Responses of Renal Cell Carcinomas to 29 Anti-Cancer Therapies
Responses of Renal Cell Carcinomas to 29 Different Systemic Anti-Cancer Therapies
Authors: Noa Goffir1, Vladimir Yurtin1, Mordechai Duduvden1, Guy Hidas1, Steve Frank2, Yakir Rottenberg1
Affiliations:
1. Department of Urology, Hebrew University of Jerusalem, Hadassah Medical Center, Faculty of Medicine
2. Department of Radiology, Hebrew University of Jerusalem, Hadassah Medical Center, Faculty of Medicine
Published: 31 July 2025
CITATION:Goffir, N., Yurtin, V., Duduvden, M., Hidas, G., Frank, S., Rottenberg, Y. (2025). Responses of Renal Cell Carcinomas to 29 Different Systemic Anti-Cancer Therapies. The European Society of Medicine, Medical Research Archives, Volume 13 Issue 7.
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.18103/mra.v13i7.6752
ISSN 2375-1924
Abstract
The prevailing view is that renal cell carcinomas (RCCs) are resistant to chemotherapy. However, it is essential to periodically reassess the effectiveness of systemic therapies that have been previously considered to be unsustainable in treating RCC. Our study aimed to evaluate the response rates of RCCs to various systemic therapies.
Keywords
renal cell carcinoma, systemic therapy, anti-cancer therapies, chemotherapy resistance
Introduction
Over a third of patients with renal cell carcinoma (RCC) present with metastatic disease or develop metastases after surgery for an apparently localized disease. The prognosis for these patients is grim.
RCC is considered chemoresistant. In the metastatic setting, its response to single or combined chemotherapy agents has been examined in several phase III studies. Single agents, including vinca alkaloids, gemcitabine, and fluoropyrimidine derivatives, have shown an objective response rate (ORR) of 0-20%. One study reported an ORR of 26% with acceptable toxicity. Combination chemotherapy, typically involving gemcitabine and fluorouracil, has not been shown to provide any meaningful benefit.
Four patients received immunotherapy, including anti-PD-1 and anti-PD-L1 agents, with an ORR of 50%. The objective response rate was calculated by subtracting the tumor’s largest diameter in the initial CT (mm) from the largest diameter on the last CT (mm), divided by the time elapsed between the studies (in years). Diameter changes within a 3 mm range were classified as “no change”. The research was approved by the institutional review board.
Methods
Patient demographics, types of malignancy, and treatment regimens were recorded and compared with the measurements taken at the follow-up period. Renal pathological specimens were evaluated using the 2002 TNM classification, histologic subclassing according to the 1997 UICC classification, and grading based on Fuhrman’s nuclear grading system.
| Num | Age | Sex | Charlson Comorbidity Index | Metastasis? | Other malignancy type | Treatment regimen |
|---|---|---|---|---|---|---|
| 1 | 57 | F | 7 | Y | Breast Cancer | trastuzumab, pertuzumab |
| 2 | 68 | M | 6 | Y | Diffuse large B-cell lymphoma | CHOP, rituximab |
| 3 | 56 | M | 4 | Y | Gastric adenocarcinoma | capecitabine |
| 4 | 68 | M | 7 | Y | Lung adenocarcinoma | cisplatin, Vinorelbine |
| Num | Histology | Follow-up of RCC | Kidney Status | Last follow-up status |
|---|---|---|---|---|
| 1 | Clear F1 | Partial nephrectomy | 131 | Alive |
| 2 | Clear F2 | Radical nephrectomy | 123 | Died Lymphoma |
| 3 | Clear F2 | Not treated | 97 | Died NED |
| 4 | Papillary | Not treated | 90 | Alive NED |
| Diameter 1 (mm) | Diameter 2 (mm) | Time difference (months) | Growth rate (mm/year) |
|---|---|---|---|
| 1 | 42.3 | 12 | 4.2 |
| 2 | 43.5 | 6 | 10.5 |
| 3 | 50.1 | 8 | 7.5 |
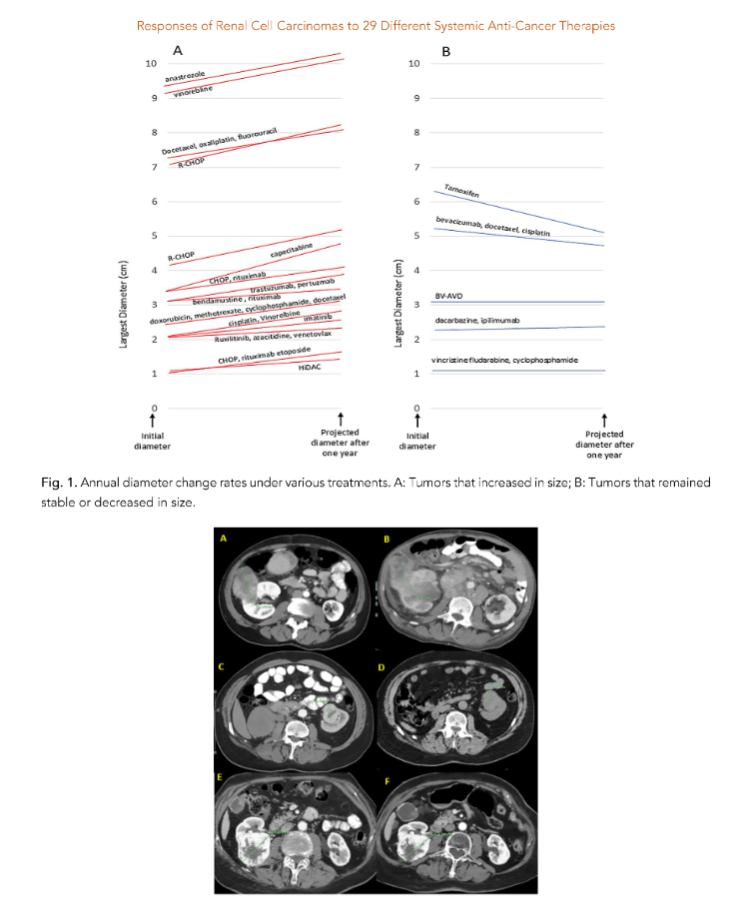
Results
These growth rates suggest that untreated RCC, as well as those treated with various systemic therapies, can exhibit significant variability in tumor response. In total, we analyzed 234 patients with untreated RCC and hemato-oncologic malignancies, showing a 10% mortality rate, which is significantly higher than that of patients with non-clear cell metastatic renal cell carcinoma. In any case, careful monitoring and addressing the RCC appears to be appropriate.
Discussion
In four patients, RCC diameter remained stable during follow-up, and in two patients, it decreased. The most significant cause of shrinkage involved a 50-year-old woman with metastatic breast cancer and a 63 mm Fuhrman grade 2 clear cell carcinoma.
References
3. Tsimafeyeu I, Demidov L, Kharkevich G, et al. Phase II, multicenter, uncontrolled trial of single-agent capecitabine in patients with non-clear cell metastatic renal cell carcinoma. Am J Clin Oncol. 2012;35:251-4. doi: 10.1097/COC.0b013e31820d bc17. PMID: 21358295.
4. Mani S, Vogelzang NJ, Bertucci D, et al. Phase I study to evaluate multiple regimens of intravenous 5-fluorouracil administered in combination with weekly gemcitabine in patients with advanced solid tumors: a potential broadly active regimen for advanced solid tumor malignancies. Cancer. 2001; 92:1567-76. doi: 10.1002/1097-0142(20010915)92 :6<1567::aid-cncr1483>3.0.co;2-l. PMID: 11745236.
5. Ryan CW, Vogelzang NJ, Stadler WM. A phase II trial of intravenous gemcitabine and 5-fluorouracil with subcutaneous interleukin-2 and interferon-alpha in patients with metastatic renal cell carcinoma. Cancer. 2002;94:2602-9. doi: 10.1002/cncr.10528. PMID: 12173327.
6. Posadas EM, Limvorasak S, Figlin RA. Targeted therapies for renal cell carcinoma. Nat Rev Nephrol. 2017;13:496-511. doi: 10.1038/nrneph.2017.82. Epub 2017 Jul 10. PMID: 28691713.
7. Tung I, Sahu A. Immune Checkpoint Inhibitor in First-Line Treatment of Metastatic Renal Cell Carcinoma: A Review of Current Evidence and Future Directions. Front Oncol. 2021;11:707214. doi: 10.3389/fonc.2021.707214. PMID: 34527581; PMCID: PMC8435744.
8. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378:1277-1290. doi: 10.1056/NEJMoa1712126. Epub 2018 Mar 21. PMID: 29562145; PMCID: PMC5972549.
9. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380:1116-1127. doi: 10.1056/NEJMoa1816714. Epub 2019 Feb 16. PMID: 30779529.
10. Motzer RJ, Escudier B, George Set al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: Updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer. 2020; 126:4156-4167. doi: 10.1002/cncr.33033. Epub 2020 Jul 16. PMID: 32673417; PMCID: PMC8415096.
11. Richard PO, Jewett MA, Bhatt JR, et al. Renal Tumor Biopsy for Small Renal Masses: A Single-center 13-year Experience. Eur Urol. 2015;68:1007-13. doi: 10.1016/j.eururo.2015.04.004. Epub 2015 Apr 18. PMID: 25900781.
12. Chawla SN, Crispen PL, Hanlon AL et al. The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol. 2006;175: 425-31. doi: 10.1016/S0022-5347 (05)00148-5. PMID: 16406965.
13. Jewett MA, Mattar K, Basiuk J, et al. Active surveillance of small renal masses: progression patterns of early stage kidney cancer. Eur Urol. 2011;60:39-44. doi: 10.1016/j.eururo.2011.03.030. Epub 2011 Apr 1. PMID: 21477920.
14. Mues AC, Haramis G, Badani K et al. Active surveillance for larger (cT1bN0M0 and cT2N0M0) renal cortical neoplasms. Urology;2010;76:620-623. doi: 10.1016/j.urology.2010.04.021. PMID: 20599256.
15. Mehrazin R, Smaldone MC, Kutikov A et al. Growth kinetics and short-term outcomes of cT1b and cT2 renal masses under active surveillance. J Urol;192:659-664. doi: 10.1016/j.juro.2014.03.038. Epub 2014 Mar 15. PMID: 24641909; PMCID: PMC4419692.
16. Staehler M, Haseke N, Stadler T et al. The growth rate of large renal masses opposes active surveillance. BJU Int;105:928-931. doi: 10.1111/j.1464-410X.2009.08840.x. Epub 2009 Sep 14. PMID: 197 51265.
17. Greene FL, Page DL, Flemming ID et al. American Joint Committee on Cancer: AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer:2002:323-5. https://link.springer.com/book/10.1007/978-1-4757-3656-4
18. Störkel S, Eble JN, Adlakha DK, et al. Classification of renal cell carcinoma: Workgroup No. 1. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC). Cancer;1997;80:987-9. doi: 10.1002/(sici)1097-0142(19970901)80:5<987::aid-cncr24>3.0.co;2-r. PMID: 9307203.
19. Fuhrman SA, Lasky LC. Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol;1982;655-653. doi: 10.1097/00000478-198210000-00007. PMID: 71 80965.
20. Mues AC, Landman J. Small renal masses: current concepts regarding the natural history and reflections on the American Urological Association guidelines. Curr Opin Urol. 2010;20:105-10.
doi: 10.1097/MOU.0b013e32833625f8. PMID: 200 75734.
21. Li XS, Yao L, Gong K et al. Growth pattern of renal cell carcinoma (RCC) in patients with delayed surgical intervention. J Cancer Res Clin Oncol. 2013;138:269-274. doi: 10.1007/s00432-011-1083-0. Epub 2011 Nov 22. PMID: 22105897.
22. Walsh N, Larkin A, Kennedy S, et al. Expression of multidrug resistance markers ABCB1 (MDR-1/P-gp) and ABCC1 (MRP-1) in renal cell carcinoma. BMC Urol. 2009;9:6. doi: 10.1186/1471-2490-9-6. PMID: 19552816; PMCID: PMC2723136.
23. McFadyen MC, Melvin WT, Murray GI. Cytochrome P450 CYP1B1 activity in renal cell carcinoma. Br J Cancer. 200491:966-71. doi: 10.103 8/sj.bjc.6602053. PMID: 15280921; PMCID: PMC240 9861.
24. Selvarajah J, Nathawat K, Moumen A, et al. Chemotherapy-mediated p53-dependent DNA damage response in clear cell renal cell carcinoma: role of the mTORC1/2 and hypoxia-inducible factor pathways. Cell Death Dis. 2013: 17;4(10):e865. doi: 10.1038/cddis.2013.395. PMID: 24136229; PMCID: PMC3920935.
25. Aweys H, Lewis D, Sheriff M, et al. Renal Cell Cancer – Insights in Drug Resistance Mechanisms. Anticancer Res. 2023;43:4781-4792. doi: 10.2187 3/anticanres.16675. PMID: 37909991.
26. Mickisch GH, Roehrich K, Koessig J, et al. Mechanisms and modulation of multidrug resistance in primary human renal cell carcinoma. J Urol. 1990; 144:755-9. doi: 10.1016/s0022-5347(17)39586-1. PMID: 1974934.
27. Amin R. Tamoxifen-induced regression of pulmonary metastases from renal cell carcinoma. Br J Radiol. 1983;56:766-7. doi: 10.1259/0007-1285-56-670-766. PMID: 6616143.
28. Atzpodien J, Kirchner H, Illiger HJ, et al. IL-2 in combination with IFN- alpha and 5-FU versus tamoxifen in metastatic renal cell carcinoma: long-term results of a controlled randomized clinical trial. Br J Cancer. 2001;85:1130-6. doi: 10.1054/bjoc.20 01.2076. PMID: 11710825; PMCID: PMC2375150.
29. Al-Sarraf M, Eyre H, Bonnet J, et al. Study of tamoxifen in metastatic renal cell carcinoma and the influence of certain prognostic factors: a Southwest Oncology Group Study. Cancer Treat Rep. 1981; 65:447-51. PMID: 7237466.
30. Bielcikova Z, Werner L, Stursa J, et al. Mitochondrially targeted tamoxifen as anticancer therapy: case series of patients with renal cell carcinoma treated in a phase I/Ib clinical trial. Ther Adv Med Oncol. 2023;30;15:17588359231197957. doi: 10.1177/17588359231197957. PMID: 377865 38; PMCID: PMC10541747.
31. Rixe O, Bukowski RM, Michaelson MD, et al. Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: a phase II study. Lancet Oncol. 2007;8:975-84. doi: 10.1016/S1470-2045(07)70285-1. Epub 2007 Oct 23. PMID: 1795 9415.
32. Johnson L, Bylund J, Strup S, et al. Concomitant Renal Cell Carcinoma and Hematologic Malignancy in Immunosuppressed Patients. Am J Med Sci. 2016; 351:480-4. doi: 10.1016/j.amjms.2016.02.027. Epub 2016 Mar 21. PMID: 27140706.

