Neuroinflammation’s Impact on Cognitive Health in T2D
Neuroinflammation and Cognitive health in Type 2 Diabetes
Arbind Kumar Choudhary1
- Department of Physiology, All India Institute of Medical Science, Raebareli, Uttar Pradesh, India
OPEN ACCESS
PUBLISHED: 31 August 2025
CITATION: Choudhary, AK., 2025. Neuroinflammation and Cognitive health in Type 2 Diabetes. Medical Research Archives, [online] 13(8). https://doi.org/10.18103/mra.v13i8.6866
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.18103/mra.v13i8.6866
ISSN 2375-1924
ABSTRACT
This review explores the complex relationship between neuroinflammation and cognitive decline in individuals with Type 2 Diabetes (T2D). There is increasing awareness that cognitive impairment, which can range from mild difficulties to severe dementia, is a significant complication associated with T2D. Extensive research indicates that chronic neuroinflammation plays a critical role in this context, linking metabolic dysfunction with neurodegenerative changes in the brain. The discussion begins with the effects of chronic hyperglycemia, insulin resistance, and metabolic disruptions that contribute to systemic inflammation. This inflammation can be intense enough to breach the blood-brain barrier (BBB), leading to neuroinflammation. The activation of essential immune cells in the brain, such as microglia and astrocytes, is accompanied by a rise in pro-inflammatory cytokines like IL-1β, IL-6, and TNF-α. These inflammatory agents disrupt synaptic function, inhibit neurogenesis, and promote neuronal death, particularly in areas critical for memory and executive functions, such as the hippocampus and prefrontal cortex. As the investigation unfolds, valuable insights are drawn from both preclinical and clinical studies. Animal research highlights the adverse effects of metabolic stress and inflammation on neuronal health, while clinical data correlate elevated inflammatory markers with reduced cognitive performance among individuals with T2D. Neuroimaging studies further support these findings by visually demonstrating microglial activation in regions essential for cognitive function.
Keywords: Neuroinflammation, Type 2 Diabetes, Cognitive Decline, Insulin Resistance, Synaptic Dysfunction, Neurodegeneration
1. Introduction
In the realm of health, type 2 diabetes (T2D) has emerged as a significant challenge, not only affecting blood sugar levels but also leading to cognitive decline. This connection poses serious difficulties for those with the condition, profoundly impacting their mental health and daily living. Increasingly emerging evidence supports the fact that T2D is not only a metabolic but also a systemic disease with severe neurological consequences resulting in high likelihood of cognitive impairment. Research indicates that individuals with T2D are at a heightened risk of developing cognitive issues, ranging from mild cognitive impairment, to more form such as vascular dementia, and Alzheimer’s disease. The cognitive domain most affected include memory, (particularly episodic memory), executive functions, attention, and processing speed, demonstrating the disease’s profound impact on mental health.
T2D-related cognitive deterioration is basically a multifactorial pathogenic issue characterized by complicated relationships among metabolic disturbances, vascular damage, and neurodegenerative complication, and among these, chronic neuroinflammation has been identified as a critical and central contributing factor. Chronic inflammation, particularly driven from activated microglial cells and astrocyte in the brain, creates a harmful cycle that deteriorates cognitive abilities. Understanding the relationship between T2D-induced neuroinflammation and cognitive health is critical. When microglia become activated, they transition from their homeostatic surveillance role to a pro-inflammatory state, releasing a cascade of detrimental mediators, including pro-inflammatory cytokines like IL-1β, IL-6, and TNF-α, along with reactive oxygen species (ROS). This ongoing inflammatory state leads to synaptic dysfunction, impaired neurogenesis, and ultimately, the loss of neurons, culminating in a decline in cognitive function.
The purpose of this review is to summarize the existing knowledge regarding T2D-induced neuroinflammation and its presence in cognitive health and, additionally, examine the underlying mechanism of this issue and its possible therapeutic targets.
2. Neuroinflammation and Type 2 Diabetes
Neuroinflammation serves as a vital response when the brain’s immune system activates in reaction to various challenges, such as injuries, infections, or systemic illnesses. This intricate process prominently involves two types of cells; microglia and astrocytes. In individuals with T2D, neuroinflammation is a key factor that can contribute to cognitive decline, underscoring the importance of addressing this issue. Chronic metabolic conditions associated with T2D, including elevated blood sugar levels and reduced insulin sensitivity, create an inflammatory environment that influences not only peripheral tissues but also significantly affects the central nervous system (CNS). This systemic inflammation may grow severe to violate the integrity of the blood-brain barrier (BBB) permitting the passage of inflammatory messengers and toxic substances into the central nervous system (brain parenchyma) and trigger and maintain neuroinflammation. To better understand this, it is crucial to examine the role of hyperglycemia, where sustained high glucose levels lead to the formation of advanced glycation end products (AGEs). These compounds accumulate within the brain and interact with receptors on microglia and astrocytes, initiating inflammatory signaling pathways such as the activation of NF-κB, which increases the production of pro-inflammatory cytokines and chemokines. Addressing this inflammation is essential to protect neurons from potential damage.
Insulin resistance, a hallmark of T2D, also plays a significant role in brain health. Insulin is essential for maintaining neuron health, synaptic flexibility, and cognitive function. By improving insulin sensitivity, we can potentially enhance neuronal protection and reduce inflammation. Furthermore, the presence of free fatty acids (FFAs) in the bloodstream can cross the blood-brain barrier, signaling the necessity for targeted interventions to modulate these levels and lessen their pro-inflammatory effects. Oxidative stress represents another significant avenue that warrants attention. When stressed or dying cells release harmful molecules, such as HMGB1, they can activate microglia, leading to a cascade of inflammatory signals. Fostering cellular health and minimizing the production of reactive oxygen species (ROS) could counteract these damaging processes. It is also essential to consider the implications of dyslipidemia, where elevated FFAs and triglycerides might provoke inflammation in the CNS. Strategies to manage lipid profiles effectively can contribute to reducing neuroinflammation and its associated cognitive impacts. A healthy blood-brain barrier is vital for maintaining CNS integrity, so addressing its compromise; often seen in T2D is crucial. Ensuring this barrier remains intact can prevent harmful substances from entering the brain and triggering inflammatory responses. Additionally, the NLRP3 inflammasome is another important player in the inflammatory response. Reducing the accumulation of factors such as AGEs and ROS could potentially prevent the activation of this inflammasome and the release of detrimental interleukins like IL-1β and IL-18. The cognitive effects of neuroinflammation are well recognized, with studies indicating that T2D patients may experience a shift in microglial behavior from a protective to a more harmful pro-inflammatory state. By promoting anti-inflammatory responses, we can support synaptic plasticity and neurogenesis, thereby enhancing cognitive function. Astrocyte activity must also be considered. While reactive astrocytes can exacerbate neuronal damage and contribute to glial scars, promoting their proper functioning can aid recovery processes such as axon regrowth and contribute to neuronal health. Chronic inflammation is closely linked to cognitive decline, similar to mechanisms observed in Alzheimer’s disease. Therefore, addressing neuroinflammation can potentially mitigate these negative cognitive outcomes and enhance neurogenesis, particularly in the hippocampus, crucial for memory. Central to neuronal health are neurotrophic factors like brain-derived neurotrophic factor (BDNF), which play an essential role in neuronal development and resilience. By fostering an environment conducive to BDNF expression, we can enhance neuronal health and combat cognitive decline associated with T2D. Cognitive impairment poses a significant challenge for individuals with Type 2 Diabetes Mellitus (T2DM). By understanding and addressing the interplay of neuroinflammatory responses within the CNS, we can initiate positive changes that support cognitive health.
3. Cognitive Decline and Type 2 Diabetes
In exploring the intricate relationship between T2D and cognitive decline, we gain valuable insights into how this condition affects brain health. Understanding the diverse cognitive challenges faced by individuals with T2D can pave the way for more effective interventions and support. Research has shown that people with diabetes experience cognitive decline more rapidly than those without the condition, highlighting the need for targeted strategies to address these issues. Cognitive impairments in T2D can manifest in several areas, beginning with episodic memory, where individuals may struggle to recall specific experiences and events. This form of memory relies heavily on the hippocampus, a brain structure sensitive to metabolic disturbances, including high blood sugar levels and inflammation. The activated inflammatory processes induced by T2D may result in the maintenance of hippocampal function, which decreases as a result of low synaptic plasticity and damages neurons, which contributes to poor episodic memory. Recognizing these symptoms early can guide proactive measures to support memory function. Working memory, which involves temporarily holding and manipulating information, is another critical area affected by T2D. Insulin resistance and elevated blood sugar may impact the prefrontal cortex, an essential region for cognitive processes. Neuroinflammation of prefrontal cortex is capable of interfering with neural circuits that support working memory, which influences the efficacy of information processing. By focusing on improving insulin sensitivity and glycemic control, we can potentially enhance working memory and overall cognitive performance in T2D patients. Executive functions, critical for planning, problem-solving, and decision-making, often present additional challenges. Microvascular damage and neuroinflammation that affect the frontal lobes can hinder these skills. The executive control related to the neuronal structures of the frontal lobes are susceptible to structural and functional changes due to the persistence of the chronic inflammatory state in T2D. Interventions aimed at improving vascular health and reducing inflammation could help individuals navigate daily tasks and enhance their decision-making abilities. Moreover, cognitive flexibility; the capacity to adapt to new information and switch between tasks; can be improved through exercises that promote mental agility. By addressing inflammation and neural pathway health, we can empower individuals to think more effectively. Attention and processing speed are also vital for daily functioning. Neuroinflammation may impair the speed of neural processing and attentional capacity due to the influence on the integrity of white matter and neurotransmitter systems. Enhancing these cognitive functions through cognitive training and lifestyle modifications may alleviate some of the challenges T2D patients face, enabling them to multitask more efficiently and make quicker decisions, ultimately improving their independence and quality of life. Visuospatial abilities, which involve understanding spatial relationships and recognizing objects, can also benefit from targeted therapies. Engaging in activities that stimulate these skills may help T2D patients better navigate their environments and improve their familiarity with surroundings and faces. Recognizing the underlying mechanisms of cognitive decline in T2D, such as chronic hyperglycemia, glycemic variability, and neural dysfunction, allows healthcare providers to develop tailored interventions. By managing blood sugar levels effectively and addressing inflammation in the brain, we can potentially mitigate cognitive deterioration. The connection between T2D and cerebrovascular health is also crucial; improving microvascular function and reducing neuroinflammatory processes can significantly contribute to cognitive preservation. Understanding the role of neuroinflammation, and how it triggers microglial activity and the release of inflammatory molecules, provides a foundation for developing innovative therapies aimed at supporting cognitive health.
4. Pathophysiology
The complex relationship between Type 2 Diabetes (T2D), neuroinflammation, and cognitive decline is significant and influenced by metabolic dysregulation, systemic inflammation, and neurodegenerative processes. Table 1 outlines how hyperglycemia, insulin resistance, oxidative stress, and blood-brain barrier (BBB) disruption are key mechanisms contributing to neuroinflammation and subsequent cognitive impairment. Chronic hyperglycemia and insulin resistance lead to the excessive production of reactive oxygen species (ROS) and advanced glycation end products (AGEs). These substances activate intracellular pathways like NF-κB, which increase the release of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α. These inflammatory mediators can compromise the integrity of the blood-brain barrier, allowing harmful substances to enter the central nervous system, which activates microglia and astrocytes. When these glial cells are activated, they perpetuate neuroinflammation, resulting in synaptic dysfunction, hindered neurogenesis, and neuronal loss; especially in the hippocampus and prefrontal cortex, areas critical for memory and executive functions.
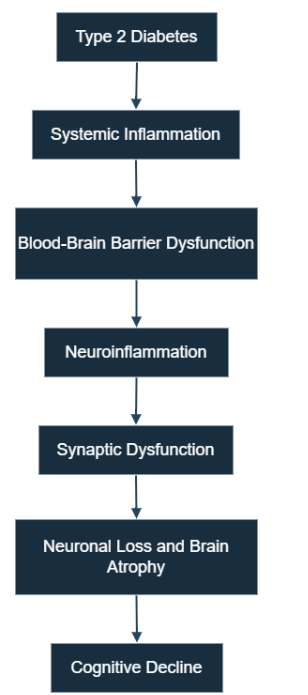
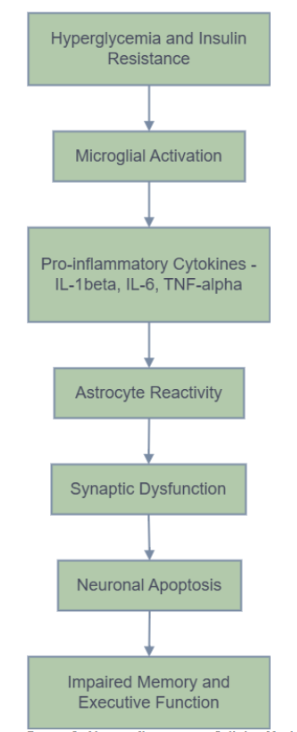
Figures 1 and 2 illustrate the systemic and cellular pathways through which T2D contributes to cognitive decline. Figure 3 provides a comprehensive overview that integrates these pathways. This figure visually summarizes how metabolic dysfunction in T2D initiates systemic inflammation, leading to blood-brain barrier disruption and subsequent neuroinflammation. It further elaborates on the activation of glial cells, including microglia and astrocytes, and the subsequent release of pro-inflammatory cytokines, culminating in synaptic damage, neuronal loss, and ultimately, cognitive impairment.
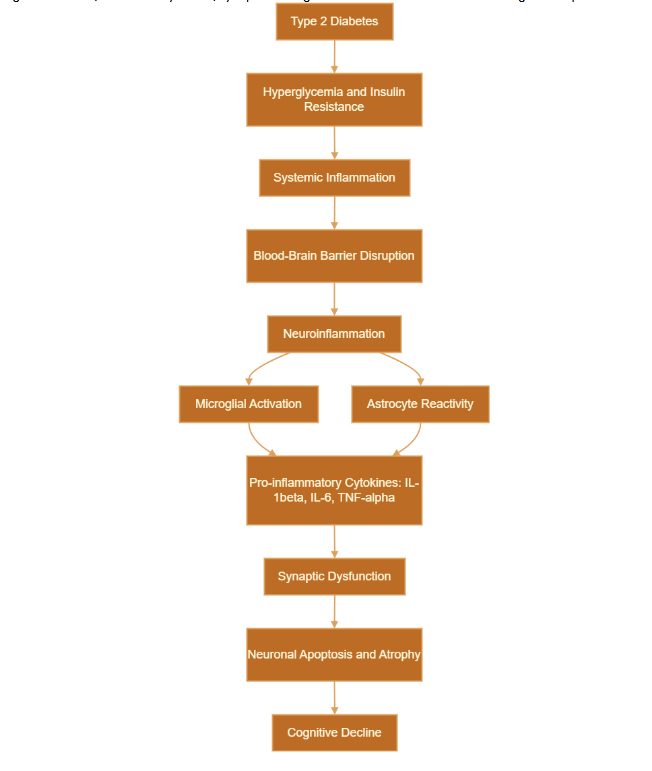
Impaired insulin signaling in the brain decreases essential synaptic proteins and neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), worsening deficits in learning and memory. The neuroinflammatory environment negatively impacts cognitive function and may also interact with the pathology of Alzheimer’s disease (AD), including the accumulation of amyloid-β and tau hyperphosphorylation, which increase the risk of dementia. Comprehensive preclinical and clinical investigations support this connection. Animal models, such as the DB/db mouse and Zucker Diabetic Fatty (ZDF) rat, show that hyperglycemic and insulin-resistant conditions drive microglia toward a pro-inflammatory M1 phenotype, adversely affecting hippocampal plasticity and neurogenesis. Human studies indicate that patients with T2D exhibit increased levels of systemic inflammatory markers, which are associated with poorer cognitive outcomes. Neuroimaging and cerebrospinal fluid analyses provide significant evidence of heightened microglial activation and elevated pro-inflammatory cytokines in regions essential for cognitive function. These findings emphasize neuroinflammation as a critical pathological mechanism contributing to cognitive decline in T2D and highlight the urgency of addressing this issue in order to protect cognitive health in diabetic populations.
5. Conclusion
The connection between neuroinflammation and cognitive decline in Type 2 Diabetes (T2D) is a crucial and emerging area of research with significant clinical implications. This review emphasizes how chronic metabolic disturbances in T2D—specifically hyperglycemia, insulin resistance, and systemic inflammation—can infiltrate the central nervous system, triggering prolonged neuroinflammatory responses. These responses, driven by activated microglia, astrocytes, and pro-inflammatory cytokines, disrupt synaptic integrity, impair neurogenesis, and promote neuronal loss, ultimately leading to progressive cognitive decline. Evidence from both preclinical and clinical studies supports the central role of neuroinflammation in cognitive dysfunction related to T2D, particularly affecting brain regions such as the hippocampus and prefrontal cortex. However, the complexity and variability of individual responses highlight the need for a personalized and multifaceted approach to understanding and managing this condition. In conclusion, it is essential to understand the pathophysiological mechanisms linking neuroinflammation with cognitive decline in T2D in order to develop effective prevention and treatment strategies. Addressing this intersection not only has the potential to preserve cognitive function in diabetic patients but also contributes to broader efforts aimed at preventing neurodegenerative diseases associated with metabolic disorders.
Acknowledgment
The authors had no acknowledgement
Conflict of interest
The authors had identified no conflicts of interest.
Table 1: Type 2 Diabetes Biological Mechanisms that Affect Cognitive Decline
| Process | Key Mediators | Pathophysiological role | Impacted Areas of the Brain | Result on Cognitive Function |
|---|---|---|---|---|
| Elevated Blood Sugar Levels and Diminished Insulin Response | Glucose, insulin, and advanced glycation end-products (AGEs) | Induces systemic inflammation, oxidative stress, and endothelial dysfunction | Comprehensive cerebral vasculature | Reduced attention and slower processing speed |
| Systemic Inflammation | C-reactive protein (CRP), Interleukin-6 (IL-6), Tumor Necrosis Factor-alpha (TNF-alpha) | Facilitates impairment of blood-brain barrier and allows peripheral cytokines to enter the central nervous system | Cerebral vasculature | Cognitive fatigue and diminished mental flexibility |
| Disruption of the Blood-Brain Barrier | Matrix metalloproteinase-9 (MMP-9), Intercellular Adhesion Molecule-1 (ICAM-1), Vascular Cell Adhesion Molecule-1 (VCAM-1) | Enables infiltration of inflammatory mediators into the brain | Hippocampus and cortex | Heightened susceptibility to neurodegenerative conditions |
| Activation of Microglia and Astrocytes | Interleukin-1 beta, Interleukin-6, Tumor Necrosis Factor-alpha | Triggers persistent neuroinflammation and causes synaptic impairment | Hippocampus and prefrontal cortex | Compromised memory and executive functioning |
| Synaptic Dysfunction and Neurotoxicity | Glutamate, reactive oxygen species, cytokines | Inhibits plasticity and neurotransmission, facilitating neuronal apoptosis | Hippocampus and frontal cortex | Deficiencies in learning and inadequate working memory |
| Loss of Neurons and Atrophy | Brain-derived neurotrophic factor, tau protein | Results in alterations to brain structure and diminished connectivity | Hippocampus and prefrontal cortex | Prolonged deterioration in cognitive function and increased potential for developing dementia. |
References
- Rachdaoui N. Insulin: the friend and the foe in the development of type 2 diabetes mellitus. International journal of molecular sciences. 2020;21(5):1770.
- Blázquez E, Hurtado-Carneiro V, LeBaut-Ayuso Y, et al. Significance of brain glucose hypometabolism, altered insulin signal transduction, and insulin resistance in several neurological diseases. Frontiers in Endocrinology. 2022;13:873301.
- Thomassen JQ, Tolstrup JS, Benn M, Frikke-Schmidt R. Type-2 diabetes and risk of dementia: observational and Mendelian randomisation studies in 1 million individuals. Epidemiology and psychiatric sciences. 2020;29:e118.
- Muraleedharan R, Dasgupta B. AMPK in the brain: its roles in glucose and neural metabolism. The FEBS Journal. 2022;289(8):2247-2262.
- Valenza S, Paciaroni L, Paolini S, et al. Mild cognitive impairment subtypes and type 2 diabetes in elderly subjects. Journal of Clinical Medicine. 2020;9(7):2055.
- Harvey PD. Domains of cognition and their assessment. Dialogues in clinical neuroscience. 2019;21(3):227-237.
- Teleanu DM, Niculescu A-G, Lungu II, et al. An overview of oxidative stress, neuroinflammation, and neurodegenerative diseases. International journal of molecular sciences. 2022;23(11):5938.
- Lénárt N, Brough D, Dénes Á. Inflammasomes link vascular disease with neuroinflammation and brain disorders. Journal of Cerebral Blood Flow & Metabolism. 2016;36(10):1668-1685.
- Gupta M, Pandey S, Rumman M, Singh B, Mahdi AA. Molecular mechanisms underlying hyperglycemia associated cognitive decline. IBRO Neuroscience Reports. 2023;14:57-63.
- Leng F, Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nature Reviews Neurology. 2021;17(3):157-172.
- Kacířová M, Zmeškalová A, Kořínková L, Železná B, Kuneš J, Maletínská L. Inflammation: major denominator of obesity, Type 2 diabetes and Alzheimer’s disease-like pathology? Clinical Science. 2020;134(5):547-570.
- Macedo RG. The Immune System and Inflammation in Type 2 Diabetes. The Diabetes Textbook: Clinical Principles, Patient Management and Public Health Issues. 2019:145-167.
- Li C, Wang Y, Xing Y, et al. Regulation of microglia phagocytosis and potential involvement of exercise. Frontiers in Cellular Neuroscience. 2022;16:953534.
- Rauf A, Badoni H, Abu-Izneid T, et al. Neuroinflammatory markers: key indicators in the pathology of neurodegenerative diseases. Molecules. 2022;27(10):3194.
- Rao JS, Kellom M, Kim H-W, Rapoport SI, Reese EA. Neuroinflammation and synaptic loss. Neurochemical research. 2012;37:903-910.
- Sun Y, Koyama Y, Shimada S. Inflammation from peripheral organs to the brain: how does systemic inflammation cause neuroinflammation? Frontiers in aging neuroscience. 2022;14:903455.
- Khalid M, Petroianu G, Adem A. Advanced glycation end products and diabetes mellitus: mechanisms and perspectives. Biomolecules. 2022;12(4):542.
- Lee J, Kim SW, Kim K-T. Region-specific characteristics of astrocytes and microglia: a possible involvement in aging and diseases. Cells. 2022;11(12):1902.
- Zhang T, Ma C, Zhang Z, Zhang H, Hu H. NF‐κB signaling in inflammation and cancer. MedComm. 2021;2(4):618-653.
- Sullivan M, Fernandez-Aranda F, Camacho-Barcia L, et al. Insulin and disorders of behavioural flexibility. Neuroscience & biobehavioral reviews. 2023;150:105169.
- Emma E, Amanda J. Dietary lipids from body to brain. Progress in lipid research. 2022;85:101144.
- de la Monte SM. Malignant brain aging: the formidable link between dysregulated signaling through mechanistic target of rapamycin pathways and alzheimer’s disease (type 3 diabetes). Journal of Alzheimer’s Disease. 2023;95(4):1301-1337.
- Lin M-m, Liu N, Qin Z-h, Wang Y. Mitochondrial-derived damage-associated molecular patterns amplify neuroinflammation in neurodegenerative diseases. Acta Pharmacologica Sinica. 2022;43(10):2439-2447.
- Andersson U, Ottestad W, Tracey KJ. Extracellular HMGB1: a therapeutic target in severe pulmonary inflammation including COVID-19? Molecular Medicine. 2020;26:1-13.
- Angelova PR. Sources and triggers of oxidative damage in neurodegeneration. Free Radical Biology and Medicine. 2021;173:52-63.
- Feingold KR, Grunfeld C. Diabetes and dyslipidemia. Diabetes And Cardiovascular Disease. Springer; 2023:425-472.
- Passarelli M, Machado UF. AGEs-induced and endoplasmic reticulum stress/inflammation-mediated regulation of GLUT4 expression and atherogenesis in diabetes mellitus. Cells. 2021;11(1):104.
- Biasizzo M, Kopitar-Jerala N. Interplay between NLRP3 inflammasome and autophagy. Frontiers in Immunology. 2020;11:591803.
- Shi X, Sun Q, Hou Y, et al. Recognition and maturation of IL-18 by caspase-4 noncanonical inflammasome. Nature. 2023;624(7991):442-450.
- Wang H, He Y, Sun Z, et al. Microglia in depression: an overview of microglia in the pathogenesis and treatment of depression. Journal of neuroinflammation. 2022;19(1):132.
- Anita NZ, Zebarth J, Chan B, et al. Inflammatory markers in type 2 diabetes with vs. without cognitive impairment; a systematic review and meta-analysis. Brain, Behavior, and Immunity. 2022;100:55-69.
- Dyer AH, McKenna L, Batten I, et al. Peripheral inflammation and cognitive performance in middle-aged adults with and without type 2 diabetes: results from the ENBIND study. Frontiers in Aging Neuroscience. 2020;12:605878.
- Edison P. Neuroinflammation, microglial activation, and glucose metabolism in neurodegenerative diseases. International Review of Neurobiology. 2020;154:325-344.
- Xu T, Liu J, Li X-r, et al. The mTOR/NF-κB pathway mediates neuroinflammation and synaptic plasticity in diabetic encephalopathy. Molecular neurobiology. 2021;58:3848-3862.
- Gaggini M, Ndreu R, Michelucci E, Rocchiccioli S, Vassalle C. Ceramides as mediators of oxidative stress and inflammation in cardiometabolic disease. International Journal of Molecular Sciences. 2022;23(5):2719.
- Boghdadi AG, Teo L, Bourne JA. The neuroprotective role of reactive astrocytes after central nervous system injury. Journal of Neurotrauma. 2020;37(5):681-691.
- Schönfeld P, Reiser G. How the brain fights fatty acids’ toxicity. Neurochemistry International. 2021;148:105050.
- Garbuz D, Zatsepina O, Evgen’ev M. Beta amyloid, tau protein, and neuroinflammation: an attempt to integrate different hypotheses of Alzheimer’s disease pathogenesis. Molecular Biology. 2021;55:670-682.
- Luo A, Xie Z, Wang Y, et al. Type 2 diabetes mellitus-associated cognitive dysfunction: advances in potential mechanisms and therapies. Neuroscience & Biobehavioral Reviews. 2022;137:104642.
- Soung AL, Davé VA, Garber C, Tycksen ED, Vollmer LL, Klein RS. IL-1 reprogramming of adult neural stem cells limits neurocognitive recovery after viral encephalitis by maintaining a proinflammatory state. Brain, behavior, and immunity. 2022;99:383-396.
- Wu A, Zhang J. Neuroinflammation, memory, and depression: new approaches to hippocampal neurogenesis. Journal of Neuroinflammation. 2023;20(1):283.
- Brown DT, Vickers JC, Stuart KE, Cechova K, Ward DD. The BDNF Val66Met polymorphism modulates resilience of neurological functioning to brain ageing and dementia: a narrative review. Brain Sciences. 2020;10(4):195.
- Colucci-D’Amato L, Speranza L, Volpicelli F. Neurotrophic factor BDNF, physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. International journal of molecular sciences. 2020;21(20):7777.
- Spinelli M, Fusco S, Grassi C. Brain insulin resistance impairs hippocampal plasticity. Vitamins and Hormones. 2020;114:281-306.
- Di Domenico F, Lanzillotta C. The disturbance of protein synthesis/degradation homeostasis is a common trait of age-related neurodegenerative disorders. Advances in Protein Chemistry and Structural Biology. 2022;132:49-87.
- Fan CL, Sokolowski HM, Rosenbaum RS, Levine B. What about “space” is important for episodic memory? Wiley Interdisciplinary Reviews: Cognitive Science. 2023;14(3):e1645.
- Davidson TL, Stevenson RJ. Vulnerability of the Hippocampus to Insults: Links to Blood–Brain Barrier Dysfunction. International Journal of Molecular Sciences. 2024;25(4):1991.
- Arvanitakis Z, Tatavarthy M, Bennett DA. The relation of diabetes to memory function. Current neurology and neuroscience reports. 2020;20:1-10.
- Patwardhan M. Gender-Specific Differences in Cognitive Functions and Alzheimer’s Risk in Type 2 Diabetes Mellitus. Adler University; 2021.
- Cui Y, Tang TY, Lu CQ, Ju S. Insulin resistance and cognitive impairment: evidence from neuroimaging. Journal of Magnetic Resonance Imaging. 2022;56(6):1621-1649.
- Jorge HMVM. Decision-Making Under Uncertainty: Linking Brain, Behavior and Family Factors in Patients with Type 1 Diabetes. Universidade de Coimbra (Portugal); 2020.
- Vinciguerra L, Lanza G, Puglisi V, et al. Update on the neurobiology of vascular cognitive impairment: from lab to clinic. International journal of molecular sciences. 2020;21(8):2977.
- Motevalli S, Salahshour HM, Bailey RP. The mediating role of cognitive flexibility in the relationship between cognitive emotion regulation strategies and mindfulness in patients with type 2 diabetes. Journal of affective disorders. 2023;339:676-682.
- Mengozzi A, de Ciuceis C, Dell’oro R, et al. The importance of microvascular inflammation in ageing and age-related diseases: a position paper from the ESH working group on small arteries, section of microvascular inflammation. Journal of Hypertension. 2023;41(10):1521-1543.
- Ryan CM, van Duinkerken E, Rosano C. Neurocognitive consequences of diabetes. American Psychologist. 2016;71(7):563.
- Jellinger KA. Pathomechanisms of vascular depression in older adults. International journal of molecular sciences. 2020;23(1):308.
- Damanik J, Yunir E. Type 2 diabetes mellitus and cognitive impairment. Acta Med Indones. 2021;53(2):213-220.
- De Felice FG, Ferreira ST. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes. 2014;63(7):2262-2272.
- Ma L, Wang J, Li Y. Insulin resistance and cognitive dysfunction. Clinica chimica acta. 2015;444:18-23.
- Sultana MA, Hia RA, Akinsiku O, Hegde V. Peripheral mitochondrial dysfunction: a potential contributor to the development of metabolic disorders and Alzheimer’s disease. Biology. 2023;12(7):1019.
- Tiehuis A, Vincken K, Van Den Berg E, et al. Cerebral perfusion in relation to cognitive function and type 2 diabetes. Diabetologia. 2008;51:1321-1326.
- Hardigan T, Ward R, Ergul A. Cerebrovascular complications of diabetes: focus on cognitive dysfunction. Clinical Science. 2016;130(20):1807-1822.
- Chung C-C, Pimentel D, Jor’Dan AJ, Hao Y, Milberg W, Novak V. Inflammation-associated declines in cerebral vasoreactivity and cognition in type 2 diabetes. Neurology. 2015;85(5):450-458.
- Fourrier C, Singhal G, Baune BT. Neuroinflammation and cognition across psychiatric conditions. CNS spectrums. 2019;24(1):4-15.
- Li H, Xue X, Li L, et al. Aluminum-induced synaptic plasticity impairment via PI3K-Akt-mTOR signaling pathway. Neurotoxicity research. 2020;37:996-1008.
- Ahmad RMAH, Ababneh NA, Al-Domi HA. Brain insulin resistance as a mechanistic mediator links peripheral metabolic disorders with declining cognition. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2022;16(4):102468.
- Choudhary AK. Type 2 Diabetes and Cognitive Decline: A Neurovascular Perspective. Chronicle of Diabetes Research and Practice. 2025;4(1):31-41.
- Li H, Ren J, Li Y, Wu Q, Wei J. Oxidative stress: The nexus of obesity and cognitive dysfunction in diabetes. Frontiers in endocrinology. 2023;14:1134025.
- Ayer A, Fazakerley DJ, James DE, Stocker R. The role of mitochondrial reactive oxygen species in insulin resistance. Free Radical Biology and Medicine. 2022;179:339-362.
- González P, Lozano P, Ros G, Solano F. Hyperglycemia and oxidative stress: an integral, updated and critical overview of their metabolic interconnections. International journal of molecular sciences. 2023;24(11):9352.
- Huang X, Hussain B, Chang J. Peripheral inflammation and blood–brain barrier disruption: effects and mechanisms. CNS neuroscience & therapeutics. 2021;27(1):36-47.
- Yu Y, Chen R, Mao K, Deng M, Li Z. The role of glial cells in synaptic dysfunction: insights into Alzheimer’s disease mechanisms. Aging and disease. 2024;15(2):459.
- Dutta BJ, Singh S, Seksaria S, Gupta GD, Singh A. Inside the diabetic brain: Insulin resistance and molecular mechanism associated with cognitive impairment and its possible therapeutic strategies. Pharmacological Research. 2022;182:106358.
- Aderinto N, Olatunji G, Abdulbasit M, et al. The impact of diabetes in cognitive impairment: A review of current evidence and prospects for future investigations. Medicine. 2023;102(43):e35557.
- Ding H, Li Y, Wen M, Liu X, Han Y, Zeng H. Elevated intracranial pressure induces IL‐1β and IL‐18 overproduction via activation of the NLRP3 inflammasome in microglia of ischemic adult rats. International Journal of Molecular Medicine. 2021;47(1):183-194.
- Kshirsagar V, Thingore C, Juvekar A. Insulin resistance: a connecting link between Alzheimer’s disease and metabolic disorder. Metabolic Brain Disease. 2021;36(1):67-83.
- Cornell J, Salinas S, Huang H-Y, Zhou M. Microglia regulation of synaptic plasticity and learning and memory. Neural regeneration research. 2022;17(4):705-716.
- Barloese MC, Bauer C, Petersen ET, Hansen CS, Madsbad S, Siebner HR. Neurovascular coupling in type 2 diabetes with cognitive decline. A narrative review of neuroimaging findings and their pathophysiological implications. Frontiers in endocrinology. 2022;13:874007.
- Biessels GJ, Nobili F, Teunissen CE, Simó R, Scheltens P. Understanding multifactorial brain changes in type 2 diabetes: a biomarker perspective. The Lancet Neurology. 2020;19(8):699-710.
- Kellar D, Register T, Lockhart SN, et al. Intranasal insulin modulates cerebrospinal fluid markers of neuroinflammation in mild cognitive impairment and Alzheimer’s disease: A randomized trial. Scientific reports. 2022;12(1):1346.
- Jönsson M, Gerdle B, Ghafouri B, Bäckryd E. The inflammatory profile of cerebrospinal fluid, plasma, and saliva from patients with severe neuropathic pain and healthy controls-a pilot study. BMC neuroscience. 2021;22:1-12.
- An Y, Xu B-t, Wan S-r, et al. The role of oxidative stress in diabetes mellitus-induced vascular endothelial dysfunction. Cardiovascular diabetology. 2023;22(1):237.
- Dludla PV, Mabhida SE, Ziqubu K, et al. Pancreatic β-cell dysfunction in type 2 diabetes: Implications of inflammation and oxidative stress. World journal of diabetes. 2023;14(3):130.
- Ma X, Nan F, Liang H, et al. Excessive intake of sugar: An accomplice of inflammation. Frontiers in immunology. 2022;13:988481.
- Zhang Z, Huang Q, Zhao D, Lian F, Li X, Qi W. The impact of oxidative stress-induced mitochondrial dysfunction on diabetic microvascular complications. Frontiers in endocrinology. 2023;14:1112363.
- Galea I. The blood–brain barrier in systemic infection and inflammation. Cellular & molecular immunology. 2021;18(11):2489-2501.
- Wątroba M, Grabowska AD, Szukiewicz D. Effects of diabetes mellitus-related dysglycemia on the functions of blood–brain barrier and the risk of dementia. International Journal of Molecular Sciences. 2023;24(12):10069.
- Takata F, Nakagawa S, Matsumoto J, Dohgu S. Blood-brain barrier dysfunction amplifies the development of neuroinflammation: understanding of cellular events in brain microvascular endothelial cells for prevention and treatment of BBB dysfunction. Frontiers in cellular neuroscience. 2021;15:661838.
- Zhao B, Yin Q, Fei Y, et al. Research progress of mechanisms for tight junction damage on blood–brain barrier inflammation. Archives of Physiology and Biochemistry. 2022;128(6):1579-1590.
- Di Benedetto G, Burgaletto C, Bellanca CM, Munafò A, Bernardini R, Cantarella G. Role of microglia and astrocytes in Alzheimer’s disease: from neuroinflammation to Ca2+ homeostasis dysregulation. Cells. 2022;11(17):2728.
- Hartmann S-M, Heider J, Wüst R, Fallgatter AJ, Volkmer H. Microglia-neuron interactions in schizophrenia. Frontiers in Cellular Neuroscience. 2024;18:1345349.
- Sun M, You H, Hu X, et al. Microglia–astrocyte interaction in neural development and neural pathogenesis. Cells. 2023;12(15):1942.
- Marrano N, Biondi G, Borrelli A, et al. Type 2 diabetes and Alzheimer’s disease: the emerging role of cellular lipotoxicity. Biomolecules. 2023;13(1):183.
- Piekarz J, Picheta N, Burdan O, Kurek M, Chroscińska-Krawczyk M. Phytotherapy in Alzheimer’s Disease—A Narrative Review. Biomedicines 2024, 12, 1812. 2024.
- Toledano Gasca A, Rodríguez-Casado A, Álvarez MI, Toledano-Díaz A. Alzheimer’s Disease, Obesity, and Type 2 Diabetes: Focus on Common Neuroglial Dysfunctions (Critical Review and New Data on Human Brain and Models). 2024.
- Banasr M, Sanacora G, Esterlis I. Macro-and microscale stress–associated alterations in brain structure: translational link with depression. Biological Psychiatry. 2021;90(2):118-127.
- Blinkouskaya Y, Weickenmeier J. Brain shape changes associated with cerebral atrophy in healthy aging and Alzheimer’s disease. Frontiers in Mechanical Engineering. 2021;7:705653.

