PNC-27: Targeting Cervical Cancer with Enhanced Efficacy
PNC-27 Kills Cervical Cancer Cells but Not Untransformed Cervical Cells, an Effect that is Enhanced by Ketone Bodies
Anna I. Miller1, Lucas Miller1*, Shabnam Seydafkan2, Steven Mc Glinchey1, Gholamali Jafari1, Miriam Silberstein1, Patryck Krzesaj1, Richard D. Feinman1, Matthew R. Pincus1
- Departments of Cell Biology and Pathology, SUNY Downstate Medical University, 450 Clarkson Avenue, Brooklyn, NY 11203
- Department of Pathology, New York University Langone Medical Center, 550 First Avenue, New York, NY 10016. *On leave, SUNY Binghamton, NY.
OPEN ACCESS
PUBLISHED: 31 May 2025
CITATION: Miller, I., A., Miller, L., Seydafkan, S., et al., 2025. PNC-27 Kills Cervical Cancer Cells but Not Untransformed Cervical Cells, an Effect that is Enhanced by Ketone Bodies. Medical Research Archives, [online] 13(5). https://doi.org/10.18103/mra.v13i5.6471
COPYRIGHT © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI https://doi.org/10.18103/mra.v13i5.6471
ISSN 2375-1924
Abstract
The anti-cancer peptide, PNC-27, is cytotoxic to cancer cells but has no effect on normal cells and has eradicated tumors in vivo with no off-target effects. We have recently treated three different cervical cancer cell lines with this peptide including two squamous cervical cancers (HTB-35 and SW756) and one endocervical cancer (HeLa) and found that it killed all three cell lines with IC50 values among the lowest values that we have found for a wide variety of cancers. On the other hand, PNC-27 had no effect on the viability or growth of PCS-480-011 primary normal cervical epithelial cells. We have recently found that lithium acetoacetate inhibits the proliferation of cancer but not normal cells without exhibiting any cytotoxicity. When administered to cancer cells with chemotherapeutic compounds, this agent causes significant lowering of the IC50 values for the chemotherapeutic compounds. We now find that lithium acetoacetate induces a lowering of the IC50 values of PNC-27 on all three cervical cancer cell lines by 200-300 percent. These results suggest that PNC-27 in acetoacetate solutions can be a powerful antitumor agent.
Keywords:
PNC-27, cervical cancer, ketone bodies, IC50 values, dose-response curves
Abbreviations: LiAcAc: lithium acetoacetate.
THE EUROPEAN SOCIETY OF MEDICINE
Medical Research Archives, Volume 13 Issue 5
RESEARCH ARTICLE
PNC-27 Kills Cervical Cancer Cells but Not Untransformed Cervical Cells, an Effect that is Enhanced by Ketone Bodies.
Introduction
PNC-27 is an anti-cancer peptide that contains the HDM-2-binding domain of the p53 protein, residues 12-26, attached to a transmembrane-penetrating peptide from antennia paedia. This peptide binds to membrane-expressed HDM-2 in cancer cells resulting in association of PNC-27-HDM-2 complexes that line trans-membrane pores that induce rapid tumor cell necrosis. Importantly, PNC-27 has no effect on untransformed cells which do not express HDM-2 in their cell membranes. This peptide induces tumor cell necrosis of a wide range of cancer cells including solid tissue tumors, i.e., human breast, pancreas, lung, colon and ovary, and hematopoietic cancer cells, including acute and chronic myelogenous leukemia cells, but has no effect on corresponding normal cells (reviewed in refs. 3 and 4). Included in the latter group are normal human hematopoietic stem cells whose differentiation under growth factors are unaffected by this peptide, suggesting that it does not suppress bone marrow as occurs with many chemotherapeutic agents. In vivo, PNC-27 has been found to eradicate a pancreatic cancer cell line (TUC-3) with high metastatic potential in nude mice and human cancer stem-cell-enriched acute myelogenous leukemia cells from nine different donors in nude mice. In both studies, no off-target effects were observed suggesting that this peptide may be an effective agent in treating cancers.
Since the tumor cell necrosis induced by PNC-27 on different cancer cell lines requires only that HDM-2 be expressed in the cancer cell membrane, this effect would appear to be independent of the particular pathways by which these cells have been transformed. To confirm this conclusion, we have incubated PNC-27 with a variety of different cancer cell lines of the same cell type but different genetic profiles that are associated with cell transformation and have found that this peptide induces tumor cell necrosis of all of these cell lines. For example, PNC-27 kills both MCF-7 breast cancer cells which express normal p53 and MDA-MB-157 cells that are p53 homozygously deficient. These results suggest that the selective anti-malignant cell effect of PNC-27 is independent of the mechanisms involved in cell transformation and may be well-suited for treating many kinds of cancers.
One type of tumor cells that have not been investigated in this fashion is squamous cell cancers. While we have found that PNC-27 is cytotoxic to HeLa cervical cancer cells, these cells are endocervical cells of the cuboidal, not squamous, epithelial type. Therefore, in the present study, we have investigated the effects of PNC-27 on several different cervical squamous cancer cell lines.
In addition, in a recent series of studies, we have found that ketone bodies such as the lithium and sodium salts of acetoacetate and beta-hydroxybutyrate inhibit the proliferation of, but are non-cytotoxic to, cancer cells. Furthermore, ketone bodies have no effect on the growth and viability of untransformed cells. These findings appear to be linked to the Warburg Effect, i.e., cancer cells tend to rely on glycolysis for ATP production while non-transformed cells utilize the Krebs Cycle and oxidative phosphorylation in addition to glycolysis. Ketone bodies are converted to acetyl co-A, that is utilized by the Krebs Cycle, but they inhibit glycolysis, which is necessary for ATP production in cancer cells, resulting in blockade of cancer cell proliferation. In contrast, untransformed cells can utilize the acetyl co-A from ketone bodies for ATP production via the Krebs Cycle resulting in ATP production.
As a consequence, these ketone bodies synergize with tumoricidal agents by decreasing cell proliferation during treatment. For example, ketone bodies significantly lower the IC50 values for rapamycin and methotrexate in killing MCF-7 breast cancer cells and SW 480 colon cancer cells and reduce the IC50 for PNC-27 for the killing of MCF-7 human breast cancer cells by a factor of over 400 percent. In vivo, two groups of mice expressing a spontaneous breast tumor with lung metastases were treated with chemotherapy; one group was placed on a regular diet while a second group was treated with a ketogenic diet. The latter group was found to undergo more rapid reduction in tumor size with increased longevity suggesting that ketone bodies generated by the ketogenic diet synergized with the chemotherapeutic agents. We therefore now investigate the effects of ketone bodies on the IC50s of PNC-27 when incubated with cervical cancer cells. We chose the lithium salt because it was found to be effective at significantly lower concentrations than the sodium salt.
Materials and Methods
Peptide: PNC-27. This a 32 amino acid peptide whose sequence is H-Pro-Pro-Leu-Ser-Gln-Glu-Thr-Phe-Ser-Asp-Leu-Trp-Lys-Leu-Leu-Lys-Lys-Trp-Lys-Met-Arg-Arg-Asn-Gln-Phe-Trp-Val-Lys-Val-Gln-Arg-Gly-OH. The bold-type residues are from the transactivating domain of p53 (residues 12-26) while the italicized residues are the leader sequence or the membrane residency peptide (MRP) and was synthesized by solid phase methods by Biopeptides (San Diego, CA, USA) and purified to > 97 percent.
Lithium Acetoacetate (LiAcAc) was purchased from TCI America through Thermo Fischer Scientific (Fairlawn, NJ, USA) (>97 percent pure) and was dissolved directly in normal saline.
Cells: HTB-35 (SiHa) human cervical squamous epithelial carcinoma cells were obtained from the American Type Culture Collection (ATCC) (Manassas, VA) and were cultured in Eagle’s Minimum Essential Medium with 10% fetal bovine serum at 37°C and 5% CO2. The 0.25% (w/v) Trypsin-0.53 mM EDTA solution were used to detach HTB-35 cells at 90% confluency. All cell type numbers were determined by hemacytometer and trypan blue protocol.
SW-756 human cervical poorly differentiated invasive squamous carcinoma cells were obtained from the American Type Culture Collection (ATCC) (Manassas, VA) Dulbecco’s Minimum Essential Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37°C in a humidified incubator with 5% CO2.
HeLa Cells were obtained from the American Type Culture Collection (ATCC) (Manassas, VA) and were incubated in DMEM supplemented with 10% FBS at 37°C in a humidified incubator with 5% CO2.
PCS-480-011 Primary normal cervical epithelial cells were obtained from the ATCC and cultured in proprietary Cervical Epithelial Cell Basal Medium (ATCC PCS-480-032) with added components in Cell Growth Kit (ATCC PCS-480-042) to make the complete medium without FBS at 37°C and 5% CO2.
Incubation of Cells with PNC-27: Dose-response curves were generated for cells incubated with different concentrations of PNC-27, over a peptide concentration range of 0-500 ug/mL, alone and in the presence of lithium acetoacetate at 10 and 15 mM. Cell viability and PNC-27-induced cell lysis were determined using the crystal violet proliferation assay kit (Abcam, cat. # ab232855) and the Cyto Tox96 LDH release assay (Promega, Madison, WI, USA), respectively, as described previously. CytoTox96 assays were performed according to manufacturer’s instructions. All incubations were performed over a 48h period.
Incubation of Cervical Cancer Cell Lines with Lithium Acetoacetate (LiAcAc). To determine whether LiAcAc is cytotoxic to the cancer cell lines in this study, we incubated each cell line with 15mM LiAcAc alone over a 48h period and determined cell counts for viable and dead cells using the trypan blue exclusion assay on the Contess 3 automatic cell counter (ThermoFischer, Fairlawn, NJ, USA).
Results
PNC-27 Exhibits Cytotoxicity to Cervical Cancer But Not Normal Cervical Cells. Figures 1 show the dose response curves for the PNC-27-induced killing of the three different cervical cancer cell lines, SW756, HeLa and HTB-35, respectively, alone (black curves) and in the presence of 10 and 15 mM LiAcAc. As can be seen in each figure, PNC-27 induces high levels of cytotoxicity of the three cervical cancer cell lines inducing almost complete cancer cell death of HTB-35 and HeLa cells and at least 80 percent cell death of SW756 cells that correlated with LDH release suggesting cancer cell membranolysis. Also shown in each figure as a blue filled circle is the effect of the maximum dose used in these experiments of PNC-27 (20 uM or 80 ug/mL) on normal human PCS-480-011 primary normal cervical epithelial cells. As can be seen in each figure, these normal cells are unaffected by the cancer-cell-cytotoxic peptide. Thus PNC-27 exhibits cytotoxicity exclusively to these cancer cells. Interestingly, the IC50 values for PNC-27 on these cell lines are among the lowest (7-17uM) that we have found in our studies of the effects of this peptide on many different cancer cell lines. For example, the IC50 for PNC-27 on several different human breast and pancreatic cancer cell lines is on the order of 33 uM.
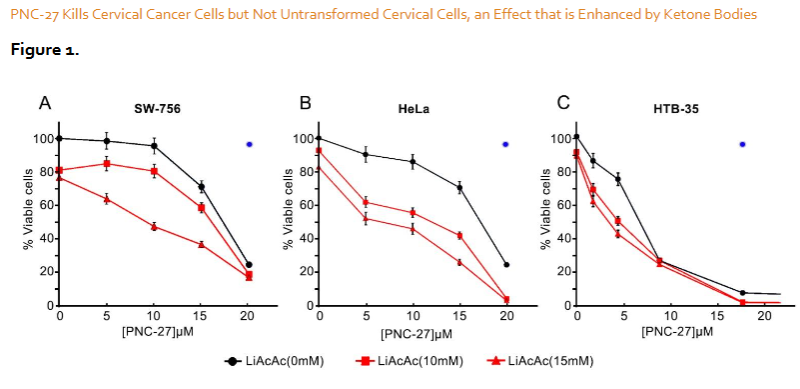
Cancer-Cell Killing by PNC-27 Is Enhanced by Ketone Bodies. We further determined if ketone bodies reduce the IC50 of PNC-27 in the killing of the three cervical cancer cell lines. As can be seen in each of Figures 1, the IC50 of PNC-27 is significantly reduced at 10 and 20 mM LiAcAc, concentrations which we found caused the maximum decreases in the IC50 values of rapamycin, methotrexate and PNC-27 in our prior studies. For SW 756 and HTB-35 cells, there is an almost two-fold reduction for the IC50 of PNC-27, respectively, and an over threefold reduction for the IC50 of PNC-27 in killing HeLa cells. These IC50 factor reductions are in the same range as factor, 4.7, that we found for PNC-27 in killing MCF-7 breast cancer cells.
It may also be noted in each figure that at an LiAcAc concentration of 20mM, there is a distinct decrease in cell viability even at low concentrations of PNC-27. Figure 1C shows that at 2 uM PNC-27, there is a drop in cell viability of HTB-35 cells from about 85% with no ketone body present to 60 percent in the presence of 20mM LiAcAc. In Figure 1A, at 5uM PNC-27 there is little effect on cell viability of SW 756 cells in the absence of LiAcAc but at 20mM LiAcAc there is a drop of 30 percent cell viability with this concentration of peptide. As shown in Figure 1B, the effect of 20mM LiAcAc on increasing the efficacy of PNC-27 is even greater for HeLa cells. At 5uM PNC-27, the decrease in cell viability is enhanced by 20 mM LiAcAc by almost 50 percent. Thus ketone bodies significantly enhance the cytotoxicity of PNC-27 to cervical cancer cells.
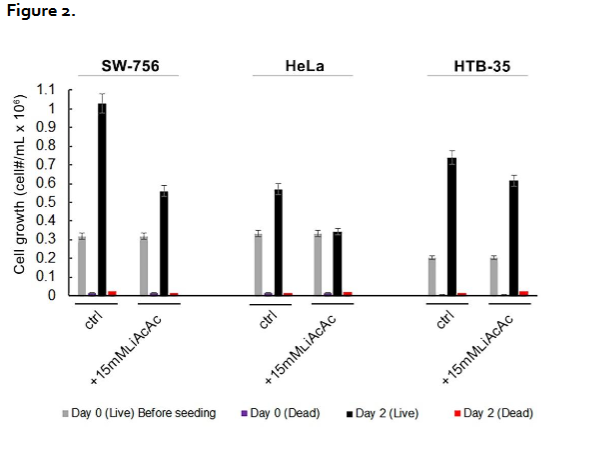
Ketone Bodies Inhibit Cervical Cancer Cell Proliferation But Do not Induce Cancer Cell Death. Figure 2 shows the effects of LiAcAc on the proliferation of each of the cervical cancer cell lines as measured by the trypan blue assay. In this figure, using as an example SW 756 cancer cells, the first set of bar graphs shows results for control cells incubated in growth medium labeled “Ctrl.” In this first set, the leftmost gray-colored bar is the initial cell count prior to incubation, together with dead cell count (dark purple bar graph next to the gray bar graph). The black bar in this cluster is the cell count after 48 h incubation of the cells in growth medium. The red bar graph shows the count for dead cells which differed minimally from the initial dead cell count. The second bar graph cluster is for the same cells incubated in incubation medium containing 15 mM LiAcAc. The black bar graph shows that there is a 50 percent decrease in cell count but a low dead cell count that is the same as the initial dead cell count suggesting that LiAcAc induces reduced cell proliferation but does not kill cancer cells. Similar results were found for the other two cancer cell lines: HeLa cell proliferation was reduced by approx 40 percent while HTB-35 cell proliferation was reduced by about 17 percent. Cell death for both cell lines was minimal and was the same as for the dead cell number in the initial cell count.
Discussion
PNC-27 Kills Cervical Cancer Cells, Regardless of Cell Type, But Not Normal Cervical Cells. PNC-27 is cytotoxic to cervical cancer cells but does not affect an untransformed human cervical squamous cell line. These findings are consistent with those of our prior studies that showed that this peptide is cytotoxic to a wide variety of solid tissue tumors (of endodermal origin) and hematopoietic tumors but does not affect corresponding untransformed cells. We have found that the specificity of PNC-27 cytotoxicity to cancer cells is due to its interaction with membrane-bound HDM-2 protein which is uniquely expressed at high levels in cancer cell membranes. Binding of PNC-27 to membrane-expressed HDM-2 induces the formation of transmembrane pores resulting in extrusion of intracellular contents and tumor cell necrosis.
An important finding of this current study is that PNC-27 is cytotoxic to cervical cancer of different origins, i.e., exocervical squamous cell cancer (HTB-35 and SW756) and endocervical endodermal (cuboidal epithelial) cell cancer (HeLa). Thus this peptide may be effective in treating cervical cancer in general with no off-target effects. In addition, PNC-27 is cytotoxic to squamous cell cervical cancer and therefore may also be effective in treating squamous cell cancers at other sites such as skin.
PNC-27 Kills Cervical Cancer Cells, Irrespective of the Mechanisms of Cell Transformation. Another significant aspect of this study is that, although all three cell lines express human papilloma viral (HPV) genomic sequences, the viral strains vary: HTB-35 cells appear to express HPV strain 16 while SW756 and HeLa cells express strain 18. In addition, there are major differences in chromosomal features among these three cell lines. HeLa cells have been found to contain abnormal multiploid karyotypes and complex translocations compared with the other two cell lines in this study. In addition, HTB-35 cells have been found to express mesenchymal phenotypes including decreased E-cadherin and increased vimentin expression not observed in SW 756 or HeLa cells suggesting that HTB-35 cells undergo epithelial to mesenchymal transition (EMT) and are more invasive and hence at a more advanced stage of tumor progression than are SW756 cells. Despite these differences in the genetic and protein expression profiles of these different cervical cell lines, PNC-27 is cytotoxic to all of them while having no effect on the untransformed human cervical PCS-480 cell lines as shown in Figures 1.
Ketone Bodies Enhance the Anti-Tumor Effects of PNC-27 by Inhibiting Cancer Cell Proliferation. Figures 1 show that LiAcAc induces a significant shift in the IC50 of PNC-27 to lower values and increases the level of cell killing in the dose response curves when the three different tumor cell lines are incubated with this peptide. Figure 2 shows that LiAcAc inhibits cancer cell proliferation without causing cancer cell death suggesting that LiAcAc increases cell killing by limiting cancer cell growth thereby reducing tumor cell burden. Importantly, in our recent studies, we found that ketone bodies do not inhibit the growth of untransformed fibroblasts in culture further indicating that their anti-proliferative effects are limited to cancer cells. These findings are consistent with our prior results with other cancer cell lines as described above and are consistent with prior studies demonstrating that LiAcAc inhibits the growth of several aggressive tumor cell lines but not untransformed fibroblast cell lines. This growth inhibition of the cancer cell lines correlated with decreased production of ATP and overexpression of uncoupling protein2 (UCP2) in each of different cancer cell lines. In contrast, LiAcAc did not affect cell growth or ATP levels in any of the untransformed cell lines. These results are consistent with the Warburg effect: cancer cells depend largely on glycolysis and not on the Krebs cycle even in the presence of oxygen to generate ATP. Since acetoacetate is converted to acetyl Co-A in cells and inhibits glycolysis, cancer cell growth is inhibited, an event that does not affect untransformed cells which utilize the Krebs cycle.
In this study, we used the lithium salt of acetoacetate as the anti-proliferative, non-cytotoxic agent rather than the sodium salt since, in a prior dose response study, we found that 20 mM LiAcAc induced about 60 percent reduction in cancer cell proliferation, a level that was achieved only at a higher concentration, 40 mM, of NaAcAc. In this prior study, we found that lithium ion itself caused non-cytotoxic inhibition of cancer cell growth since LiCl induced significant inhibition possibly explaining its increased efficacy. Importantly, both agents LiAcAc and NaAcAc, were found not to inhibit the growth of a normal human fibroblast (HSF2617) cell line even at the highest concentrations (20 and 40 mM, respectively) of each agent. Thus both agents may be effective adjuncts in treating cancers with PNC-27 with no off-target effects. An important correlate of these findings is the in vivo study performed on mice with syngeneic breast cancers which were treated with chemotherapeutic agents and were divided into two groups: those fed normal control diets and those placed on a ketogenic diet. The latter group survived for significantly longer periods than the mice fed the control diet, suggesting that ketone bodies enhanced the chemotherapeutic agents. Since we have found that a ketone body enhances the cytotoxicity of PNC-27 against cervical cancer cells, treatment of cancers using PNC-27 would likely be aided using concurrent ketogenic diets.
Conclusion
PNC-27 kills cervical cancer cells of both exo and endocervical origin but has no effect on normal cervical cells. This selective cytotoxic effect of PNC-27 is independent of cancer cell karyotype, specific activated signal transduction pathway and HPV genotype and is consistent with its known effect of binding to membrane-expressed HDM-2 in the cervical cancer cells that does not occur in normal cell lines. The cytotoxic effect of PNC-27 is enhanced by the presence of the ketone body, LiAcAc, which significantly inhibits cancer cell mitosis but does not cause tumor cell death. This anti-mitotic effect of LiAcAc is specific for cancer cells in agreement with prior studies.
Conflict of Interest
None.
Funding Statement
This research was supported by funds from The Nutrition and Metabolism Society, The Research Foundation of SUNY and the Alumni Foundation of SUNY Downstate Health Sciences Center, Brooklyn, NY.
Acknowledgements
We wish to thank the Nutrition and Metabolism Society, the Research Foundation of SUNY and the Alumni Association of SUNY Downstate Medical University, Brooklyn, NY, for supporting this study.
References
- Sarafraz-Yazdi E, Bowne, WB, Adler, V, Sookraj KA, Wud V, Shteyler V, Patel H, Oxbury W, Brandt-Rauf W, Zenilman ME, Michl J, Pincus, MR. Anticancer Peptide PNC-27 Adopts an HDM-2-Binding Conformation and Kills Cancer Cells by Binding to HDM-2 in their Membranes. Proc Natl Acad Sci USA. 2010; 107:1918-1923.
- Sarafraz-Yazdi E, Mumin S, Cheung D, Fridman D, Lin B, Wong L, Rosal R, Rudolph R, Frenkel M, Thadi A, Morano WF, Bowne WB, Pincus MR, Michl JP. PNC-27, a Chimeric p53-Penetratin Peptide Binds to HDM-2 in a p53 Peptide-like Structure, Induces Selective Membrane-Pore Formation and Leads to Cancer Cell Lysis. Biomedicines. 2022;10: 945. https://doi.org/10.3390/biomedicines10050945.
- Pincus MR, Bowne WB, Sarafraz-Yazdi, E. Poptosis: A Novel Mechanism for the Selective Killing of Cancer Cells. Clin Oncol. 2023; 7 (6): 1-13 , ISSN: 2640-1037.
- Pincus MR, Silberstein M, Zohar N, Sarafraz-Yazdi E, Bowne WB. Poptosis or Peptide-Induced Transmembrane Pore Formation: A Novel Way to Kill Cancer Cells without Affecting Normal Cells. Biomedicines. 2024;12: 1144. https://doi.org/10.3390/biomedicines12061144.
- Michl J, Scharf B, Schmidt A, Hannan R, von Gizycki H, Friedman FK, Brandt-Rauf PW, Fine RL, Pincus MR. PNC-28, a p53 Peptide that Is Cytotoxic To Cancer Cells, Blocks Pancreatic Cancer Cell Growth in vivo. Int J Cancer. 2006; 119, 1577-1585.
- Wang H, Zhao D, Nyguyen LX, Wuz H, Ling L, Dong D, Troadec E, Zhu Y, Hoang DH, Stein AS, et al. Targeting Cell Membrane HDM2: A Novel Therapeutic Approach for Acute Myeloid Leukemia. Nat. Leuk. 2019; 34, 75–86.
- Kanovsky M, Raffo A, Drew L, Rosal R, Do T, Friedman FK, Rubinstein P, Visser I, Robinson R, Brandt-Rauf PW, Michl J, Fine RL, Pincus MR. Peptides from the Amino Terminal mdm-2 Binding Domain of p53, Designed from Conformational Analysis, Are Selectively Cytotoxic to Transformed Cells. Proc Natl Acad Sci USA. 2001;98, 12438-12443.
- Miller A, Lin B, Pincus MR, Fine E and Feinman RD. Selective Enhancement of Chemotherapeutic Agent-Induced Tumor Cell Killing by Acetoacetate and 3-Hydroxybutyrate. EC Pharmacology and Toxicology 2021;9, 29-34.
- Miller AI, Diaz D, Lin B, Krzesaj PK, Ustoyev S, Shim A, Fine EJ, Sarafraz-Yazdi E, Pincus MR, Feinman RD, Ketone Bodies Induce Unique Inhibition of Tumor Cell Proliferation and Enhance the Efficacy of Anti-Cancer Agents. Biomedicines. 2023; 11, 2515. https://doi.org/10.3390/biomedicines11092515.
- Fine EJ, Miller A, Quadros EV, Sequeira JM, Feinman RD. Acetoacetate Reduces Growth and ATP Concentration in Cancer Cell Lines Which Over-Express Uncoupling Protein 2. Cancer Cell Int. 2009: 9, 14.
- Warburg O. On the origin of cancer cells. Science. 1956;123: 309-314.
- Zo Y, Fineberg S, Pearlman A, Feinman RD, Fine EJ. The Effect of a Ketogenic Diet and Synergy with Rapamycin in a Mouse Model of Breast Cancer. PLoS ONE. 2020:15, e0233662.
- Schwartz E, Freese UK, Gissman L, Mayer W, Roggenbuck B, Stremlau A, Zur Hausen H. Structure and Transcription of Human Papillomavirus Sequences in Cervical Carcinoma Cells. Nature. 1985: 314, 111-114.
- Macville M, Schrock E, Padilla-Nash H, Keck C, Ghadimi BM, Zimonic D, Popescur N, Ried T. Comprehensive and Definitive Molecular Cytogenetic Characterization of HeLa Cells by Spectral Karyotyping. Cancer Res. 1999: 59 (1), 141–150.
- Miekus K, Pawlowska M, Sekuła M, Drabik G, Madeja Z, Adamek D, Majka M. MET Receptor Is a Potential Therapeutic Target in High Grade Cervical Cancer. Oncotarget. 2015: Apr 30;6(12),10086-10101. doi: 10.18632/oncotarget.3161. PMID: 25888626; PMCID: PMC4496342.

