRH receptor, during early pregnancy, GHR106 as GnRH antagonist will cause suppressions of HCG which will lead to the eventual pregnancy loss due to the insufficient support of the downstream reproductive hormones. The tissue-specific targeting of GnRH receptor of GHR106 as GnRH antagonist can be used to treat numerous gynecological diseases due to improper modulations of GnRH receptors and/or fertility regulations. These include: premenstrual syndrome, precocious puberty, endometriosis, uterine fibroids and termination of ectopic pregnancy, etc.
On the other hand, universal expressions of GnRH receptor among most of cancer cells or tissues are known for decades. Most of these receptor expressions carried no apparent association with reproductive hormones or functions. Upon interactions with GnRH antagonist, such as GHR106, cellular apoptosis can be induced to almost all cancer cells. These observations make GHR106 a useful drug for numerous applications in cancer immunotherapy. Among different types of cancer, some of which are associated with reproductive hormones and may be suitable for targeting in cancer immunotherapy including breast cancer, gynecological cancers, and prostate cancer etc. In addition, the CDC and ADCC reactions of GHR106 may be beneficial to cancer therapeutics. Recently, advancement in cancer immunotherapy has enabled us to expand the utility/efficacy of GHR106 as anti-cancer drugs for therapeutic applications in the future. These include the formulations of GHR106-CAR-T cell construct, bispecific antibodies, as well as antibody drug conjugates.
Acknowledgements
This study was supported in parts by Vancouver Biotech Ltd. (Vancouver, Canada). The supports of IRAP and NSERC during 2010-2015 were acknowledged.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
References
- Schally AV, Arimura A, Kastin AJ, et al. Gonadotropin-releasing hormone: one polypeptide regulates secretion of luteinizing and follicle-stimulating hormones. Science. 1971;173(4001):1036-1038. doi: 10.1126/science.173.4001.1036.
- Nagy A, Schally AV. Targeting of cytotoxic luteinizing hormone-releasing hormone analogs to breast, ovarian, endometrial, and prostate cancers. Biology of Reproduction. 2005;73(5):851-859. doi: 10.1095/biolreprod.105.043489.
- Ezzati M, Carr BR. Elagolix, a novel, orally bioavailable GnRH antagonist under investigation for the treatment of endometriosis-related pain. Womens Health (Lond). 2015;11(1):19-28. doi: 10.2217/whe.14.68.
- Shirley M. Relugolix: a review in advanced prostate cancer. Target Oncol. 2023;18(2):295–302. doi: 10.1007/s11523-022-00944-4.
- Peng B, Abdellatif L, Klausen C, et al. The role of GNRH antagonists in a novel primary ectopic pregnancy cell model. Fertility & Sterility. 2017;108(3):Suppl e103-e104.
- Yeh J. New solutions for medical treatment of ectopic pregnancies: dawn of novel therapeutic agents and “Ectopic Cocktails”. Current Women`s Health Reviews. 2021;17(1)2-3. doi: 10.2174/157340481701201221093653.
- Emons G, Pahwa GS, Brack C, et al. Gonadotropin releasing hormone binding sites in human epithelial ovarian carcinomata. Eur J Cancer Clin Oncol. 1989;25(2):215-221. doi: 10.1016/0277-5379(89)90011-4.
- Casati L, Ciceri S, Maggi R, et al. Physiological and pharmacological overview of the gonadotropin releasing hormone. Biochemical Pharmacology. 2023; 212:115553. doi.org/10.1016/j.bcp.2023.115553.
- Lee G, Ge B. Growth inhibition of tumor cells in vitro by using monoclonal antibodies against gonadotropin-releasing hormone receptor. Cancer Immunol Immunother. 2010;59(7):1011-1019. doi: 10.1007/s00262-010-0823-3.
- Lee G, Cheung AP, Ge B, et al. CA215 and GnRH receptor as targets for cancer therapy. Cancer Immunol Immunother. 2012;61(10):1805-1817. doi: 10.1007/s00262-012-1230-8.
- Lee G. RP215 and GHR106 monoclonal antibodies and potential therapeutic applications. Open Journal of Immunology. 2023;13(3):61-85. doi: 10.4236/oji.2023.133005.
- Lee G. “Proof of Concept” rabbit experiments demonstrating GHR106 (hIgG4) as GnRH antagonist. Int J Zoo Animal Biol. 2022;5(2):1-9. doi: 10.23880/izab-16000371.
- Tang Y, Zhang H, Lee G. Similar gene regulation patterns for growth inhibition of cancer cells by RP215 or anti-antigen receptors. J Cancer Sci Ther. 2013;5(6):200-208. doi:10.4172/1948-5956.1000207.
- Lee G, Tang Y, Zhang H. Anti-GnRH receptor monoclonal antibodies as bioequivalent analogs of GnRH. In: Sillis ES, editor. Gonadotropin-Releasing Hormone (GnRH): Production, Structure & Functions. Los Angeles. Novel Biomedical. Chapter X: 159-174. https://www.kriso.ee/db/9781628084726.html.
- Lee G. Chimeric antigen receptor (CAR) with scFv genes of humanized anti-GnRH receptor for immunotherapy of gynecologic cancer. Nessa Journal of Gynecology (NJG), 2017;1: 1-14.
- Shim H. Bispecific antibodies and antibody–drug conjugates for cancer therapy: technological considerations. Biomolecules. 2020;10(3):360. doi: 10.3390/biom10030360.
- Hong Y, Nam S-M, Moon A. Antibody–drug conjugates and bispecific antibodies targeting cancers: applications of click chemistry. Archives of Pharmacal Research. 2023;46:131–148.

