Aesthetic Impacts of Hyaluronidase: Case Report Insights
Aesthetic Impacts of Elective Hyaluronidase Dissolution: A Case Report Highlighting the Restorative Benefits of Dermal Fillers
Sylvia Paz Baens Ramirez, MD, MPH, MBA ¹; Gunther Scherz, MBA ¹
¹ Cutis Medical Laser Clinics, 9 Scotts Road #08-07, Singapore 228210
OPEN ACCESS
PUBLISHED: 30 June 2025
CITATION Ramirez, SPB., and Scherz, G., 2025. Aesthetic Impacts of Elective Hyaluronidase Dissolution: A Case Report Highlighting the Restorative Benefits of Dermal Fillers. Medical Research Archives, [online] 13(6). https://doi.org/10.18103/mra.v13i6.6650
COPYRIGHT © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.18103/mra.v13i6.6650
ISSN 2375-1924
Abstract
Introduction and Importance: Dermal fillers are pivotal in aesthetic enhancement, offering minimally invasive solutions for facial rejuvenation. This case underscores the stark contrast in appearance following elective hyaluronidase dissolution of long-term fillers and examines the challenges of transitioning to fat transfer as an alternative.
Presentation of Case: A 50+ year-old Asian female, with a history of dermal fillers over 10 years, sought hyaluronidase dissolution to facilitate fat transfer. The dramatic aging effects post-dissolution left the patient deeply dissatisfied. Subsequent fat transfer in another country failed to meet aesthetic expectations, despite undergoing 2 sessions.
Clinical Discussion: This report highlights the transformative benefits of dermal fillers, the risks associated with high-dose hyaluronidase, and the complexities of achieving comparable outcomes with fat transfer.
Conclusion: Properly placed fillers provide profound aesthetic benefits. The case underscores the importance of careful evaluation before administration of hyaluronidase.
Keywords:
Hyaluronidase, Dermal Fillers, Post-Hyaluronidase Syndrome, Aesthetic Medicine, Fat Transfer, Facial Rejuvenation
1. Introduction
Dermal fillers, particularly those composed of hyaluronic acid (HA), have become an indispensable tool in aesthetic medicine, offering effective solutions for addressing the signs of aging and enhancing facial contours. Hyaluronic acid (HA) fillers mimic the natural substance found in human skin, making them highly versatile for applications such as restoring volume in cheeks, sculpting jawlines, filling nasolabial folds, and rejuvenating lips. Their biocompatibility and ability to integrate seamlessly into the dermis have made HA fillers the most widely used class of fillers, with over 4 million HA filler procedures performed globally in 2020 alone. In addition to their aesthetic benefits, HA fillers have also shown promise in medical applications, such as addressing lipoatrophy in HIV patients and reconstructing facial asymmetry in trauma cases. These advancements underscore the transformative potential of dermal fillers when administered judiciously.
However, the increasing popularity of dermal fillers has brought to light concerns regarding overfilling, where excessive product use can lead to an unnatural or distorted appearance. Overfilling, colloquially referred to as “filler fatigue”, is often driven by either unrealistic patient expectations or practitioners’ overzealousness in delivering exaggerated results. Studies have shown that overfilled faces can appear aged or deformed due to altered facial proportions and excessive tissue expansion. This phenomenon has sparked widespread discussions about the need for moderation, with a focus on subtle, restorative approaches that prioritize maintaining facial harmony, a perspective further amplified by high-profile media coverage of celebrities with visibly overfilled faces, such as the public critique of filler use among reality television stars, thus increasing awareness and caution among both patients and practitioners.
The emergence of hyaluronidase, an enzyme that degrades hyaluronic acid by cleaving its β1,4-glycosidic bonds, has reshaped the management of complications and aesthetic dissatisfaction in filler treatments. Hyaluronidase is an enzyme that catalyzes the breakdown of hyaluronic acid, facilitating the rapid dissolution of HA-based fillers. Initially employed for medical emergencies, such as vascular occlusion resulting from inadvertent filler injection into blood vessels, its use has expanded to address aesthetic concerns like asymmetry, nodules, and overfilling. For example, a study by DeLorenzi highlighted the life-saving potential of hyaluronidase in cases of filler-induced vascular compromise, emphasizing its critical role in mitigating severe complications. Beyond emergencies, hyaluronidase has become a “go-to solution” for patients seeking to reverse the effects of poorly executed or unwanted filler treatments, with many clinics offering it as part of routine aesthetic services. Despite its utility, the application of hyaluronidase carries inherent risks that require careful consideration. Adverse reactions, including localized swelling, bruising, and allergic responses, are well-documented, with rare cases of anaphylaxis reported in patients with hypersensitivity to the enzyme. Additionally, the enzymatic action of hyaluronidase is non-selective, meaning it can degrade both filler material and endogenous hyaluronic acid, potentially compromising native tissue integrity. A study by Alam et al. emphasized the importance of precise dosing and placement to minimize unintended tissue damage, particularly in areas with thin skin or limited native HA reserves.
Excessive or indiscriminate application of hyaluronidase to dissolve dermal fillers may paradoxically result in suboptimal aesthetic outcomes. The enzyme, while effective in degrading hyaluronic acid-based fillers, can unintentionally affect the surrounding extracellular matrix, including endogenous hyaluronic acid and other structural components critical for skin integrity and appearance. This unintended action may lead to volume loss, uneven skin texture, or changes in tissue elasticity, ultimately compromising the desired cosmetic results. Careful dosing and precise localization are essential to mitigate these risks and maintain favorable aesthetic outcomes.
In this case report, we describe the hyaluronic acid filler treatment of a patient, achieving high patient satisfaction, followed by elective dissolution of the dermal fillers in preparation for fat transfer and other surgical procedures. This case report aims to illustrate the restorative benefits of dermal fillers by exploring the outcomes of elective hyaluronidase dissolution in a patient who sought to reassess her aesthetic goals. This case underscores the stark contrast in appearance following elective hyaluronidase dissolution of long-term fillers and examines the challenges of transitioning to fat transfer as an alternative. By reversing previous filler treatments, the case highlights the return to a baseline appearance and underscores the transformative potential of properly administered dermal fillers. The report seeks to contribute to the ongoing conversation about the role of fillers in achieving balanced and harmonious aesthetic outcomes, demonstrating that when used judiciously, they can enhance not only appearance but also patient confidence and satisfaction.
2. Complete Treatment over last 5 years
The SCARE Guidelines were used to ensure completeness of this case report submission.
3. Patient Information
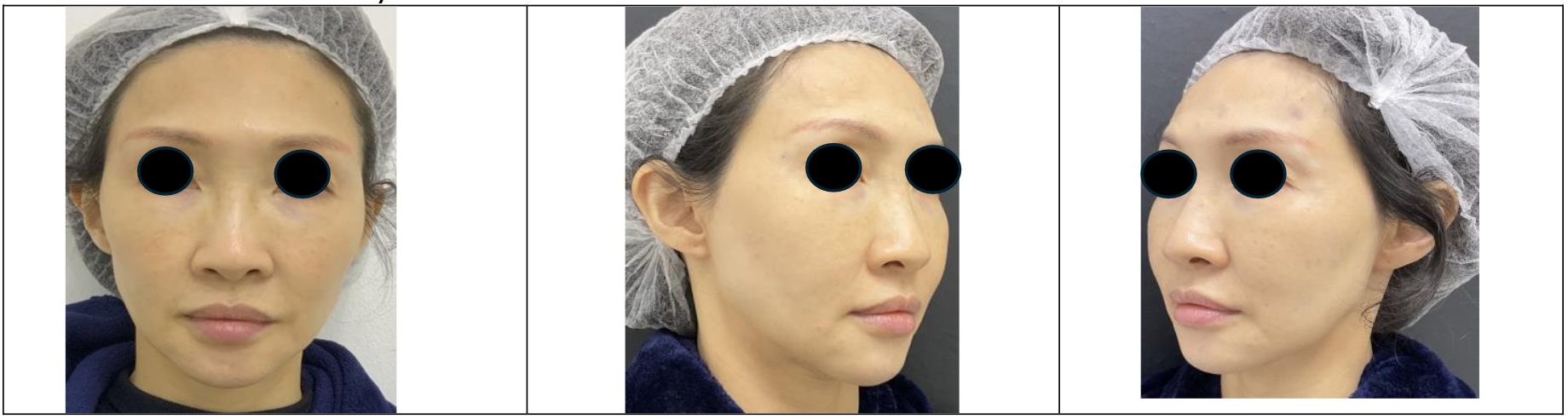
The patient was a 50+ year-old Asian female who had received regular hyaluronic acid dermal fillers over the preceding 10 years for aesthetic purposes. Treatment areas included the temples, cheeks, tear troughs, nasolabial folds, marionette lines, jawline, chin, and lips. Fillers from the Juvederm Vycross range were used to address volume loss and contour deficiencies with a total volume over ten years as shown in Table 1 below. The most recent post filler treatment images (pre-hyaluronidase) are shown in Figure 1a.
| Filler Composition | Areas of Treatment | Years of Treatment | Total Volume in 10 years | Last Treatment Volume in Last Treatment |
|---|---|---|---|---|
| Vycross 20mg/ml and Vycross 17.5mg/ml | Midface | 2014, 2018 | 4ml | 2018 0.5ml |
| Vycross 20mg/ml | Temple | 2014, 2016, 2018, 2019, 2020 | 9ml | 2020 2ml |
| Vycross 20mg/ml and Vycross 15 mg/ml | Lower Face | 2014, 2019, 2020, 2023, 2024 | 14ml | 2024 2ml |
| Vycross 17.5mg/ml and Vycross 15 mg/ml | Nasolabial fold | 2014, 2018, 2019, 2023, 2024 | 5ml | 2024 1ml |
| Vycross 15 mg/ml and Vycross 12 mg/ml | Tear Trough | 2014, 2018, 2017, 2019 | 5ml | 2019 1ml |
| Vycross 20 mg/ml | Chin | 2014, 2017, 2018, 2023 | 4ml | 2023 1ml |
| Vycross 17.5mg/ml and Vycross 15 mg/ml | Marionette Lines | 2020, 2023 | 2ml | 2023 1ml |
Note: Summary of dermal fillers received by the patient from initial treatment in 2014 to most recent treatment in 2024 by treatment area. All fillers received were from the Juvederm Vycross range with hyaluronic acid concentrations ranging from 12 to 20 mg/ml, following manufacturer’s guidelines on types of fillers recommended by treatment area.
Over a 10-year period, the patient received a total of 43 ml of dermal fillers, with 22 ml administered in the last five years (2019-2024), including a final treatment of 4 ml in May 2024. Her most recent post-filler photograph where the patient reported high levels of patient satisfaction is shown in Figure 1a.
In September 2024, the patient expressed a desire to transition to autologous fat transfer for long-term facial rejuvenation. She intended to undergo the fat transfer in Korea, a popular destination for plastic surgery for patients from Singapore. In her goal of achieving a permanent volumization with fat transfer, existing dermal fillers needed to be dissolved for accurate assessment of fat transfer volume requirements for full face treatment.
Patient consulted a plastic surgeon, who initiated aggressive filler dissolution using hyaluronidase to achieve an accurate anatomical baseline for fat transfer. While the exact dosage and technique were not disclosed, the dissolution was completed in a single session. Patient recalls hyaluronidase administration in the temple, midface, tear trough, lower face, marionette, nasolabial folds and chin areas. The patient was not counselled on the potential for post-hyaluronidase dissolution volume loss or associated aesthetic changes.
4. Clinical Findings
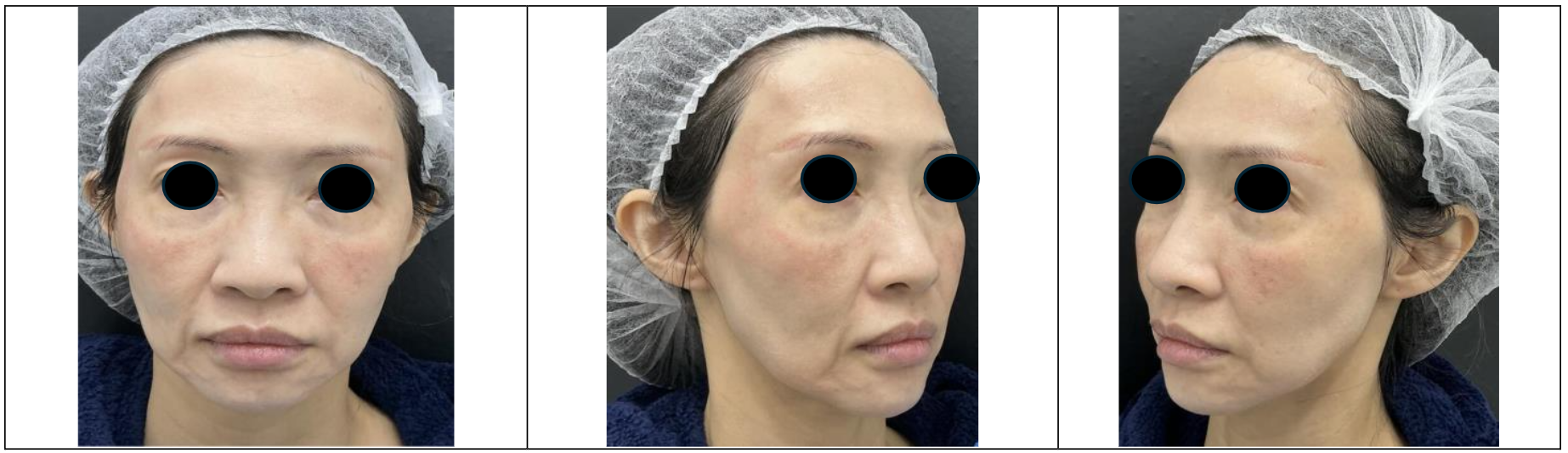
After the high-dose hyaluronidase treatment, the patient returned for consult under extreme distress. More specifically, her quoted statement is as follows: “I deeply regret dissolving my fillers. I had no idea how much they improved my appearance until they were gone.”
On assessment, profound facial changes were observed after hyaluronidase treatment including an overall appearance of looking extremely tired, aged, and sad. Specific changes noted include:
- Tear troughs: Pronounced hollowing with visible under-eye bags.
- Midface and cheeks: Severe volume loss, leading to sagging and deepened nasolabial folds.
- Temples: Significant hollowing.
- Jawline and lower face: Loss of definition, contributing to a prematurely aged appearance.
The patient estimated that these changes made her look approximately 10 years older. She expressed shock and regret, stating she was unprepared for the dramatic effects of filler removal.
5. Diagnostic Assessment
The diagnosis of post-hyaluronidase syndrome was made based on clinical examination and patient history, consistent with reports of adverse outcomes following aggressive filler dissolution. The high dose of hyaluronidase likely contributed to accelerated degradation of endogenous HA, compounding the effects of filler removal.
6. Fat Transfer
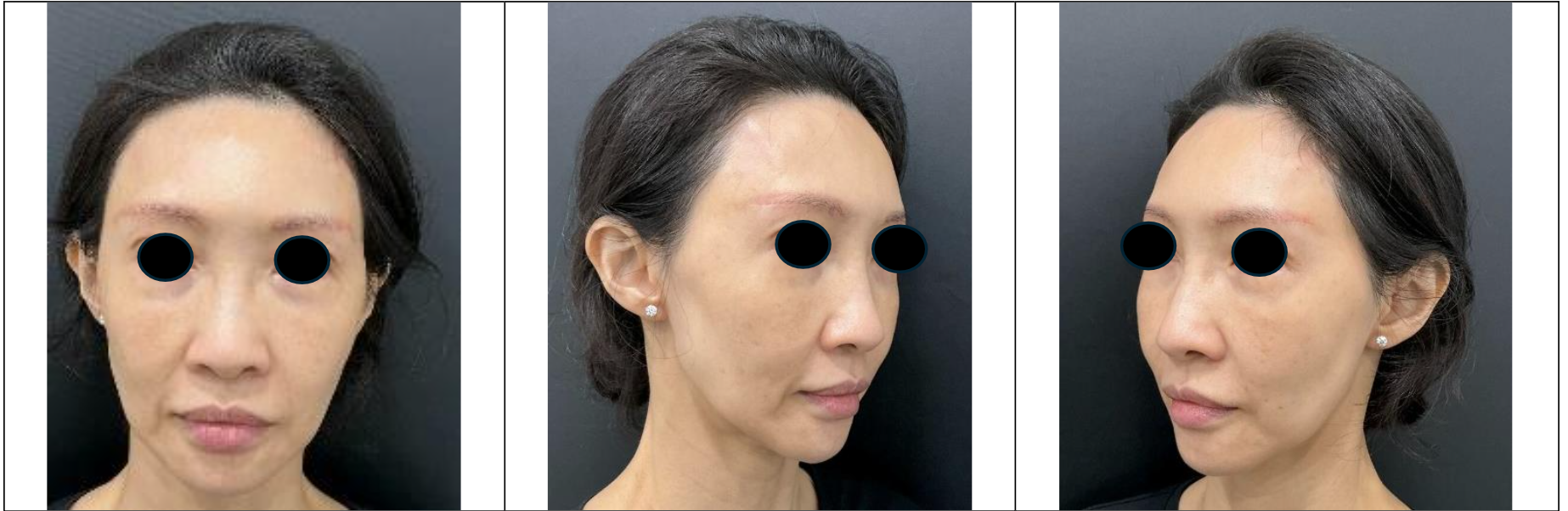
Approximately two months later, the patient underwent fat transfer in Korea. Fat was harvested from her abdomen and thighs, then processed and injected into the face to address volume deficiencies in the temples, midface, nasolabial folds, and jawline. Despite the procedure, the patient reported suboptimal outcomes, particularly in the midface, where the fat grafting failed to restore the natural fullness previously achieved with fillers.
7. Outcomes
While the fillers had provided consistent and predictable rejuvenation, the fat transfer results were uneven and failed to address all aesthetic concerns. The patient remains incompletely treated despite 2 sessions of fat transfer and continues to look older than her pre-dissolution state.
8. Patient Perspective
Her quoted statement after fat transfer is as follows – “The fat transfer sessions didn’t help as much as I hoped, and I still feel incomplete. I wish I had been better informed.”
9. Discussion
Against this backdrop, this case report explores the aesthetic and psychological outcomes of elective hyaluronidase dissolution in a patient who specifically desired filler reversal in order to undergo facial fat transfer. Complete hyaluronic acid filler dissolution was required prior to any such surgical intervention to achieve a more accurate assessment of volume requirements with fat transfer. By reversing previous filler treatments, the patient achieved a renewed appreciation for her features resulting from treatment with hyaluronic acid fillers. The patient was notably struck by the aging changes of her natural features had fillers not been administered in the first place. This highlights the role of properly placed fillers which offer profound rejuvenative effects, masking volume loss and enhancing facial contours. Their reversibility, when performed judiciously, adds to their appeal.
Our case also demonstrates that aggressive dissolution of fillers can unmask underlying aging changes, leading to patient dissatisfaction. The enzyme’s effects on native HA may exacerbate volume loss and skin laxity; however, its application is not without controversy. There is currently no standardized protocol for the concentration or dosage of hyaluronidase in elective filler reversal, and dosage adjustments often depend on the filler’s resistance to enzymatic degradation, which varies with modifications such as cross-linking. For instance, highly cross-linked fillers, including the VYCROSS range, demonstrate greater resistance, necessitating higher or repeated doses for dissolution. The mean total dose used in a published study was 403 ± 191 units, with a wide range of 19–900 units, and an average of 35 units of hyaluronidase was used to treat every 0.1 mL of filler where the volume was known.
The degradation kinetics of fillers depend significantly on their manufacturing technology, as highlighted in a comparative study of 16 fillers using various cross-linking approaches. VYCROSS fillers, such as Juvéderm Voluma and Volux, were the most resistant to hyaluronidase-mediated degradation, likely due to their dense cross-linking and tightly packed hyaluronic acid chains. Conversely, fillers employing the Cohesive Polydensified Matrix (CPM) technology, like Belotero Balance, showed the least resistance to degradation. The study observed that the Preserved Network Technology (PNT) fillers, such as RHA products, demonstrated a more proportional and predictable degradation profile relative to their cross-linking degree and hyaluronic acid concentration.
Interestingly, while higher elastic modulus (G’) generally correlated with increased resistance to degradation for monophasic fillers, this trend was not observed for biphasic fillers like those using Non-Animal Stabilized Hyaluronic Acid (NASHA) technology. This indicates that filler composition and rheological properties interact uniquely with hyaluronidase. For example, NASHA fillers, despite having the highest G’ among the tested products, exhibited uniform degradation profiles due to their particulate structure, which facilitates enzymatic penetration. These findings suggest that the selection of filler type and associated manufacturing technology must account for their differential reversibility in clinical scenarios, especially in high-risk or delicate anatomical regions.
The phenomenon of post-hyaluronidase syndrome, characterized by facial hollowing, loss of skin elasticity, and worsening rhytids, was observed in 18% of patients in a retrospective study, with significant correlations identified between the syndrome and the volume and duration of filler in situ. Notably, the study found no relationship between hyaluronidase concentration or dose and the incidence of adverse outcomes. However, the term “post-hyaluronidase syndrome” was applied broadly, encompassing a range of adverse outcomes without standardized diagnostic criteria, which limits the precision of these findings. The duration of filler in situ before dissolution was notably longer in affected patients, with a mean duration of 60 ± 35 months compared to 24 ± 35 months in asymptomatic individuals. Larger volumes of filler, averaging 4 ± 2.2 mL in patients with adverse outcomes compared to 1.5 ± 2.0 mL in others, were also significantly associated with negative aesthetic effects.
Magnetic resonance imaging has demonstrated that HA fillers can persist for years—up to 12 years in some cases—depending on injection site, filler properties, and patient metabolism. These findings underscore the potential for long-term tissue changes during the filler’s presence and its implications upon dissolution. Furthermore, natural age-related changes that occur while fillers are in situ likely contribute to the post-dissolution appearance, making it unclear whether these changes are purely due to hyaluronidase or an interplay of factors. These adverse outcomes highlight the importance of precise technique and judicious application of hyaluronidase by experienced practitioners.
Hyaluronidase’s utility remains invaluable. In our patient, its use reversed dermal filler application that notably enhanced her appearance. The outcome underscores that while hyaluronidase is a powerful tool for resetting and recalibrating aesthetics, the process often reveals the critical role of fillers in maintaining a youthful appearance. Enhanced patient counseling and a phased approach to dissolution could mitigate adverse outcomes. Ultrasound-guided hyaluronidase injection and staged fat transfer may offer improved results.
Adhering to popular media trends and influencer-driven recommendations advocating for the avoidance or dissolution of dermal fillers may inadvertently limit the aesthetic enhancements achievable through non-invasive procedures. Such generalized guidance, often lacking individualized clinical evaluation, can discourage the optimal use of dermal fillers, which are integral to restoring facial volume, enhancing symmetry, and improving skin contours. Overgeneralized dissolution practices or avoidance may impede tailored treatment plans, thereby reducing the potential for personalized, high-quality aesthetic outcomes.
This case also demonstrates the importance of skilled filler application to avoid complications and optimize outcomes. Proper placement of fillers, consideration of anatomical nuances and patient-specific aging patterns, ensures results that enhance rather than distort facial aesthetics. Tailored application minimizes the risks of overfilling and the need for corrective dissolution.
10. Conclusion
By examining the outcomes of elective filler dissolution, this case report seeks to contribute to the ongoing conversation about the evolving standards of beauty and the role of aesthetic medicine in achieving balanced results. It emphasizes the importance of thorough consultation, precise technique, and patient-centered care in ensuring optimal aesthetic outcomes. Furthermore, it highlights the critical role of hyaluronidase in safeguarding against overfilling, enabling both practitioners and patients to recalibrate their approach to facial enhancement.
In conclusion, this case underscores the potential for dermal fillers to deliver transformative results when administered judiciously as well as the importance of hyaluronidase as a corrective tool. It calls for continued education on the risks and benefits of filler reversal, advocating for a nuanced understanding of how these interventions can support the pursuit of aesthetic harmony and patient satisfaction. By documenting this patient’s journey, the report aims to inspire greater awareness and responsibility in the application of aesthetic treatments, ultimately advancing the practice of cosmetic medicine.
Ethical Approval
The case report was conducted in accordance with the SCARE 2023 Guidelines. Ethical approval was not required for this type of report.
Informed Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Funding
None.
Conflicts of Interest
Sylvia Ramirez is a consultant, speaker, and investigator for Allergan Aesthetics, an AbbVie company. Gunther Scherz declares no conflict of interest.
Author Contributions
Dr. Sylvia Ramirez conceptualized the report, conducted the clinical work, and led manuscript preparation. Gunther Scherz contributed to literature review, manuscript editing, and formatting.
11. References
- C. de la Guardia, A. Virno, M. Musumeci, A. Bernardin, and M. B. Silberberg, “Rheologic and Physicochemical Characteristics of Hyaluronic Acid Fillers: Overview and Relationship to Product Performance,” Facial Plast. Surg. FPS, vol. 38, no. 2, pp. 116–123, Apr. 2022, doi: 10.1055/s-0041-1741560.
- E. Papakonstantinou, M. Roth, and G. Karakiulakis, “Hyaluronic acid: A key molecule in skin aging,” Dermatoendocrinol., vol. 4, no. 3, pp. 253–258, Jul. 2012, doi: 10.4161/derm.21923.
- “American Society of Plastic Surgeons,” American Society of Plastic Surgeons. Accessed: May 13, 2025. [Online]. Available: https://www.plasticsurgery.org/
- D. Ho and J. Jagdeo, “Safety and Efficacy of a Volumizing Hyaluronic Acid Filler for Treatment of HIV-Associated Facial Lipoatrophy,” JAMA Dermatol., vol. 153, no. 1, pp. 61–65, Jan. 2017, doi: 10.1001/jamadermatol.2016.3827.
- R. J. Rohrich et al., “Facial soft-tissue fillers: Assessing the state of the science conference – Proceedings report,” Plast. Reconstr. Surg., vol. 127, no. SUPPL. 4 S, pp. 9S-21S, Apr. 2011, doi: 10.1097/PRS.0b013e31820578b9.
- S. Cotofana, R. H. Gotkin, K. Frank, N. Lachman, and T. L. Schenck, “Anatomy Behind the Facial Overfilled Syndrome: The Transverse Facial Septum,” Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. Al, vol. 46, no. 8, pp. e16–e22, Aug. 2020, doi: 10.1097/DSS.0000000000002236.
- “The filler industry bombshell that triggered a panicked ‘glow-down’ trend,” Cosmopolitan. Accessed: May 13, 2025. [Online]. Available: https://www.cosmopolitan.com/uk/beauty-hair/celebrity-hair-makeup/a35708681/celebrities-dissolve-filler-amount-tweakment/
- H. Jung, “Hyaluronidase: An overview of its properties, applications, and side effects,” Arch. Plast. Surg., vol. 47, no. 4, pp. 297–300, Jul. 2020, doi: 10.5999/aps.2020.00752.
- D. Funt and T. Pavicic, “Dermal fillers in aesthetics: an overview of adverse events and treatment approaches,” Plast. Surg. Nurs. Off. J. Am. Soc. Plast. Reconstr. Surg. Nurses, vol. 35, no. 1, pp. 13–32, 2015, doi: 10.1097/PSN.0000000000000087.
- F. D and P. T, “Dermal fillers in aesthetics: an overview of adverse events and treatment approaches,” PubMed, 2013, Accessed: May 30, 2025. [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/24363560/
- C. DeLorenzi, “Complications of Injectable Fillers, Part 2: Vascular Complications,” Aesthet. Surg. J., vol. 34, no. 4, pp. 584–600, May 2014, doi: 10.1177/1090820X14525035.
- G. Murray, C. Convery, L. Walker, and E. Davies, “Guideline for the Safe Use of Hyaluronidase in Aesthetic Medicine, Including Modified High-dose Protocol,” J. Clin. Aesthetic Dermatol., vol. 14, no. 8, pp. E69–E75, Aug. 2021.
- M. Alam et al., “Effectiveness of Low Doses of Hyaluronidase to Remove Hyaluronic Acid Filler Nodules: A Randomized Clinical Trial,” JAMA Dermatol., vol. 154, no. 7, pp. 765–772, Jul. 2018, doi: 10.1001/jamadermatol.2018.0515.
- C. Yz, P. G, and A.-N. F, “Dermal fillers: pathophysiology, prevention and treatment of complications,” PubMed, Accessed: May 30, 2025. [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/27662522/
- M. King, C. Convery, and E. Davies, “This month’s guideline: The Use of Hyaluronidase in Aesthetic Practice (v2.4),” J. Clin. Aesthetic Dermatol., vol. 11, no. 6, pp. E61–E68, Jun. 2018.
- D. B. K and H. I, “Patient factors influencing dermal filler complications: prevention, assessment, and treatment,” PubMed, 2015, Accessed: May 30, 2025. [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/25926750/
- M. Landau, “Hyaluronidase Caveats in Treating Filler Complications,” Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. Al, vol. 41 Suppl 1, pp. S347-353, Dec. 2015, doi: 10.1097/DSS.0000000000000555.
- C. Sohrabi et al., “The SCARE 2023 guideline: updating consensus Surgical CAse REport (SCARE) guidelines,” Int. J. Surg. Lond. Engl., vol. 109, no. 5, pp. 1136–1140, May 2023, doi: 10.1097/JS9.0000000000000373.
- “S. Korea sees surge in foreign patients for beauty care, plastic surgery | The Straits Times.” Accessed: May 13, 2025. [Online]. Available: https://www.straitstimes.com/asia/east-asia/s-korea-sees-surge-in-foreign-patients-for-beauty-care-plastic-surgery
- C. L. Wilde, K. Jiang, S. Lee, and D. G. Ezra, “The Posthyaluronidase Syndrome: Dosing Strategies for Hyaluronidase in the Dissolving of Facial Filler and Independent Predictors of Poor Outcomes,” Plast. Reconstr. Surg. Glob. Open, vol. 12, no. 4, p. e5765, Apr. 2024, doi: 10.1097/GOX.0000000000005765.
- R. L, R. C, C. L, Z. Us, K. H, and C. L, “Adverse reactions to injectable soft tissue fillers,” PubMed, Accessed: May 30, 2025. [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/21167403/
- J. Mlw, L. Mk, and M. Es, “The Kinetics of Reversible Hyaluronic Acid Filler Injection Treated With Hyaluronidase,” PubMed, Accessed: May 30, 2025. [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/28498207/
- J. Faivre, K. Wu, M. Gallet, J. Sparrow, F. Bourdon, and C. J. Gallagher, “Comparison of Hyaluronidase-Mediated Degradation Kinetics of Commercially Available Hyaluronic Acid Fillers In Vitro,” Aesthet. Surg. J., vol. 44, no. 6, pp. NP402–NP410, Feb. 2024, doi: 10.1093/asj/sjae032.
- S. B, B. F, H. V, E. R, W. L, and R. B, “The current state of treatment of adverse reactions to injectable fillers,” PubMed, Accessed: May 30, 2025. [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/20969667/
- M. M, “Hyaluronic Acid Filler Longevity and Localization: Magnetic Resonance Imaging Evidence,” PubMed, 2021, Accessed: May 30, 2025. [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/33002985/
- Jung GS. Thoughts and Concepts in Ultrasonography-guided Hyaluronidase Injection Technique. Plast Reconstr Surg Glob Open. 2023 Jun 13;11(6):e5041. doi: 10.1097/GOX.0000000000005041. PMID: 37325378; PMCID: PMC10263199.
- V. M, V. A, and N. K, “Dermal Fillers: Do’s and Dont’s,” PubMed, Accessed: May 30, 2025. [Online]. Available: https://pubmed.ncbi.nlm.nih.gov/20606986/

