Lifestylopathy: Ethical Pharmaceuticals and Halal Compliance
Lifestylopathy and Ethical Pharmaceuticals: A Halalopathy Perspective
Jawad Alzeer, PhD
- College of Medicine and Health Sciences, Palestine Polytechnic University, Hebron, Palestine.
OPEN ACCESS
PUBLISHED: 31 March 2025
CITATION: Alzeer, J., 2025. TITLEHERE. Medical Research Archives, [online] 13(3). https://doi.org/10.18103/mra.v13i3.6384
COPYRIGHT © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI https://doi.org/10.18103/mra.v13i3.6384
ISSN 2375-1924
Abstract
Pharmaceutical ethics is a critical pillar of healthcare, ensuring that medicines are developed, tested, and distributed with integrity, safety, and adherence to ethical principles. In response to the growing demand for ethically conscious healthcare solutions, Lifestylopathy emerges as a holistic framework that integrates ethical, natural, and sustainable approaches to medicine, emphasizing the alignment of pharmaceuticals with cultural, religious, and personal health values. Within this paradigm, Halalopathy addresses the specific ethical and religious concerns of the Muslim community, ensuring that pharmaceuticals comply with Halal (lawful ingredients) and Tayyib (clean and pure process) standards by excluding non-Halal ingredients, preventing cross-contamination, and enforcing transparency in sourcing and production. However, disintegrated Halal certification systems, ingredient verification complexities, and limited awareness among healthcare professionals and consumers present significant barriers to its widespread adoption. Despite these challenges, the growing demand for Halal, Kosher, Vegan, and other ethical pharmaceuticals presents vast opportunities for biotechnological advancements, the development of plant-based excipients, synthetic alternatives, and the establishment of ethical supply chain models. By integrating pharmaceutical ethics with the principles of Lifestylopathy, the industry can meet the needs of an increasingly diverse and ethically conscious consumer base, while maintaining scientific rigor, regulatory compliance, and market competitiveness.
Keywords
Ethical Pharmaceuticals, Lifestylopathy, Halalopathy, Halal-Tayyib, Vegan, Personalized Medicine
Introduction
The pharmaceutical industry is currently navigating a transformative period characterized by the convergence of ethical imperatives, religious values, and holistic healthcare philosophies. This shift is largely driven by scientific advancements that enhance pharmaceutical innovation and elevate consumer expectations for ethical, transparent, and culturally inclusive healthcare solutions. Modern consumers are increasingly prioritizing not just the efficacy of medical treatments but also their alignment with personal ethical beliefs, which may stem from religious observance, sustainability concerns, or individual health philosophies.
In this context, the concept of Lifestylopathy, along with its specialized subset Halalopathy, emerges as a significant paradigm that integrates ethical, cultural, and scientific principles into pharmaceutical development. This approach ensures that medicines are developed in a manner that respects diverse consumer needs while adhering to regulatory and scientific standards. Lifestylopathy posits that health is a dynamic interplay of physical, emotional, psychological, and spiritual well-being, thereby offering a holistic framework for ethical drug development. This is particularly pertinent in regions where cultural and religious factors heavily influence healthcare decisions, addressing longstanding ethical dilemmas in the pharmaceutical sector and fostering inclusive, patient-centered treatment models.
The increasing prevalence of various lifestyle choices, including Halal, Kosher, Vegan, Vegetarian, Gluten-free, Glucose-free, and Lactose-free diets, has significantly reshaped consumer demand within the pharmaceutical industry. Individuals adhering to these dietary and ethical principles are actively seeking products, including medications, that resonate with their specific needs and values. This shift towards plant-based, cruelty-free, and lifestyle-compliant medicines reflects a broader consumer trend favoring ethical and sustainable production practices across multiple sectors.
Consequently, pharmaceutical companies are responding by innovating new formulations, such as plant-based excipients and synthetic alternatives, while prioritizing production methods that minimize or eliminate animal products and animal testing.
Within this expansive framework, Halalopathy plays a crucial role in addressing the ethical and religious healthcare needs of the Muslim community. This approach ensures that pharmaceuticals comply with the principles of Halal and Tayyib, thereby aligning medical treatments with Islamic values. The growing global Muslim population is driving substantial demand for Halal-certified pharmaceuticals, indicating a market poised for significant expansion in the coming years.
To address the growing intersection of ethical, religious, and scientific considerations in modern healthcare, this manuscript explores the concept of Lifestylopathy as a holistic framework that integrates personalized medicine with ethical pharmaceutical practices. By emphasizing Halalopathy as a specialized subset, we aim to highlight its role in ensuring pharmaceutical compliance with Halal-Tayyib principles while also accommodating broader lifestyle-based preferences such as Kosher, Vegan, and other ethical standards. This study examines the evolving landscape of consumer-driven pharmaceutical demands, the challenges of certification and regulatory harmonization, and the opportunities for innovation in biotechnological advancements, plant-based excipients, and synthetic alternatives. Through this comprehensive analysis, we seek to establish a foundation for ethically aligned pharmaceutical solutions that accommodate to diverse global populations while maintaining scientific rigor and regulatory compliance.
Lifestylopathy: A Holistic Approach to Pharmaceutical Ethics
Lifestylopathy offers a comprehensive framework for integrating ethical considerations into pharmaceutical development, ensuring that medicines are not only effective but also aligned with the values and beliefs of diverse populations. This holistic approach acknowledges that healthcare is deeply personal, shaped by cultural, religious, and ethical principles that vary across individuals and communities. By incorporating multiple perspectives, including Halal, Kosher, Vegan, Vegetarian, Gluten-free, Glucose-free, and Lactose-free preferences, Lifestylopathy creates an inclusive foundation for ethical and sustainable pharmaceutical innovation. It emphasizes balance and stability in both physical and non-physical aspects of health, recognizing that well-being is a dynamic interplay of mental, metabolic, and energetic processes.
A key element of Lifestylopathy is the careful balance between disorder (entropy) and order (potential energy), ensuring homeostasis and optimal biological function. By understanding and respecting the body’s unique metabolic and physiological needs, this approach allows for the development of pharmaceuticals that align with different lifestyle principles, such as avoiding animal-derived ingredients for Vegans, adhering to specific processing methods for Kosher and Halal consumers, or accommodating clean-label, non-GMO, and organic preferences. This balance is essential not only for enhancing the body’s resilience against disease but also for creating healthcare solutions that are ethically sound, environmentally sustainable, and culturally appropriate.
By embracing the principles of personalized medicine, Lifestylopathy adapts interventions to individuals’ values and specific health needs. It integrates scientific consistency with ethical integrity, ensuring that pharmaceuticals align with faith-based dietary restrictions, environmental sustainability concerns, and broader wellness philosophies. This adaptable framework paves the way for a more compassionate, consumer-conscious pharmaceutical industry, where healthcare is no longer a one-size-fits-all model but a tailored, holistic approach that respects diversity in all its forms. Through Lifestylopathy, the future of medicine becomes not only more effective but also more inclusive, bridging the gap between science, ethics, and lifestyle choices.
Halalopathy: Addressing Ethical and Religious Compliance
Halalopathy, as a specialized subset of Lifestylopathy, is dedicated to ensuring that pharmaceuticals adhere to the ethical and religious principles of Halal (lawful ingredients) and Tayyib (clean and pure process) as outlined in Islamic teachings. This approach plays a crucial role in bridging the gap between modern medicine and faith-based healthcare, ensuring that Muslim consumers have access to treatments that align with their beliefs. One of its key elements is rigorous ingredient sourcing and verification, which guarantees that all pharmaceutical components comply with Halal standards. This requires careful analysis to exclude any non-Halal substances, such as alcohol or porcine-derived ingredients, while also ensuring ethical sourcing and transparency throughout the supply chain. By integrating these principles, Halalopathy promotes confidence and trust in pharmaceuticals, fostering an industry that respects religious and ethical considerations.
Beyond ingredient selection, Halalopathy upholds strict adherence to Islamic guidelines throughout the manufacturing process. This includes preventing cross-contamination with non-Halal substances, maintaining dedicated production lines where necessary, and ensuring that all equipment, facilities, storage conditions, lubricants, cleaning reagents, and primary packaging materials comply with Halal standards throughout the manufacturing process. These thorough manufacturing protocols align with Tayyib principles, emphasizing not only religious permissibility but also the overall purity, safety, and quality of pharmaceutical products. In this way, Halalopathy extends beyond religious compliance, advocating for clean, ethical, and high-quality pharmaceutical production that benefits consumers on a broader scale.
Ethical supply chain management is another cornerstone of Halalopathy, reinforcing transparency, traceability, and accountability at every stage of pharmaceutical development, from raw material sourcing to final distribution. This framework ensures that Halal-certified medicines meet international regulatory requirements while also addressing ethical concerns related to animal welfare, environmental sustainability, and humane labor practices. By integrating ethical sourcing, stringent manufacturing processes, and transparent supply chains, Halalopathy sets a precedent for responsible pharmaceutical development. As demand for Halal-compliant healthcare continues to rise globally, this approach not only serves the needs of Muslim consumers but also contributes to a more ethical, inclusive, and sustainable pharmaceutical industry.
The Power of Lifestyle-Compatible Medicine
Medicine has traditionally been regarded as a purely scientific discipline, centered on diagnosing diseases and formulating treatments through rationally designed, chemically prepared therapeutic drugs that target physiological symptoms. However, healthcare is more than just chemistry; it is deeply linked with culture, ethics, and personal identity. A growing realization in modern medicine is that patients are not just biological entities but individuals shaped by their beliefs, values, and lifestyles. This understanding has led to a paradigm shift from standardized treatments to lifestyle-compatible medicine, where therapies align with personal, cultural, and ethical preferences.
Research indicates that holistic approaches to healing, which consider the psychological, social, and spiritual dimensions of health, are increasingly recognized as essential for effective patient care. A person’s lifestyle choices, including diet, spiritual practices, and ethical considerations, are fundamental to their well-being. When medicine respects these aspects, it fosters trust, compliance, and psychological comfort, ultimately enhancing treatment efficacy. For instance, a Muslim patient seeking Halal-certified medicine, a Jewish patient needing Kosher pharmaceuticals, or a Vegan patient avoiding animal-derived ingredients all share a common need: treatment that aligns with their values. Ignoring these preferences creates hesitation, reduces adherence, and, in some cases, forces patients into ethical dilemmas, which can negatively impact recovery. Holistic healing is not just about the drug itself; it is about the entire therapeutic experience. When patients feel that their beliefs and values are respected, their mental and emotional state improves, contributing to better therapeutic responses and long-term health outcomes.
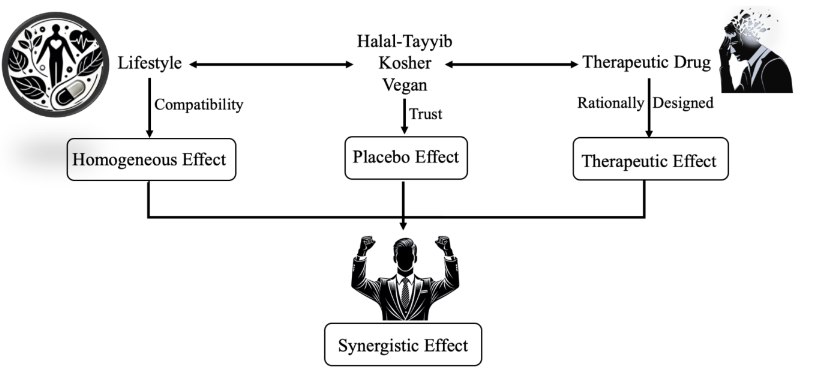
In this evolving context, Halalopathy emerges as a revolutionary approach to personalized medicine, embodying the essence of lifestyle-compatible healthcare. Unlike conventional models that primarily focus on disease management, Halalopathy integrates cultural and spiritual elements into the healing process, creating a patient-centered approach that promotes holistic recovery. It recognizes that patients are more than their symptoms; they are complex beings whose well-being depends on the synergy between their body, mind, and values. Treatment is not just about prescribing the most effective drug; it involves establishing favorable circumstances that encourage active patient participation in the healing process, supported by a healthcare system that aligns with their personal beliefs.
The trust-building process in Halalopathy plays a crucial role in maximizing treatment efficacy, as it acknowledges the deep psychological and emotional connection between belief, medicine, and healing. When a patient trusts their physician, believes in their treatment, and knows that their medication aligns with their ethical and religious framework, the placebo effect and therapeutic effect work in synergy, enhancing overall health outcomes. This concept of compatibility ensures that medicine does not disrupt the patient’s ethical or lifestyle balance, reducing stress, anxiety, and physiological resistance to treatment. By fostering an environment of trust, compliance, and holistic integration, Halalopathy goes beyond symptom management, shifting the focus to comprehensive care that respects individuality. In doing so, it bridges the gap between science and faith, reinforcing the idea that medicine is most powerful when it heals both the body and the soul.
Scientific research strongly supports the role of belief and patient trust in enhancing treatment efficacy, particularly through the placebo effect. Studies in psychoneuroimmunology reveal that patients who perceive their treatment as aligned with their values and beliefs exhibit higher adherence rates, improved psychological well-being, and even measurable physiological improvements. Within Halalopathy, the integration of faith-conscious treatment approaches strengthens patient confidence, creating a positive therapeutic environment where psychological and pharmacological mechanisms work synergistically.
Challenges and Opportunities
The pharmaceutical industry is shifting toward personalized and ethically conscious healthcare, aligning with the principles of Lifestylopathy, which emphasizes health practices that respect individual beliefs, ethics, and cultural values. This movement goes beyond conventional medicine, addressing the growing demand for pharmaceuticals that align with religious and ethical dietary laws. As consumers become more informed and conscious of what they consume, not only in food but also in medicine, the industry faces both challenges and opportunities in developing compliant formulations. The demand for faith-based and ethical pharmaceuticals is rapidly increasing, driven by heightened awareness, technological advancements, and regulatory pressure for greater transparency.
In the context of Vegan and Kosher pharmaceuticals, key challenges arise from ingredient sourcing, certification complexity, and manufacturing transparency. Vegan consumers seek medications free from animal-derived excipients, such as gelatin in capsules and lactose in coatings, but alternatives remain costly and not widely available. Similarly, Kosher-certified medicines must avoid non-Kosher animal derivatives and meet strict production standards, but cross-contamination risks in multi-purpose pharmaceutical facilities complicate certification. Despite these hurdles, the Vegan pharmaceutical market is expanding, with biotech companies developing synthetic stabilizers, plant-based gelatin, and cruelty-free alternatives. The Kosher pharmaceutical sector is also seeing growth, particularly in North America and Israel, where demand is driven by an increasing preference for certified products and consumer trust in transparent labeling.
Halalopathy, a specialized branch of Lifestylopathy, faces significant challenges due to the absence of a globally standardized Halal pharmaceutical framework. One of the primary concerns in Halal pharmaceutical production is the verification of excipients and active ingredients, ensuring they are free from porcine-derived substances, alcohol-based solvents, and cross-contamination risks. Additionally, the widespread use of Fetal Bovine Serum (FBS) in cell culture media poses compliance issues in biotech-based treatments, including vaccines, insulin, and monoclonal antibodies. These challenges are further exacerbated by the lack of regulatory harmonization among Halal certification bodies, as different countries enforce varying standards and certification processes, making compliance complex and inconsistent for pharmaceutical manufacturers.
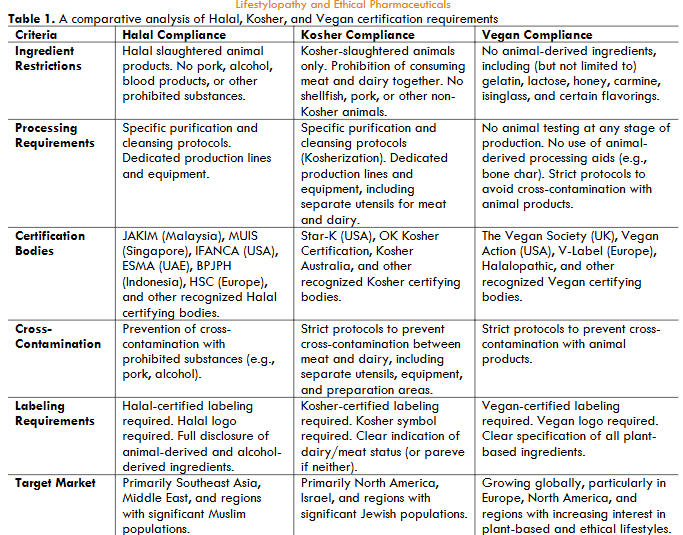
| Criteria | Halal Compliance | Kosher Compliance | Vegan Compliance |
|---|---|---|---|
| Ingredient Restrictions | Halal slaughtered animal products. No pork, alcohol, blood products, or other prohibited substances. | Kosher-slaughtered animals only. Prohibition of consuming meat and dairy together. No shellfish, pork, or other non-Kosher animals. | No animal-derived ingredients, including (but not limited to) gelatin, lactose, honey, carmine, isinglass, and certain flavorings. |
| Processing Requirements | Specific purification and cleansing protocols. Dedicated production lines and equipment. | Specific purification and cleansing protocols (Kosherization). Dedicated production lines and equipment, including separate utensils for meat and dairy. | No animal testing at any stage of production. No use of animal-derived processing aids (e.g., bone char). Strict protocols to avoid cross-contamination with animal products. |
| Certification Bodies | JAKIM (Malaysia), MUIS (Singapore), IFANCA (USA), ESMA (UAE), BPJPH (Indonesia), HSC (Europe), and other recognized Halal certifying bodies. | Star-K (USA), OK Kosher Certification, Kosher Australia, and other recognized Kosher certifying bodies. | The Vegan Society (UK), Vegan Action (USA), V-Label (Europe), Halalopathic, and other recognized Vegan certifying bodies. |
| Cross-Contamination Prevention | Prevention of cross-contamination with prohibited substances (e.g., pork, alcohol). | Strict protocols to prevent cross-contamination between meat and dairy, including separate utensils, equipment, and preparation areas. | Strict protocols to prevent cross-contamination with animal products. |
| Labeling Requirements | Halal-certified labeling required. Halal logo required. Full disclosure of animal-derived and alcohol-derived ingredients. | Kosher-certified labeling required. Kosher symbol required. Clear indication of dairy/meat status (or pareve if neither). | Vegan-certified labeling required. Vegan logo required. Clear specification of all plant-based ingredients. |
| Target Market | Primarily Southeast Asia, Middle East, and regions with significant Muslim populations. | Primarily North America, Israel, and regions with significant Jewish populations. | Growing globally, particularly in Europe, North America, and regions with increasing interest in plant-based and ethical lifestyles. |
Addressing this disintegration, the Standards and Metrology Institute for Islamic Countries (SMIIC), operating under the Organization of Islamic Cooperation (OIC), is leading efforts to establish a globally unified Halal certification system, including standards for pharmaceuticals. By creating a single, internationally recognized regulatory framework, SMIIC aims to facilitate cross-border trade, simplify compliance for manufacturers, and enhance consumer trust in Halal-certified medicines. A harmonized global Halal pharmaceutical certification system, modeled after the widely accepted Kosher certification standards, would streamline regulatory requirements, reduce market entry barriers, and ensure consistency in Halal compliance worldwide, benefiting both industry stakeholders and consumers seeking reliable, ethically produced medicines.
Despite the challenges, Halal-certified pharmaceuticals represent a significant market opportunity, particularly in Southeast Asia, the Middle East, and Muslim-majority regions, where demand for ethically compliant medicine is on the rise. Interestingly, Halal and Vegan pharmaceutical standards share common principles, such as the avoidance of animal-derived excipients and contamination-free processing, making Vegan-certified medicines naturally compatible with Halal requirements, provided they are free from alcohol. The development of the Halal Positive List, a pre-approved database of Halal-compliant pharmaceutical ingredients, is expected to streamline certification, reduce regulatory complexity, and accelerate market entry for compliant products. Additionally, advancements in AI-driven monitoring and blockchain traceability are enhancing supply chain transparency, ensuring medicines remain free from contamination and fully traceable from source to consumer. As biotechnology evolves, the rise of plant-based biosimilars, synthetic excipients, and alternative stabilizers will allow pharmaceutical companies to meet both regulatory and consumer demands. Companies that invest in Halal pharmaceutical innovation, ethical compliance, and international collaboration will lead the next generation of ethical medicine, reshaping the way faith-based and lifestyle-conscious consumers access healthcare worldwide.
Future Directions and Recommendations
To further the development of ethical pharmaceuticals through the frameworks of Lifestylopathy and Halalopathy, focused attention is needed across several key areas. First, research and development efforts must be significantly increased. This includes substantial investment in identifying alternative ingredients and pioneering production methods that adhere to Halal principles without compromising therapeutic efficacy. Specifically, the exploration of plant-based alternatives to animal-derived ingredients and the development of novel synthesis methods for active pharmaceutical ingredients (APIs) that meet Halal standards are crucial.
Second, education and awareness initiatives are essential for driving acceptance and demand for ethically compliant pharmaceuticals. Targeted training programs for healthcare providers and pharmacists, coupled with public awareness campaigns, can effectively disseminate the principles of Lifestylopathy. Integrating these concepts into medical and pharmaceutical curricula will further solidify their importance and ensure future practitioners are well-versed in these ethical considerations. Collaboration among regulatory bodies, religious authorities, and pharmaceutical companies is necessary to establish comprehensive and globally recognized standards for Halal pharmaceuticals. This includes creating clear guidelines for certification processes and establishing mechanisms for the international recognition of Halal certifications, facilitating smoother trade and access to these vital medications.
Finally, continuous investment in technological innovation is vital. Solutions like blockchain technology for enhanced supply chain transparency and artificial intelligence for sophisticated ingredient analysis can significantly improve the verification of Halal compliance throughout the pharmaceutical production lifecycle. These technological advancements can also contribute to more efficient drug discovery and development, aligning with the core tenets of Lifestylopathy and Halalopathy. International collaboration, fostering partnerships and knowledge sharing, is equally essential. Such collaboration between companies, research institutions, and regulatory bodies across nations will accelerate the development and global adoption of Halal-compliant pharmaceuticals by facilitating the sharing of best practices and innovative solutions.
Conclusion
The future of pharmaceuticals is shifting toward a more inclusive, ethical, and personalized approach, where faith-based, cultural, and ethical considerations are no longer secondary but integral to healthcare innovation. Lifestylopathy and Halalopathy provide a structured framework that harmonizes scientific advancements with ethical integrity, ensuring that medications align with diverse consumer needs without compromising efficacy or safety. While regulatory disintegration, ingredient verification, and standardization challenges persist, technological innovations such as AI-driven compliance monitoring and blockchain-based traceability are paving the way for transparent and trustworthy pharmaceutical supply chains. The rising demand for Halal, Kosher, and Vegan medicines, particularly in Southeast Asia, the Middle East, and North America, signals a significant market opportunity for companies that prioritize ethical sourcing, regulatory harmonization, and consumer education. By embracing ethical pharmaceutical development, the industry can enhance consumer trust, expand global access, and redefine medicine as not only a tool for healing the body but also a system that respects cultural and ethical diversity, fostering holistic well-being worldwide.
Conflicts of interests: None
Funding statement: The authors have no funding to report.
References
- Saxena K, Balani S, Srivastava P. The relationship among corporate social responsibility, sustainability and organizational performance in pharmaceutical sector: a literature review. Int J Pharm Healthc Mark. 2021;15(4):572-597. https://doi.org/10.1108/ijphm-12-2020-0104
- Chomać-Pierzecka E. Pharmaceutical companies in the light of the idea of sustainable development—an analysis of selected aspects of sustainable management. Sustainability. 2023;15(11):8889. https://doi.org/10.3390/su15118889
- Alzeer J. Lifestylopathy as personalized medicine: a holistic approach to health. Med Res Arch. 2025;13(1). https://doi.org/10.18103/mra.v13i1.6209
- Alzeer J. Halalopathic: a new concept in medicine. J Mol Genet Med. 2018;12(2). https://doi.org/10.4172/1747-0862.1000353
- Alzeer J. Halalopathy: a science of trust in medicine. J Integr Med. 2019;17(3):150-154. https://doi.org/10.1016/j.joim.2019.03.005
- Moermond C, Puhlmann N, Brown A, Owen S, Ryan J, Snape J, Kümmerer K. Greener pharmaceuticals for more sustainable healthcare. Environ Sci Technol Lett. 2022;9(9):699-705. https://doi.org/10.1021/acs.estlett.2c00446
- Ngah A, Ramayah T, Ali M, Khan M. Halal transportation adoption among pharmaceuticals and cosmetics manufacturers. J Islam Mark. 2019;11(6):1619-1639. https://doi.org/10.1108/jima-10-2018-0193
- Alzeer J. Permissible medicine and rationalization of halal pharma. Halalsphere. 2021;1(1):43-52. https://doi.org/10.31436/hs.v1i1.18
- Lewis J, Lipworth W, Kerridge I. Ethics, evidence and economics in the pursuit of “personalized medicine”. J Pers Med. 2014;4(2):137-146. https://doi.org/10.3390/jpm4020137
- Osborne T. Power and persons: on ethical stylisation and person‐centred medicine. Sociol Health Illn. 1994;16(4):515-535. https://doi.org/10.1111/1467-9566.ep11347638
- Brothers K, Rothstein M. Ethical, legal and social implications of incorporating personalized medicine into healthcare. Pers Med. 2015;12(1):43-51. https://doi.org/10.2217/pme.14.65
- Alzeer J. Lifestylopathy: unlocking potential by embracing duality and homeostasis for improved healthcare. Int J Regen Med. 2023;1-6. https://doi.org/10.31487/j.rgm.2023.02.02
- Mohamad T. Individualizing medicinal therapy post heart stent implantation: tailoring for patient factors. Cureus. 2023. https://doi.org/10.7759/cureus.43977
- Alzeer J. Balancing potential energy and entropy: the foundations of lifestylopathy and homeostasis. J Public Health Emerg. 2024;8:8-8. https://doi.org/10.21037/jphe-23-140
- Alzeer J. Lifestylopathy: a holistic approach to healthcare. J Altern Med Ther. 2024;1(1):1-4. https://doi.org/10.59462/jamt.1.1.101
- Olorunsogo T, Balogun O, Ayo-Farai O, Ogundairo O, Maduka C, Okongwu C, Onwumere C. Bioinformatics and personalized medicine in the U.S.: a comprehensive review: scrutinizing the advancements in genomics and their potential to revolutionize healthcare delivery. World J Adv Res Rev. 2024;21(1):335-351. https://doi.org/10.30574/wjarr.2024.21.1.0016
- Curtis E, Jones R, Tipene‐Leach D, Walker C, Loring B, Paine S, Reid P. Why cultural safety rather than cultural competency is required to achieve health equity: a literature review and recommended definition. Int J Equity Health. 2019;18(1). https://doi.org/10.1186/s12939-019-1082-3
- Santaló J, Berdasco M. Ethical implications of epigenetics in the era of personalized medicine. Clin Epigenet. 2022;14(1). https://doi.org/10.1186/s13148-022-01263-1
- Evers K. Personalized medicine in psychiatry: ethical challenges and opportunities. Dialogues Clin Neurosci. 2009;11(4):427-434. https://doi.org/10.31887/dcns.2009.11.4/kevers
- Alzeer J, Rieder U, Abou Hadeed K. Rational and practical aspects of Halal and Tayyib in the context of food safety. Trends Food Sci Technol. 2018;71:264-267. https://doi.org/10.1016/j.tifs.2017.10.020
- Alzeer J. Halalopathy: improving potential energy and minimising entropy offer an integrative approach for more effective treatment. MCMS. 2022. https://doi.org/10.55162/mcms.02.027
- Alzeer J. Holistic approach to personalised medicine: a focus on halalopathy. Halalsphere. 2024;4(2):22-28. https://doi.org/10.31436/hs.v4i2.97
- Yang C, Smith T, Knowlton A. Cancer patient perspectives on the meaning of healing and the clinician as a healer. Am J Hosp Palliat Med. 2023;41(6):658-663. https://doi.org/10.1177/10499091231191697
- Alzeer J, Benmerabet H. The development of human personality: a comprehensive overview. Psychol Disord Res. 2023;1-8. https://doi.org/10.31487/j.pdr.2023.01.01
- Dahlgren S, Reyna-Martínez M, Méte M, Dutton M. Healing narratives from the holistic healing arts retreat. Traumatology Int J. 2020;26(1):40-51. https://doi.org/10.1037/trm0000212
- Gannotta R, Malik S, Chan A, Urgun K, Hsu F, Vadera S. Integrative medicine as a vital component of patient care. Cureus. 2018. https://doi.org/10.7759/cureus.3098
- Namisango E, Luyirika E, Matovu L, Berger A. The meaning of healing to adult patients with advanced cancer. Int J Environ Res Public Health. 2023;20(2):1474. https://doi.org/10.3390/ijerph20021474
- Drury C, Hunter J. The hole in holistic patient care. Open J Nurs. 2016;6(9):776-792. https://doi.org/10.4236/ojn.2016.69078
- Lee A, Wong Y, Neo X. Personality and psychoneuroimmunology: patient perspectives on mind-body health. JPPR. 2023;1(3):34-40. https://doi.org/10.61838/kman.jppr.1.3.6
- Alzeer J, Benmerabet H. Potentiality to actuality: quantum physics inspires creative innovation. J Pijar Mipa. 2025;20(1):1-6. https://doi.org/10.29303/jpm.v20i1.8176
- Jaber D, Hasan H, Alkaderi A, Alkilani A, El-Sharif A. Assessment of the knowledge, attitude, and perception of healthcare providers regarding halal pharmaceuticals. Open Public Health J. 2024;17(1). https://doi.org/10.2174/0118749445296459240
- Naimat N. Challenges and opportunities in the halal pharmaceutical industry in Malaysia. Inf Manag Bus Rev. 2024;15(4(I)):73-78. https://doi.org/10.22610/imbr.v15i4(i).3565
- Nekha A. Halal pharmaceutical development in Indonesia. Islam Econ Methodol. 2024;2(2). https://doi.org/10.58968/iem.v2i2.353
- Ngah A, Ramayah T, Ali M, Khan M. Halal transportation adoption among pharmaceuticals and cosmetics manufacturers. J Islam Mark. 2019;11(6):1619-1639. https://doi.org/10.1108/jima-10-2018-0193
- Alzeer J, Abou Hadeed K. Halal certification of food, nutraceuticals, and pharmaceuticals in the Arab world. In: Laher I, ed. Handbook of Healthcare in the Arab World. Cham: Springer; 2020. https://doi.org/10.1007/978-3-319-74365-3_36-1
- Hasnan N. Halal supply chain: challenges of halal certification in Japan. 2023. https://doi.org/10.15405/epsbs.2023.11.02.60
- Alzeer J, Abou Hadeed K. Ethanol and its halal status in food industries. Trends Food Sci Technol. 2016;58:14-20. https://doi.org/10.1016/j.tifs.2016.10.018
- Alzeer J, Rieder U, Abou Hadeed K. Good agricultural practices and its compatibility with halal standard. Trends Food Sci Technol. 2020;102:237-241. https://doi.org/10.1016/j.tifs.2020.02.025
- Alzeer J, Abou Hadeed K, Tufail F. The halal positive list: streamlining the path to certification. Halalsphere. 2025;5(1):1-7. https://doi.org/10.31436/hs.v5i1.109
- Kasri R, Ahsan A, Widiatmoko D, Hati S. Intention to consume halal pharmaceutical products: evidence from Indonesia. J Islam Mark. 2021;14(3):735-756. https://doi.org/10.1108/jima-06-2021-0192
- Silalahi S, Fachrurazi F, Fahham A. Factors affecting intention to adopt halal practices: case study of Indonesian small and medium enterprises. J Islam Mark. 2021;13(6):1244-1263. https://doi.org/10.1108/jima-05-2020-0152
- Al-Kwifi O, Farha A, Ahmed Z. Dynamics of Muslim consumers’ behavior toward halal products. Int J Emerg Mark. 2019;14(4):689-708. https://doi.org/10.1108/ijoem-11-2017-0486
- Handayani D, Masudin I, Haris A, Restuputri D. Ensuring the halal integrity of the food supply chain through halal suppliers: a bibliometric review. J Islam Mark. 2021;13(7):1457-1478. https://doi.org/10.1108/jima-10-2020-0329

