Long-Acting Injectable Aripiprazole in High-Risk Pregnancy
Long-Acting Injectable Aripiprazole in High-Risk Pregnancy: A Case Report on Relapse Prevention, Obstetric Outcomes, and Infant Development
Dr. Parinda Parikh1, Kanuj Soofi1, Himani J Suthar1, Arnesh Shukla1, Parthiv Pansuriya1, Shaurya Kumar Singh1, Zoe Geller1, Sahiya Maneppallil1, Mahya Budhwarapu1, Arush Kaushik Chandra1, Anika Shrivastava1, Dr. Mina Oza1
- Department of Psychiatry, Well Cornell Medical College, New York, USA
OPEN ACCESS
PUBLISHED: 31 August 2025
CITATION: PARIKH, Dr. Parinda et al. Long-Acting Injectable Aripiprazole in High-Risk Pregnancy: A Case Report on Relapse Prevention, Obstetric Outcomes, and Infant Development. Medical Research Archives, [S.l.], v. 13, n. 8, sep. 2025. Available at: <https://esmed.org/MRA/mra/article/view/6905>.
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ISSN 2375-1924
ABSTRACT
Pregnancy in women with bipolar disorder high risk, with significant relapse rates. This case report aims to explore the utilization of long-acting injectable aripiprazole (LAI) in a pregnant woman, to enhance treatment adherence and maintain a stable mood throughout the perinatal period. The patient experienced a weight gain of 45 lbs within the initial month period and subsequently developed gestational diabetes. The patient delivered a healthy baby boy via cesarean section at 39 weeks, weighing 9 lbs and 7 oz, and exhibiting an APGAR score of 9. The patient exhibited no significant symptoms of paranoia along with no risk of rehospitalization. This case highlights the importance of considering LAIs in pregnant women with bipolar disorder to prevent relapse and ensure favorable obstetric outcomes.
Keywords: Long-acting injectable aripiprazole, bipolar disorder, pregnancy, relapse prevention, obstetric outcomes
Introduction
Pregnancy in women with bipolar disorder (BD) is considered high-risk due to various clinical and pharmacotherapeutic factors. The administration of pharmacological treatment during pregnancy requires a thorough evaluation of the exposure to psychotropic drugs and the risk of BD relapse. According to a recent systematic review published in the American Journal of Psychiatry, 37% of patients with a history of bipolar disorder relapsed during pregnancy.
Failure to address bipolar disorder during pregnancy can lead to adverse outcomes for both the mother and the fetus. This case report aims to explore the utilization of long-acting injectable aripiprazole (LAI) in a pregnant woman to enhance treatment adherence and maintain a stable mood throughout the perinatal period.
Case Report
A 28-year-old female with a history of manic, psychotic, or depressive episodes was referred to our clinic for management of her bipolar disorder. Her monthly Young Mania rating scale (YMRS) ranged from the range of 4-8. In addition to her primary treatment, the patient reported experiencing anxiety regarding her pharmacological needs. Regular monitoring by a multidisciplinary team, comprising her psychiatrist and obstetrician, revealed no major concerns about the growing fetus. Notably, the patient experienced a weight gain of 45 lbs within the initial month period and subsequently developed gestational diabetes. The patient delivered a healthy baby boy via cesarean section at 39 weeks, weighing 9 lbs and 7 oz, and exhibiting an APGAR score of 9. The patient exhibited no significant symptoms of paranoia along with no risk of rehospitalization.
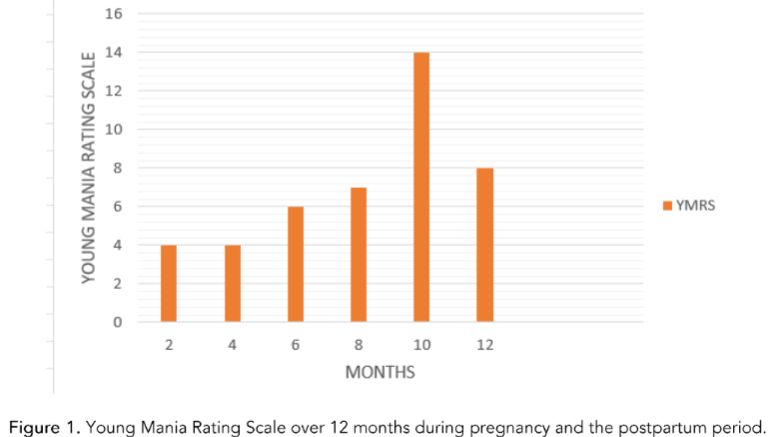
Discussion
Long-acting injectable (LAI) antipsychotics play a crucial role in enhancing medication adherence and reducing the recurrence of psychiatric symptoms. In contrast to oral antipsychotics, LAIs have demonstrated greater efficacy in preventing psychiatric hospitalization. Additional advantages of LAIs include heightened awareness among clinicians regarding medication adherence, reduced risk of overdose, and improved, standardized interaction between clinicians and patients. The majority of available data suggest that the adverse effects of LAIs are comparable to those of oral counterparts, except for long-acting injections, which necessitate a three-hour observation period following injection.
Conclusion
In the case of long-acting injectable antipsychotics (LAIs), there is a greater tendency for clinicians not to begin or discontinue LAI prescriptions during pregnancy. Given the prevalence of significant psychiatric conditions and their associated risks of decompensation during the perinatal period, healthcare providers must be at ease with the use of antipsychotic medications during pregnancy. While acknowledging the limited safety data on long-acting injectable (LAI) formulations during pregnancy, we recommend transcending apprehensions related to prescribing LAIs to pregnant women when substantial risks of psychiatric decompensation outweigh the absence of data.
References
- McNeil TJ, Kauffman RM. The role of lithium in pregnancy. Am J Psychiatry. 1984; 141: 1101-1109. doi:10.1176/ajp.141.8.1101.
- Veselska R, Kampman M, Munt-Oslen T, Pop VJ, Kushner SA, Bergvik R. Risk of Postpartum Relapse in Bipolar Disorder and Postpartum Psychosis: A Systematic Review and Meta-Analysis. Am J Psychiatry. 2016;173(2):117-127. doi:10.1176/appi.ajp.2015.1500164.
- Rosen MN, Berg M, Begley C. Bipolar disorder in pregnancy and childbirth: a systematic review of the literature. Arch Womens Ment Health. 2016;19(3):331-341. Published online 2016 Dec 10. doi:10.1007/s00737-016-0628-4.
- Reinstatler SA, Cozzolino K, Malekshahi T, Deligiannidis KM. Long-acting Injectable Antipsychotic Use During Pregnancy: A Brief Review and Concise Guide for Clinicians. J Clin Psychiatry. 2020;81(6):20r13059. Published 2020 Nov 24. doi:10.4088/JCP.20r13059.
- Caregiver discussion regarding long-acting injectables (LAIs): a treatment option for individuals living with bipolar disorder and schizophrenia. Otus Patient Education. Accessed June 16, 2025. Link.

