Risk Factors for Acute Kidney Injury in CABG Patients
Risk factors and prognostic significance of acute kidney injury in coronary artery bypass graft surgery: Insights from the KDIGO criteria.
Sumiran Mahajan ¹, Abhishek Prabhu ², Gananjay G. Salve ², Sweta G. Sooragonda ³, Jabbar Momin ³, Mohan D. Gan ², Mallikarjun Karishetti (Khanpet) ¹
² Department of Cardiovascular & Thoracic Surgery, Jawaharlal Nehru Medical College, KLES Academy of HigherEducation & Research, Belgaum, 590010, Karnataka, India
³ Department of Cardiac Anaesthesia, Jawaharlal Nehru Medical College, KLES Academy of Higher Education & Research, Belgaum, 590010, Karnataka, India
OPEN ACCESS
CITATION Mahajan, S., Prabhu, A., Salve., G., G., Sooragonda S., G., Momin, J., Gan, M., D., Karishetti. M., Risk factors and prognostic significance of acute kidney injury in coronary artery bypass graft surgery: Insights from the KDIGO criteria. Medical Research Archives, [online] 13(8). https://doi.org/10.18103/mra.v13i8.6805.
ISSN: 2375-1924
ABSTRACT
Purpose: To assess the risk factors and prognostic implications of patients with normal preoperative renal parameters, and who developed acute kidney injury following coronary artery bypass grafting.
Methods: This observational study involved 69 patients, who underwent elective isolated coronary artery bypass grafting with normal preoperative renal function between February 2023 and July 2024, and who subsequently developed acute kidney injury within 7 days postoperatively. The association between clinical and demographic characteristics and the outcome was assessed using the Chi-square or Fisher’s exact test. Univariable and multivariable linear regression were used to test for associations between various risk factors and the development of acute kidney injury.
Results: Total of 57% (n= 39) of patients were >60 years, with male preponderance (77%; n=53). Associated comorbidities included Type 2 diabetes mellitus (77%; n=53), hypertension (62%; n=43), atrial fibrillation (3%; n=2), stroke (9%; n=6), peripheral vascular disease (7%; n=5), anaemia (< 12gm%) (54%; n=37), hypoalbuminemia (52%; n=36)(serum albumin levels < 3.5 gm%), and hyperuricemia (33%; n=23) (serum uric acid level > 7mg%). Hospital mortality was observed in 7 patients (10%) with age >60 years (p=0.02), hypertension (p=0.04), use of cardiopulmonary bypass (p=0.02), and Stage 2/3 acute kidney injury (p=0.00005) being significantly associated. Hemodialysis was required in 4 patients (6%). Multivariate regression identified hyperuricemia as an independent predictor (p=0.02) for development of Stage 2/3 acute kidney injury (Odds ratio 42.33; 95% confidence interval 10.24-59.64).
Conclusions: Age >60 years, hypertension, on-pump coronary artery bypass grafting, and development of acute kidney injury are risk factors for early mortality in coronary artery bypass grafting patients. Preoperative hyperuricemia is a significant predictor of postoperative acute kidney injury.
Keywords: Coronary artery bypass grafting; acute kidney injury; KDIGO; predictors; outcomes
INTRODUCTION
Coronary artery bypass grafting (CABG) remains the most frequently performed cardiac surgery globally, with approximately 200,000 isolated procedures conducted annually in the United States alone. In Western Europe, the procedure has an average incidence rate of 62 per 100,000 residents. Due to the increasing prevalence of ischemic heart disease in the Indian population, the number of coronary artery bypass graft surgeries has risen significantly. Although percutaneous interventions are becoming more common, CABG remains the most reliable and optimal method of revascularization for coronary artery disease, as it extends life and offers greater symptom relief.
Acute kidney injury (AKI) is a frequent complication following coronary artery bypass grafting (CABG) and significantly heightens both short- and long-term risks of morbidity and mortality. The incidence of AKI after cardiac surgery ranges from 1% to 30%. Furthermore, AKI is linked to higher in-hospital mortality and an increased likelihood of progressing to chronic kidney disease (CKD). When AKI after cardiac surgery requires renal replacement therapy, the associated mortality rate can be as high as 25%. Even modest increases in postoperative serum creatinine levels are associated with considerable negative outcomes.
Therefore, identifying risk factors for AKI and developing effective preventive strategies are essential to reduce complications. Moreover, predicting potential mortality outcomes becomes increasingly important if these risk factors are non-modifiable. While some studies have explored the incidence and risk factors of AKI after CABG surgery using the Acute Kidney Injury Network (AKIN) and RIFLE criteria (risk, injury, failure, loss, and end-stage), most of these investigations are retrospective in nature. Fewer international studies have focused on the risk factors for AKI following cardiac surgery, including CABG, using the Kidney Disease: Improving Global Outcomes (KDIGO) classification. However, many of these studies are limited by small sample sizes and do not exclusively focus on CABG patients. Furthermore, there is a significant gap in research regarding the Indian population, where very few studies have assessed the risk factors for AKI after CABG using KDIGO criteria or explored the prognostic value of these factors in predicting hospital mortality.
We aimed to assess the risk factors and prognostic implications of AKI in CABG patients, under the KDIGO guidelines, specifically, who had normal preoperative renal function but developed AKI in the immediate postoperative period.
MATERIALS AND METHODS:
Ethics Statement
The JNMC Institutional Ethics Committee of KLES Academy of Higher Education and Research approved this study on 18.03.2023 and individual patient consent was obtained (Ref. No. MDC/JNMCIEC/76).
Patients
This observational study involved 69 patients who underwent elective isolated CABG with normal preoperative renal function, as indicated by serum creatinine levels and glomerular filtration rate (GFR), and who subsequently developed AKI within 7 days postoperatively. The study was conducted between February 2023 and July 2024. Eligibility criteria included patients over 18 years of age, with normal preoperative renal function and satisfactory urine output, who underwent elective isolated CABG. The inclusion threshold for left ventricular ejection fraction was set at >30%. Exclusion criteria included patients with preoperative renal dysfunction, a history of nephrotoxic drug use within one week prior to surgery, a history of preoperative hemodialysis, death within 12 hours post-surgery, a history of renal stones, or preoperative proteinuria.
Definition of AKI
The Kidney Disease: Improving Global Outcomes (KDIGO) criteria was used to define cardiac surgery-associated acute kidney injury:
- A rise in serum creatinine level by ≥ 0.3mg/dl (≥26.5μmol/l) within 48 hours of surgery.
- A rise in serum creatinine level to ≥1.5 times the baseline levels, which is known or presumed to have occurred within the prior 7 days.
- Urine volume <0.5ml/kg/hr for 6hr.
Acute kidney injury is staged for severity according to the following KDIGO criteria:
- Stage 1: Serum creatinine 1.5–1.9 times baseline OR ≥ 0.3mg/dl (≥ 26.5mmol/l) increase. Urine output < 0.5ml/kg/h for 6–12 hours.
- Stage 2: Serum creatinine 2.0–2.9 times baseline. Urine output < 0.5ml/kg/h for ≥ 12 hours.
- Stage 3: Serum creatinine 3.0 times baseline OR Increase in serum creatinine to ≥ 4.0mg/dl (≥ 353.6mmol/l) OR Initiation of renal replacement therapy OR, In patients < 18 years, decrease in eGFR to < 35ml/min per 1.73 m2. Urine output < 0.3ml/kg/h for ≥ 24 hours OR Anuria for ≥ 12 hours.
Methods
Informed written consent was obtained from all participants before inclusion in the study. Subjects were recruited based on the established inclusion criteria. A comprehensive medical history was taken, followed by a thorough physical examination of each patient. Patients diagnosed with AKI after CABG were evaluated using the KDIGO criteria within 7 days post-surgery. Basic laboratory tests, including complete blood counts, random blood glucose, renal function tests, liver function tests, HbA1c levels, lipid profile, and routine urine analysis with microscopic examination, were performed. Additional tests, such as serum procalcitonin, blood cultures, and urine cultures, were carried out as clinically indicated.
Patients were monitored for a period of 30 days, either in the ICU, hospital wards, or via telephonic follow-up when appropriate, to assess the mortality outcome.
Statistical Analysis
The data were de-identified and analyzed using SPSS statistical software (version 20.0). For continuous quantitative variables, the mean and standard deviation were computed. Intergroup continuous variables were compared using the unpaired Student’s t-test, while paired Student’s t-test was used to compare two quantitative variables within the same group. Categorical data were presented as rates, ratios, and percentages. The association between clinical and demographic characteristics and the outcome was assessed using the Chi-square test or Fisher’s exact test, as appropriate. Discrete variables were represented by their median values, and nonparametric tests were applied to compare them. Relevant graphical representations were employed to illustrate the comparisons. The association of risk factors for hospital mortality was assessed by either relative risk or odds ratio with a 95% confidence. Univariable and multivariable linear regression analyses were used to test for associations between various risk factors and the development of AKI. A p-value of less than 0.05 was considered statistically significant for all tests. The confidence interval was set at 95%, with a 5% probability of Type I error (α = 0.05). The study was powered at 80%.
The study sample size was calculated using the following formula: N = ZPQ/ E², where Z= the reliability coefficient at 95% confidence interval (3.841); P = the prevalence of disease considered as 58%; Q = (100- p) = 42; E = range of confidence interval (i.e. 20% of 58= 11.6).
RESULTS:
Study sample size
The value of N derived from the above formula was 73. Over the past three years, an average of 2-3 patients suffered AKI every month following CABG and were referred to the Nephrology Department at KLES Dr. Prabhakar Kore Hospital, Belgaum. Based on this trend, we included 69 patients in our study from February 2023 to July 2024.
Demographic Characteristics
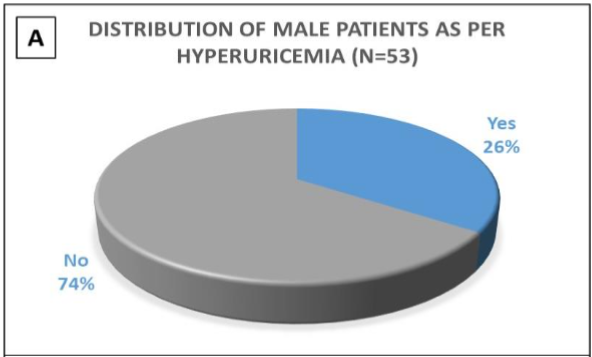
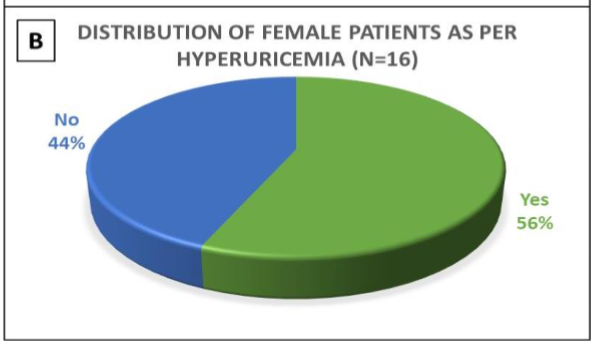
In this study, 57% (n= 39) of the patients were over 60 years of age, with male preponderance (77%; n= 53). The spectrum of comorbidities included Type 2 diabetes mellitus (77%; n= 53), hypertension (62%; n= 43), presence of atrial fibrillation (3%; n= 2), history of stroke (9%; n= 6), history of peripheral vascular disease (7%; n= 5), and presence of anaemia (< 12gm%) (54%; n= 37) including 3 females & 34 males. 52% of the patients had hypoalbuminemia (serum albumin levels < 3.5 gm%), including 8 females and 28 males. 92% of diabetic patients had uncontrolled blood sugar levels, with HbA1c values ranging from 6.6 to 14.2%. Hyperuricemia was present in 23 patients (33%) (serum uric acid level > 7mg%). Gender-wise distribution of hyperuricemia patients is depicted in Figures 1A and 1B.
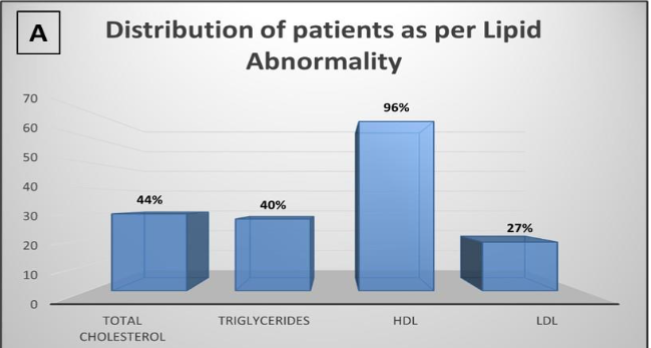
Total cholesterol was high in 30 patients (43%), triglycerides were high in 28 patients (41%), HDL cholesterol was low in 66 of them (96%), and LDL cholesterol was high in 19 patients (28%). The distribution of patients with various lipid abnormalities has been depicted in Figure 2A. There was no association between positive procalcitonin value and mortality or Stages of AKI in these patients (p > 0.05). All except one of our study participants had a left ventricular ejection fraction greater than 30%, with 49% (n= 34) falling within the 31-50% range and another 49% (n= 34) within the 51-60% range. None of the patients required intra-aortic balloon pump insertion in the perioperative period.
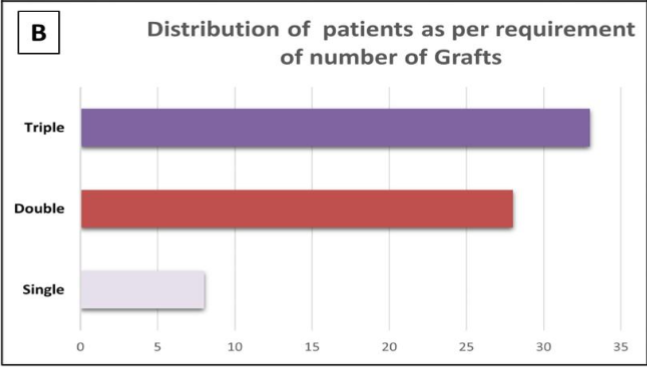
A total of 33 patients (48%) underwent triple bypass graft surgery, 28 patients (40%) received double bypass grafts, and 8 patients (12%) had a single bypass graft, as shown in Figure 2B. A total of 56% of patients (n=39) underwent surgery with cardiopulmonary bypass (CPB), indicating a balanced distribution of the data. Among these, 23 patients (59%) required a procedure time on CPB ranging from 70 to 110 minutes, while 15 patients (38%) had an on-pump time between 111 and 150 minutes. Only one patient (3%) had a pump time exceeding 150 minutes. Regarding aortic cross-clamp times, 16 patients (41%) had a cross-clamp duration between 30 to 60 minutes, 21 patients (54%) experienced a cross-clamp time of 60 to 90 minutes, and 2 patients (5%) had a cross-clamp time greater than 90 minutes.
Hospital mortality
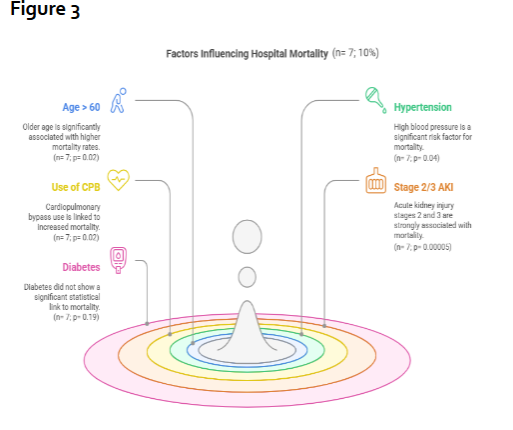
Hospital mortality was observed in 7 patients (10%), and 4 of them (6%) required hemodialysis postoperatively. Table 1 illustrates the correlation between various clinical parameters and hospital mortality. Statistically significant associations with hospital mortality were found with age > 60 years (p=0.02), hypertension (p=0.04), use of CPB (p=0.02), and Stage 2/3 AKI (p=0.00005). Figure 3 reveals all the factors that influenced hospital mortality. Surprisingly, all 7 patients were > 60 years old, had hypertension, underwent on-pump CABG, and suffered from Stage 2/3 AKI. Interestingly, while all seven deceased patients had diabetes, this association did not reach statistical significance (p=0.19). Other factors, including low left ventricular ejection fraction (LVEF) (p=0.55), anaemia (p=0.3), hypoalbuminemia (p=0.78), hyperuricemia (p=0.57), hypercholesterolemia (p=0.37), hypertriglyceridemia (p=0.5), and LDL dyslipidemia (p=0.95), were not significantly associated with mortality.
| Parameters | Mortality | Total | Fisher exact p-value | |
|---|---|---|---|---|
| Age >60 | 7 | 32 | 39 | 0.02 |
| ≤60 | 0 | 30 | 30 | |
| Sex F | 3 | 13 | 16 | 0.34 |
| M | 4 | 49 | 53 | |
| DM Yes | 7 | 46 | 53 | 0.19 |
| No | 0 | 16 | 16 | |
| HTN Yes | 7 | 36 | 43 | 0.04 |
| No | 0 | 26 | 26 | |
| AF Yes | 0 | 2 | 2 | 0.99 |
| No | 7 | 60 | 67 | |
| Stroke Yes | 0 | 6 | 6 | 0.99 |
| No | 7 | 56 | 63 | |
| PVD Yes | 0 | 5 | 5 | 0.99 |
| No | 7 | 57 | 64 | |
| CPB off | 0 | 30 | 30 | 0.02 |
| on | 7 | 32 | 39 | |
| AKI Stage I | 0 | 50 | 50 | 0.00005 |
| Stage II/ III | 7 | 12 | 19 |
Among the four deceased patients who required hemodialysis (6%), three exhibited both elevated procalcitonin levels and positive blood cultures, indicative of a systemic infectious process. Notably, all four patients also had preoperative hyperuricemia, with a mean serum uric acid level of 11.5 ± 0.77 mg%. While these findings may suggest a potential association between hyperuricemia/sepsis and adverse postoperative outcomes, the small sample size precluded any definitive statistical conclusion. Of the 3 deceased patients who did not require hemodialysis, 2 suffered from significant hypotension during surgery, and one had elevated procalcitonin levels and a positive blood culture.
Staging by KDIGO Criteria & Predictors for AKI
Approximately 20% of patients required a blood transfusion either during or after surgery, and these patients were subsequently diagnosed with acute kidney injury (AKI). Among the entire cohort, 50 patients (72%) developed Stage 1 AKI, 9 patients (13%) experienced Stage 2 AKI, and 10 patients (15%) progressed to Stage 3 AKI.
| Parameters | Mortality | RR(95%CI) | p-value | ||
|---|---|---|---|---|---|
| Age >60 | 11 | 28 | 39 | 0.99(0.27-3.66) | 0.89 |
| ≤60 | 8 | 22 | 30 | ||
| Sex Female | 8 | 8 | 16 | 4.89(1.04-23.00) | 0.045 |
| Male | 11 | 42 | 53 | ||
| DM Yes | 13 | 40 | 53 | 0.30(0.06-1.48) | 0.14 |
| No | 6 | 10 | 16 | ||
| HTN Yes | 13 | 30 | 43 | 1.58(0.39-6.36) | 0.52 |
| No | 6 | 20 | 26 | ||
| AF Yes | 1 | 1 | 2 | 2.11(0.17-22.66) | 0.56 |
| No | 17 | 49 | 66 | ||
| Stroke Yes | 1 | 5 | 6 | 0.58(0.12-3.88) | 0.45 |
| No | 18 | 45 | 63 | ||
| LVEF <50% | 9 | 23 | 32 | 1.16(0.33-4.06) | 0.81 |
| 50-60% | 10 | 27 | 37 | ||
| BT Yes | 6 | 8 | 14 | 2.12(0.44-6.17) | 0.86 |
| No | 13 | 42 | 55 | ||
| CPB off | 5 | 25 | 30 | 0.339(0.08-1.34) | 0.12 |
| on | 14 | 25 | 39 | ||
| Anemia Yes | 10 | 23 | 33 | 3.96(0.87-17.96) | 0.08 |
| No | 9 | 27 | 36 | ||
| Hypoalbuminemia Yes | 10 | 26 | 36 | 1.12(0.32-4.05) | 0.86 |
| No | 9 | 24 | 33 | ||
| Hyperuricemia Yes | 17 | 6 | 23 | 42.33(10.24-59.64) | 0.02 |
| No | 2 | 44 | 46 | ||
| Total cholesterol dyslipidemia | 9 | 22 | 31 | 1.29(0.10-16.76) | 0.85 |
| Normal | 10 | 28 | 38 | ||
| Triglycerides dyslipidemia | 8 | 20 | 28 | 2.00(0.16-34.51) | 0.64 |
| Normal | 11 | 30 | 41 | ||
| LDL dyslipidemia | 5 | 14 | 19 | 0.43(0.06-3.14) | 0.41 |
| Normal | 14 | 36 | 50 | ||
| HDL dyslipidemia | 2 | 1 | 3 | 2.26(0.19-7.18) | 0.86 |
| Normal | 17 | 49 | 66 | ||
| Hypotension Yes | 1 | 6 | 7 | 4.36(0.33-57.38) | 0.99 |
| No | 40 | 22 | 62 |
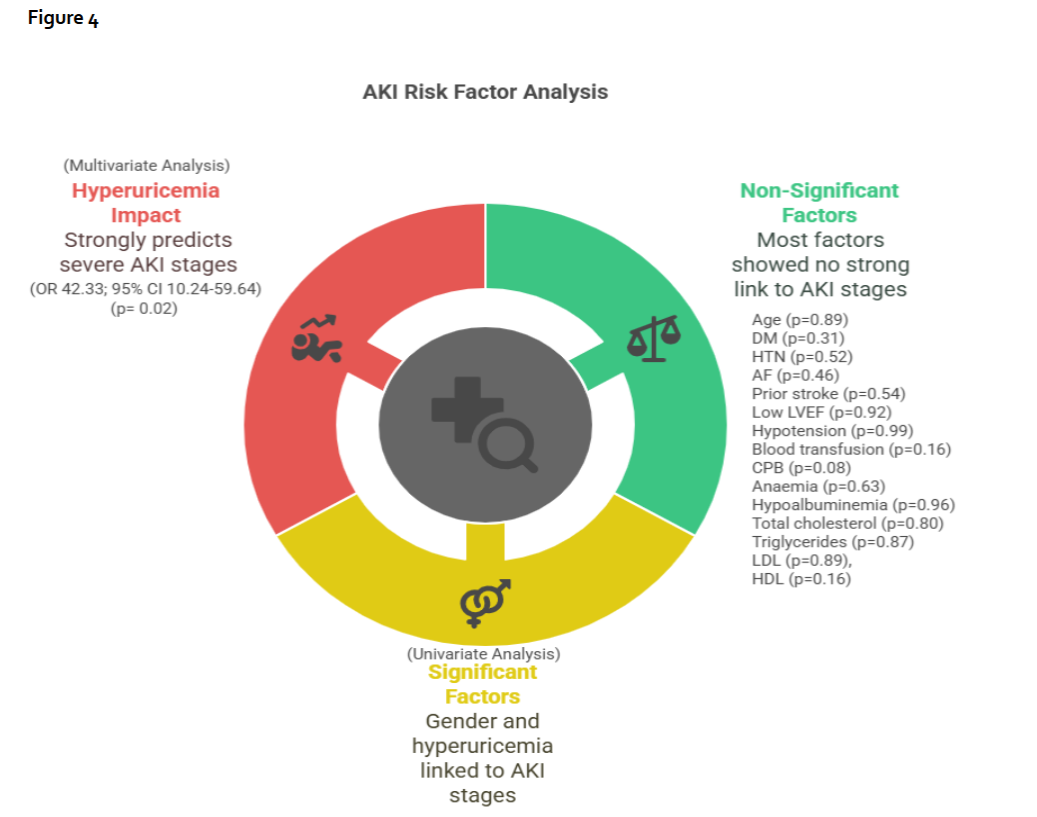
Further analysis through multivariate regression identified hyperuricemia as a strong predictor for the development of Stage 2 and Stage 3 AKI. Patients with hyperuricemia had odds of developing these more severe stages of AKI that were 42.33 times higher compared to those without hyperuricemia.
DISCUSSION:
Acute kidney injury (AKI) following coronary artery bypass grafting (CABG) surgery remains a significant concern due to its association with increased short-term and long-term morbidity and mortality. AKI is defined by a sudden decline in kidney function, with varying degrees of severity, and its occurrence after CABG has been linked to several factors, including the duration of cardiopulmonary bypass (CPB), pre-existing kidney dysfunction, and intraoperative complications.
The incidence of AKI after CABG varies depending on the criteria used for its definition, ranging from mild elevations in serum creatinine to the need for dialysis. Several studies report an incidence of AKI following CABG ranging from 10% to 40%, with the severity of AKI also varying widely. A study by Brown et al. found that 16.3% of patients undergoing CABG developed AKI, with 5.4% requiring dialysis. This is in line with a study by Rydén et al., which demonstrated that AKI following CABG was associated with a 2- to 3-fold increase in long-term mortality.
Acute kidney injury after CABG is also associated with an increased risk of cardiovascular events, including myocardial infarction (MI), and adverse outcomes such as prolonged mechanical ventilation, extended ICU stays, and higher rates of infection. A recent study by Cheruku et al. noted that AKI following cardiac surgery not only increases the immediate postoperative mortality but also contributes significantly to long-term kidney dysfunction, potentially leading to end-stage renal disease. The long-term survival of CABG patients with AKI can be severely impacted, even with mild increases in serum creatinine. This underscores the need for early identification and management of AKI to improve outcomes.
A total of 19 patients (28%) in our study group developed AKI following CABG. Among these, 4 patients (6%) required hemodialysis and 7 patients (10%) were deceased. Among the deceased patients, age > 60 years, comorbid hypertension, use of CPB, and AKI itself were significant associates. This highlights the critical importance of proactive management and treatment of hypertension preoperatively, as well as a crucial need to reconsider the use of the CPB during CABG.
Risk Factors for AKI
The development of AKI after CABG is influenced by several risk factors. Some of these risk factors are modifiable, while others are inherent to the patient’s preoperative condition. Intraoperative factors such as CPB, prolonged cross-clamp times, and low cardiac output are well-established contributors to AKI. According to the study by Karim et al., the duration of CPB and the cross-clamp time were strongly correlated with the development of AKI. Prolonged CPB time leads to systemic inflammatory responses and microcirculatory disturbances that compromise renal perfusion, contributing to AKI. Similarly, low cardiac output during the postoperative period can further exacerbate renal ischemia and lead to kidney dysfunction.
Preoperative conditions such as pre-existing chronic kidney disease (CKD), diabetes mellitus, and hypertension are also significant risk factors for AKI after CABG. Patients with CKD are at a higher baseline risk for developing AKI due to reduced renal reserve and pre-existing endothelial dysfunction. Furthermore, diabetes and hypertension contribute to the pathophysiology of AKI by promoting endothelial injury, oxidative stress, and glomerular hyperfiltration, all of which increase the risk of kidney injury. In particular, the presence of diabetes has been shown to increase the incidence and severity of AKI following CABG.
| Risk Factors | Rydén L et al | Hou et al | Yang et al | Our Study |
|---|---|---|---|---|
| DM | 30% | 40% | 40% | 77% |
| HTN | 67% | 65% | 23% | 62% |
| AF | – | 3.2% | – | 3% |
| Stroke | – | 11% | – | 9% |
| PVD | 13% | 10% | 7% | – |
In addition to these preoperative and intraoperative factors, several biochemical markers have been identified as predictors of AKI. Serum creatinine and blood urea nitrogen (BUN) levels are the standard biomarkers that have been shown to correlate with AKI severity and outcomes. A study by Zakkar et al. found that elevated preoperative serum uric acid levels were significantly associated with an increased risk of AKI after CABG. Similar findings were noted by Tang H. et al. and Kaufeld T. et al. Hyperuricemia, which often accompanies comorbidities like hypertension and diabetes, may play a role in renal tubular injury and oxidative stress, both of which contribute to the development of AKI.
In this study, preoperative hyperuricemia emerged as the sole independent predictor of postoperative AKI after performing multivariate regression analysis. This highlights the importance of effective preoperative management of hypertension, diabetes, and serum uric acid levels to potentially reduce the risk of postoperative AKI.
Mechanisms of AKI
The pathophysiology of AKI after CABG is multifactorial, involving both ischemic and non-ischemic mechanisms. The most common etiology is acute tubular necrosis (ATN), which occurs due to a combination of hypoperfusion, inflammation, and endothelial dysfunction. During CABG, the use of CPB causes a systemic inflammatory response that can lead to endothelial injury and microvascular thrombosis, impairing renal perfusion and oxygenation. These changes can contribute to renal ischemia, which is exacerbated by the hemodynamic instability often observed in the postoperative period.
In addition to ischemic injury, other mechanisms such as oxidative stress, inflammation, and tubular injury play significant roles in the development of AKI. The systemic inflammatory response triggered by CPB is characterized by the release of pro-inflammatory cytokines, which can induce endothelial dysfunction and increase vascular permeability, further compromising renal perfusion. Furthermore, the activation of the renin-angiotensin-aldosterone system during CABG surgery can exacerbate renal vasoconstriction and contribute to the progression of kidney injury.
In some cases, the use of nephrotoxic agents, such as certain antibiotics and contrast agents, can also contribute to the development of AKI after surgery. However, the contribution of these agents is less significant compared to the intraoperative and postoperative hemodynamic changes and systemic inflammatory responses associated with CABG.
Preventive Strategies and Management
Preventing and managing AKI after CABG remains a major challenge. Several strategies have been proposed to mitigate the risk of AKI, including optimization of hemodynamic parameters, such as maintaining adequate perfusion pressure and minimizing the duration of CPB. Preoperative optimization of renal function, such as ensuring adequate hydration and managing comorbid conditions like diabetes and hypertension, can reduce the risk of AKI. Pharmacological interventions, including the use of renoprotective agents such as statins and angiotensin-converting enzyme (ACE) inhibitors, have also shown promise in reducing the incidence and severity of AKI.
In the postoperative period, careful monitoring of kidney function and the early identification of AKI are critical for improving outcomes. The use of biomarkers such as urinary NGAL (neutrophil gelatinase-associated lipocalin) has been suggested as an early predictor of AKI, allowing for earlier intervention and potentially reducing the risk of long-term renal damage. Dialysis remains the primary treatment for patients with severe AKI requiring renal replacement therapy, but the optimal timing and modality of dialysis are still subjects of ongoing research.
This study is one of the few from India that specifically examines AKI in post-CABG patients with normal preoperative renal function, as defined by KDIGO guidelines. A key strength of our study lies in the stratification of patients based on AKI stages, which enabled us to analyze the risk factors associated with each stage systematically. This approach provided a deeper understanding of the progression of AKI and helped pinpoint specific risk factors relevant to different stages. Furthermore, we assessed mortality rates and identified the corresponding risk factors, offering valuable insights into the determinants of patient outcomes following CABG.
LIMITATIONS
This was a single-centre, prospective study with a relatively small sample size, which may have limited the statistical power and precision of our findings. Although AKI was diagnosed using KDIGO criteria, urine output was not included as a diagnostic criterion since many post-surgical patients were receiving diuretics. Additionally, most patients were on multiple medications, including antibiotics, which could have contributed to acute interstitial nephritis. However, detailed data on this potential factor was not available and fell outside the scope of this study.
CONCLUSIONS
Age over 60 years, hypertension, on-pump CABG, and the development of AKI have been identified as independent risk factors for mortality in CABG patients. Mortality rates are notably higher in patients requiring renal replacement therapy compared to those who do not, emphasizing the critical need for preventing severe AKI. Additionally, preoperative hyperuricemia is strongly associated with the occurrence of postoperative AKI following CABG. This study highlights the importance of early identification and proactive management of these risk factors to improve outcomes in post-CABG care.
Conflict of Interest:
The authors have no relevant financial or non-financial interests to disclose.
Funding Statement:
None.
Data availability:
Data transparency is provided; the master chart will be shared on request.
Ethics approval:
The JNMC Institutional Ethics Committee of KLES Academy of Higher Education and Research approved this study on 18.03.2023, and individual patient consent was obtained (Ref. No. MDC/JNMCIEC/76).
Statement of human and animal rights:
This dissertation upholds human and animal rights principles. All research activities were conducted following the highest ethical standards to ensure the dignity, welfare, and rights of all human participants, and no animals were involved.
ACKNOWLEDGEMENTS:
None
Glossary of Abbreviations:
- AKI Acute Kidney Injury
- AKIN Acute Kidney Injury Network
- CABG Coronary Artery Bypass Grafting
- CKD Chronic Kidney Disease
- CPB Cardiopulmonary Bypass
- GFR Glomerular Filtration Rate
- HDL High density lipoprotein
- ICU Intensive Care Unit
- KDIGO Kidney Disease- Improving Global Outcomes
- LDL Low density lipoprotein
- LVEF Left Ventricular Ejection Fraction
- RIFLE Risk, Injury, Failure, Loss, and End-Stage
Figure Legends:
Figure 1- 1A is a pie diagram showing the incidence of hyperuricemia in the male population of the study group. 1B is another pie diagram showing the incidence of hyperuricemia in the female population of the study group.
Figure 2- 2A is a bar diagram showing the distribution of the study population as per the different lipid abnormalities. 2B is another bar diagram showing the number of patients as per the number of grafts received during the coronary artery bypass grafting.
Figure 3-Demonstrates the factors influencing hospital mortality in this study group. AKI Acute kidney injury; CPB Cardiopulmonary bypass.
Figure 4-Demonstrates the risk factors for the development of acute kidney injury (AKI) following univariate and multivariate analysis. AF Atrial fibrillation; BT Blood transfusion; CPB Cardiopulmonary bypass; DM Diabetes mellitus; HDL High density lipoprotein; HTN Hypertension; LDL Low density lipoprotein; LVEF Left ventricular ejection fraction.

