Lab-on-a-Chip Biosensors for Drug Safety and Health
Smarter Chips, Safer Lives: Lab-on-a-chip Biosensors for Pharmacological Applications and Healthcare Transformation
Ankit Kumar Singh1 and Ida Tiwari2
OPEN ACCESS
PUBLISHED: 31 March 2025
CITATION: SINGH, Ankit Kumar; TIWARI, Ida. Smarter Chips, Safer Lives: Lab-on-a-chip Biosensors for Pharmacological Applications and Healthcare Transformation. Medical Research Archives, [S.l.], v. 13, n. 3, mar. 2025. Available at: <https://esmed.org/MRA/mra/article/view/6337>.
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.18103/mra.v13i3.6337.
ISSN 2375-1924
ABSTRACT
Rapid advancements in industries like genetics, biotechnology, and medicine have raised the demand for chemical and biochemical analysis and led to the development of useful chip applications for the use of sophisticated equipment, labs, and traditional procedures. A new technique called lab-on-a-chip displays a miniature laboratory onto a tiny coin-sized chip. Reagent mixing, dilution, electrophoresis, separation, staining, and detection can all be accomplished on a sensor chip. One or several analyses are made possible by this sensor technology, which also produces high sensitivity, quick diagnostic times, improved process control, inexpensive fabrication costs, portability, and safety. The detection of drugs is currently being performed using lab-on-a-chip technology, which may accept a variety of biological and non-biological materials. A number of detection techniques were used by lab-on-a-chip devices, with immunoassays being most frequently used. The usage of real-world samples should be increased, validation should be enhanced, and practicality should be further examined in terms of providing information on cost, speed of analysis, and convenience of use. A wide variety of lab-on-a-chip techniques are already accessible, which suggests that these devices could be used as portable, quick, and affordable detection systems. Lab-on-a-chip systems can be utilised both within and outside of hospitals for clinical purposes. Numerous benefits were provided by Lab-on-a-chips over existing tests, including as the ability to do point-of-care diagnostics with minimal fluid volumes, the use of small quantities of costly chemicals and samples, controlled flow rate, short diffusion distance, fast mixing time, and inexpensive fabrication costs.
Keywords
lab-on-a-chip, biosensors, pharmacological applications, healthcare transformation, drug testing
1. Introduction
The pharmaceutical sector is facing significant obstacles due to rising expenses and inefficient medication research. The low predictive capacity of current preclinical models is the reason behind drug failures in trials, hence drug development researchers have confirmed the urgent need for novel testing methods that can reliably anticipate drug safety and efficacy in people¹. According to data from the 2019 United Nations Office on Drugs and Crime (UNODC) World Drugs Report, 271 million persons worldwide abused drugs in 2017, resulting in approximately 585,000 drug-related deaths². This demonstrates not only the substantial number of drug users but also the considerable number of drug-related deaths that occur worldwide.
Thus, devices and methods for identifying and monitoring harmful substances are essential for ensuring human health. In this age of rapid material evolution, it is critical to describe and evaluate the toxicity associated with pharmaceutical residues. Toxic pharmaceuticals must be closely monitored to prevent any risks to human health, even if national authorities have strict control over these systems. One important element that boosts the impact of treatment is early detection³. Despite ongoing advancements in drug screening techniques, only a small percentage of drug candidates are approved for clinical use by the U.S. Food and Drug Administration (FDA)⁴.
Drugs in various samples can be analysed using a variety of contemporary laboratory-based methods, including mass spectrometry⁵, chromatography⁶, immunoassays⁷, spectrophotometry⁸, and colorimetry⁹. These advanced methods, however, are typically linked to difficulties with sample handling, miniaturisation, or skilled lab personnel. When millions of distinct chemical combinations must be tested during drug discovery, the need for numerous read-outs puts a significant strain on the testing procedures. Small chemical volumes and parallel operation are required for a high-throughput system to handle these many samples while maintaining an inexpensive development cost¹⁰.
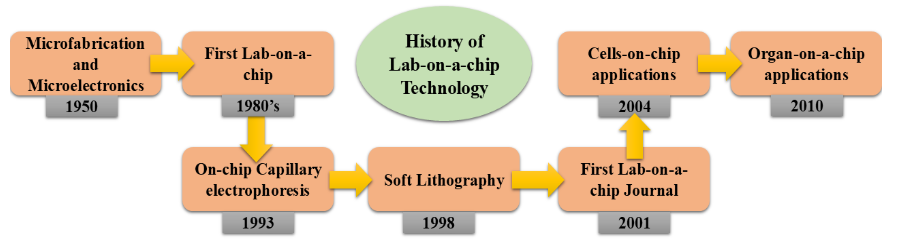
There is a growing trend of miniaturisation using chemical and biological analytical techniques. An approach that shows promise for analyte detection and for enabling wireless communication of analysis results to remote areas is chip-based sensing technology. These instruments are affordable, portable, ensure speedy findings, and can be included into biological systems that are responsive and sophisticated¹¹,¹². The advancement in the miniaturisation of these analysis techniques is directly reflected in lab-on-a-chip (LOC) technology. Devices that are capable of controlling and modifying fluid flows at the micro level are known as LOC devices. They offer a wide range of fascinating applications in a number of domains, such as drug release investigations, environmental analysis, clinical diagnostics, and food control¹³. Numerous laboratory procedures, biological processes, DNA sequencing, and chemical production all make use of these microdevices. By combining microfluidic devices with biosensors, they can be used in industries like environmental monitoring, food processing and safety, pharmaceuticals, medical diagnostics, and agriculture. A few examples of applications where LOC platforms can be useful include the analysis of ions from various compositions used in forensics, explosives identification, water quality assessment, bodily fluid research, agriculture, and pollution level detection¹⁴,¹⁵. The history in the development and applications of LOC techniques is represented in Fig. 1.
Lab-on-a-chip tools provide automation and high-throughput analysis by combining various laboratory processes onto a single chip. The LOC technologies have the following key characteristics¹⁶,¹⁷: (i) Transporting liquid samples containing bioparticles, such as cells, proteins, and DNA, into a field with electrode molecules that have been previously stored; (ii) Electrode and extracting non-specific and lossy binding, as well as mixing and binding reactions of extracted bioparticles; and (iii) Detecting the
corresponding change in chemical, physical, electrical, mechanical, or magnetic signals¹⁸. These methods provide portable equipment by performing several laboratory operations on a smaller scale, such as chemical synthesis and analysis on a single chip. Developments in micro and nanotechnology have made it possible to fabricate LOC tools in smaller sizes. This is comparable to the revolution in semiconductors, which has been significantly influenced by lithographic methods.
The integration of many laboratory processes on a micro or nanoscale facilitates automation and high-throughput screening. A number of factors, such as capillary forces, electrokinetics (EKs), and pressure gradients, affect LOC manufacturing. By controlling low-volume samples, microfluidic systems can achieve high analysis rates while saving money and time. It can be applied to chemical and biological studies, as well as chemical synthesis¹⁹.
complexity of the field. Indeed, it is frequently and incorrectly believed that a biosensor is a device that detects the existence and usually the amount of a biomolecule such a protein or a DNA strand or possibly a cell. The biosensors field is large and complicated. The number of findings reported in the literature and now being studied virtually reaches astronomical proportions when we include the potential to integrate a biosensing element with microfluidic sample handling capabilities.
The last few years have seen a significant development of LOC platforms for biosensor development, with a particular emphasis on three-dimensional (3D) designs. As device sizes decrease, small quantities offer several advantages, such as lower reagent costs and higher analytical accuracy. Single cell analysis and other cellular biological investigations are made possible by these glass or polymer-based biochips. The quasi-realistic reproduction of the physiological systems in these 3D in vitro models may make them viable substitutes for in vivo investigations and animal sacrifices²⁶. Point-of-care (POC) tests have the potential to significantly improve the standard of healthcare. Clinical diagnostic technologies have advanced significantly and been implemented successfully in a number of fields during the last ten years²⁷. The latest developments in MIP-based LOC detection technologies using a variety of biomolecule samples are observed. With the new MIP technology, which enables direct detection of target biomolecules and sensitive and selective detection of biomolecules in a sampling of biofluids, we firmly believe that the current difficulties of LOC can be resolved¹¹,²⁶.
The urgent need for a novel method to more accurately replicate human drug responses in preclinical research has driven the development of Organoids-on-chips (OOC) technologies or microphysiological systems (MPS)⁴. The OOC is a new and creative concept that combines sophisticated organ-on-chip technology with stem cell-derived organoids to create biomimetic micro-physiological systems in vitro. The synergistic union surpasses the constraints of conventional methods for safety evaluation and drug screening, including animal models and 2D cell cultures.
Although few reviews are available on chip-based devices for drug detection²⁷,²⁸,²⁹, but these include only limited descriptions and do not summarize the advantages and limitations of their clinical approaches. Here, we focus on a set of recently developed LOC and OOC techniques that facilitate high-throughput analysis and review their applications in drug detection for healthcare transformations. We highlighted the important features and virtues of LOC devices, clarifying their power to genuinely imitate human physiology, so addressing contemporary hurdles. We comprehensively overviewed the recent endeavors where LOC and OOC have been harnessed for drug screening and safety assessment and delved into potential opportunities and challenges for resolving sophisticated, near-physiological chips. Based on recent successes, we go on to explore how multidisciplinary convergent innovation can improve the viability of chips and hasten the transition from preclinical to clinical stages in industry and healthcare³⁰. We also provide an outlook into their key features and various fabrication approaches. Additionally, it discusses the limitations of current methods that must be addressed in the future for healthcare transformation.
2. Lab-on-a-chip sensors
Microfluidic approach can be included into a LOC device to enable the miniaturisation of certain laboratory-based analytical methods³¹,³². The manipulation of fluids in micrometer-scale channels is known as microfluidics. In addition to providing notable benefits over more conventional approaches, this also makes new advancements viable that would not be feasible on a wider scale. The general benefits include reduced sample requirements, low reagent consumption, and less waste effluent, quicker reaction times because of a higher surface area to volume ratio, greater portability, and cost-effectiveness in terms of equipment required and no need
specialised staff³¹,³²,³³. Microfluidics has developed into a very interdisciplinary topic of study in recent years. The development of fully integrated ‘sample in–answer out’ LOC devices has focused on areas such as clinical diagnostics³³, but one fully integrated LOC for forensic purposes is the RapidHIT ID System, which analyses buccal swab samples for human identification purposes and can generate a DNA profile in just 90 minutes³⁴.
The first commercially accessible LOC system was released around two years after Hopwood et al.³⁵ published the first research journal article in 2010 describing a fully integrated LOC device that might generate a DNA profile. Since the Rapid DNA Act of 2017, law enforcement has been using this type of LOC technology to analyse reference samples³⁵. This indicates that the employment of LOC systems in forensic investigations may prove to be beneficial.
Analytical instruments are made smaller by using LOC technology, which packs numerous lab tools onto a tiny single chip¹². Two essential concepts of engineering research that are miniaturization and integration are important aspects of LOC technology. The main reason for this significant growth is the quick development of integrated circuit (IC) technology. Miniaturisation makes it possible to replace traditional heavy, costly equipment with more affordable, portable alternatives¹². These days, embedded artificial intelligence technology and smartphone integration are popular.
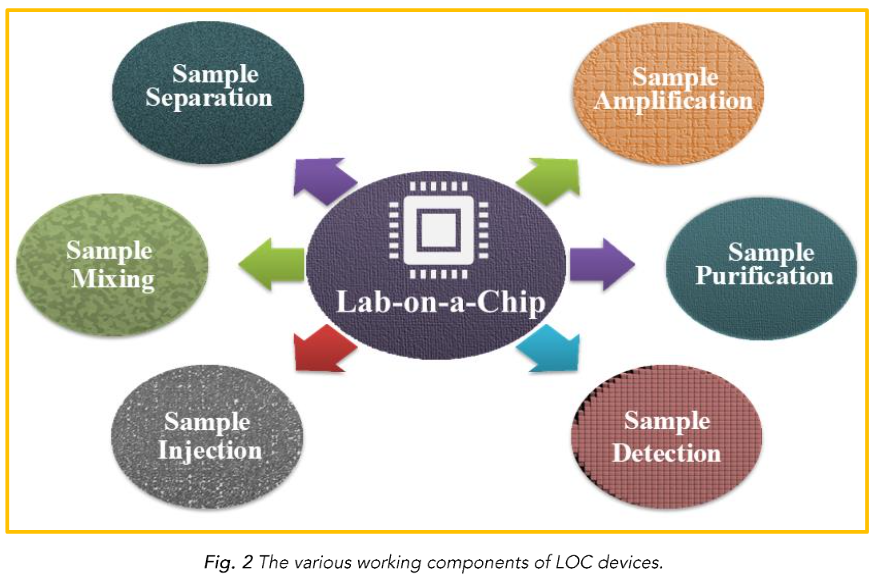
Depending on the intended use, the literature contains a wide variety of design types. Lab-on-a-chip systems have certain basic components, but the types of components differ depending on the application. The working parts include an injector, a transporter, a preparator, a mixer, a reactor, a separator, a detector, a controller, and a power source. Since the components are represented by appropriate symbols, designers can display their LOC products consistently¹⁴. To elucidate these counters, the elements of a LOC device are shown in Fig. 2.
3. Key Features of Lab-on-a-chip Sensors
The LOC systems differ from conventional laboratory equipment in a number of significant ways, including microfluidics, integration and miniaturization¹²,¹⁵,²⁰,³⁶.
-
Microfluidics: The technology used in LOC devices is microfluidic, which manipulates fluids in channels that range in size from tens to hundreds of micrometres. Microfluidics makes it possible to precisely control fluid flow, mixing, and reactions, which makes sample processing and analysis more effective¹²,³¹.
-
Integration: The LOC devices combine several laboratory processes onto a single chip, including sample preparation, separation, reaction, and detection. This integration speeds up and increases the accuracy of the analysis while lowering the need for manual intervention and consuming fewer samples and reagents¹².
-
Miniaturization: The LOC instruments drastically cut down on the quantity of necessary samples and reagents by minimising laboratory procedures, which lowers expenses and waste production. Additionally, miniaturisation makes it possible to create POC and portable diagnostic tools¹²,³⁷.
4. Fabrication of lab-on-a-chip sensors
A wide range of inorganic, organic, and composite materials are being employed to build the fundamental structure of LOC devices. Among the most widely used materials in fabrication include crystalline minerals like silicon and glass, polymers including photoresists, acrylate, and thermoplastics, and elastomers like polydimethylsiloxane³⁸. Silicon used to rule the microfabrication industry because of its semiconducting characteristics. Due to its well-documented surface engineering capabilities based on the silanol group (SiOH), silicon is seen as a viable material for LOCs devices³⁸.
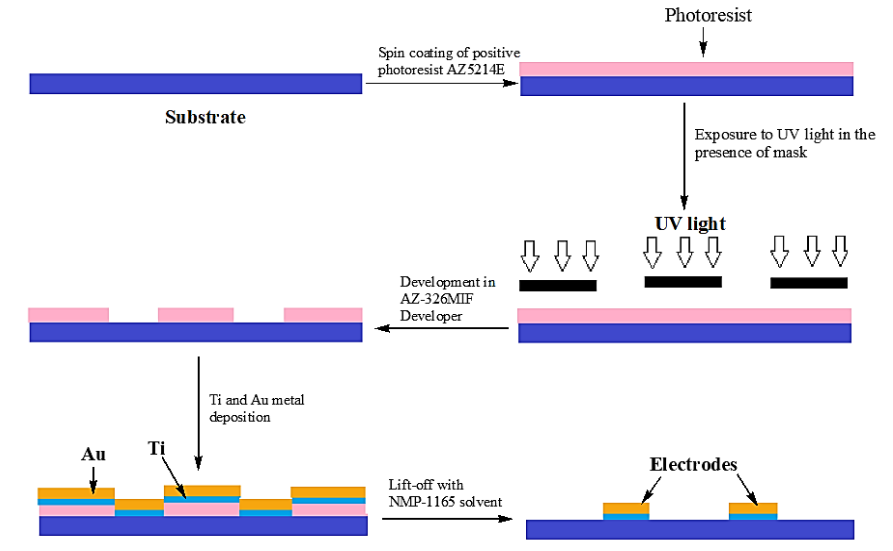
There are several techniques such as photolithography, soft lithography, laser micromachining, screen printing, 3D printing, and nano imprinting for fabrication of LOC devices³⁹,⁴⁰. The term chip refers to the initial manufacturing process, which used an enhanced type of photolithographic etching to create computer microchips and allowed for uniform control over the sizes and shapes of surface features³⁹. The various steps involved in photolithography method of LOC manufacturing is illustrated in Fig. 3.
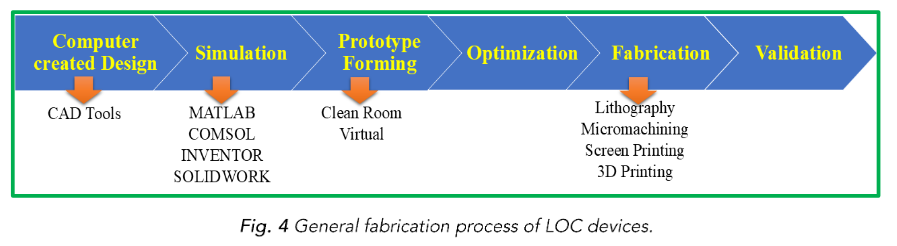
Usually, microfabrication methods taken from the semiconductor industry are used to create LOC devices³⁷ and the general fabrication process are represented in Fig. 4. The following are the primary steps in LOC fabrication:
-
Design: Computer-aided design (CAD) software is used to create the functionality and layout of the LOC devices. Reaction chambers, integrated sensors or detectors, and microfluidic channels are all part of the design.
-
Master Fabrication: The planned LOC device is made into a master mould by employing either soft lithography or photolithography methods. For the microfluidic structures to be replicated, the master acts as a template.
-
Replication: Polydimethylsiloxane (PDMS), a flexible and optically transparent polymer, is one of the materials used to recreate the microfluidic structures from the master mould. The entire LOC device is then created by bonding the duplicated structures to a substrate, like glass or plastic.
-
Integration: As required, further parts are added to the LOC device, such as electrodes, sensors, or microvalves. These parts make it possible to identify and analyse samples as well as regulate and track fluid flow.
Figure 3: Steps involved in photolithography method for fabrication of lab-on-a-chip.
5. Nanoscale materials in fabrication of lab-on-a-chip
The choice of materials becomes crucial because LOC devices are meant to carry out very specialized tasks. This might affect the inherent characteristics of the device as well as the construction method that is chosen. The literature provides an invaluable summary of materials for LOC and microfluidics¹¹,¹². Here, few materials are briefly emphasised.
Polydimethylsiloxane: The PDMS, a silicon-based elastomer that cures from 40 °C to 70 °C, provides a practical and affordable platform for LOC. The PDMS is easily sealed, has good flexibility, gas permeability, and a low surface tension. Its high absorption capability, however, makes surface modification necessary.
Epoxy resins (SU-8): The benefits of thermosetting materials, such as their excellent durability at high temperatures, chemical susceptibility, and transparency, are somewhat outweighed by their expensive cost.
Silicon: Many characteristics of silicon crystals are similar to those of glass, but unlike amorphous glass, which has spherical walls, silicon crystals have vertical sidewalls and opaque chips. Additionally, silicon chips have good resistance to solvents and thermal stability.
Glass: The major advantages of glass include the optical transparency, chemical resistance, electroosmotic mobility, and poor electrical conductivity. Its main drawbacks, however, are its extreme hardness and high construction costs.
Several alternative metals, metal thin films, thermoplastics such as poly(methyl methacrylate) (PMMA), and hydrogels are emerging as significant materials for LOC applications in addition to the four most popular ones. A new class of LOC devices, known as paper-based microfluidics, uses hydrophobically modified cellulose and cellulose. The very porous structure of this matrix makes it possible to take advantage of capillary activities more successfully. Despite their ease of production, paper LOCs need to be improved in the areas of channel resolution, integration with other components, and, eventually, detection sensitivity. An overview of primary physical-chemical characteristics of these materials as well as the processing methods currently used to create devices are described in Table 1.
Table 1. Characteristics of the materials and processing methods commonly employed in the production of LOC devices.
| Material/Property | Elastomers | Thermosets | Thermoplastics | Silicon/Glass | Hydrogel | Paper |
|---|---|---|---|---|---|---|
| Thermal stability | medium | high | medium to high | very high | low | medium |
| Hydrophobicity | hydrophobic | hydrophobic | hydrophobic | hydrophilic | hydrophilic | amphiphilic |
| Optical transparency | high | high | medium to high | no/high | low to medium | low |
| Chemical resistance | moderate | good | moderate to good | excellent | low | low |
| Microfabrication technique | casting | casting, photopolymerization | thermo-molding | photolithography, laser assisted etching | casting, photopolymerization, 3D bioprinting | photolithography, printing |
| Throughput | high | high | high | medium to high | low to medium | high |
6. Applications of lab-on-a-chip devices
Quick developments in the field of microfluidic systems have made it possible to create low-cost, portable, disposable, and easy-to-use platforms that are ideal for LOC applications and health status monitoring¹²,⁴². Multidisciplinary collaboration across engineering, chemistry, physics, biochemistry, microtechnology, nanotechnology, and biotechnology is encouraged via microfluidics-based LOC systems.
The portability of microfluidic devices makes them useful for on-site testing⁴³. A single microfluidic biosensor can do all the analysis, including preconcentration and treatment, continuous sampling, sample separation and mixing, and more because microsystems are small⁴⁴. Additionally, these microfluidic biosensors enhance analytical performance, throughput, rapid reaction rates, portability, and real-time detection. This makes it possible to convert detection to home-testing
techniques, which is very desirable in both developed and developing nations⁴⁵. Continuous microfluidic systems have steadily improved their performance during the past 20 years. Today, microfluidics can study biological systems from molecules to tiny multicellular organisms since technology has advanced to that stage. Because microfluidics can detect small amounts of analytes, it has given sensing devices a substantial advantage over previous techniques. This has resulted in an integration of chemical and biological processes on a single platform, with reduction in prices, waste, and energy⁴⁶.
A wide range of research fields, such as (a) system biology and analytical chemistry, (b) drug development and biological screening, (c) clinical diagnostics, (d) POC devices for environmental, and (e) medicinal applications, have recently seen a surge in interest in continuous microfluidics⁴⁵. The benefits of merging biosensors with LOC technology and microfluidics systems have sparked a lot of interest recently¹⁶. Combining biosensors and microfluidic devices results in a comprehensive and compact substitute for conventional repeated laboratory procedures³³. Microfluidic-based biosensors can also lower costs while improving detection sensitivity and specificity when compared to conventional detection techniques⁴⁷,⁴⁸.
The capacity of LOC systems to handle samples and manipulate fluids in combination with extremely sensitive biosensor designs promises to produce powerful instruments for analytical and, more precisely, diagnostic applications⁴⁹. It is beneficial for a number of reasons to combine microfluidic circuits with biosensor designs in order to improve the overall performance of sensing system. The main goal is to improve the transit of analytes from the sample volume to the biorecognition element, particularly for sensing components that are surface-bound. Microfluidic channels can analyse blood droplets or even the contents of individual cells because of their smaller sizes and volumes, which enable them to work with significantly less sample than would otherwise be possible. More importantly, the shorter distances between the biorecognition components and the analyte molecules lead to a significant increase in response time, which greatly improves the conditions for diffusion-limited processes⁵⁰,⁵¹.
Lab-on-a-chip technology is used in many different fields (c.f. Fig. 5), such as:
-
Biomedical Diagnostics: For POC diagnostics, LOC devices are especially well-suited, allowing for the quick and precise identification of illnesses, infections, and genetic disorders. Even in environments with limited resources, LOC devices can deliver prompt and accurate diagnostic results by combining sample preparation, amplification, and detection onto a single chip¹⁵,¹⁹.
-
Drug Discovery and Development: High-throughput screening of drug candidates is made possible by LOC technology, which speeds up the drug discovery and development process. Drug development workflow efficiency can be increased and the requirement for animal testing can be decreased by using microfluidic devices to test the toxicity and efficacy of medications on tissue models or cell cultures¹¹,²⁰,²⁹.
-
Environmental Monitoring: Environmental contaminants like heavy metals, pesticides, and infectious biological agents can be monitored on-site with LOC devices. The LOC devices enable early pollution identification and cleanup by combining sample pretreatment, separation, and detection onto a single chip, allowing for quick and sensitive analysis of environmental samples⁵²,⁵³.
7. Lab-on-a-chip Sensors in Pharmacological Applications
In order to eventually replace animals in preclinical testing, microfluidic LOCs may be utilised as drug testing platforms, disease modelling, biomarker identification, and distinct cellular and molecular mechanisms¹¹,⁵⁴,⁵⁵. As a result, LOCs can provide suitable conditions for assessing medication efficacy and toxicity, cellular activity, and drug metabolism⁵⁶. The use of such testing microdevices in pharmacokinetic applications has been examined in a number of studies aimed at developing innovative strategies for customised cancer treatments⁵⁷,⁵⁸,⁵⁹.
For example, it was proposed to use a LOC platform to evaluate up to five drugs against osteosarcoma cells in real time⁵⁷. Other LOC microdevices were developed to perform single-cell cytotoxic and genotoxic experiments and simulate a hypoxic cancer microenvironment⁶⁰. Anti-inflammatory medicines were also assessed using a ‘small airway-on-a-chip’ model of human lung inflammation with dynamic flow conditions⁶¹. It should be mentioned that LOC technology has been authorised by the U.S. Food and Drug Administration for use in pharmaceutical medicine security testing and screening⁶²,⁶³.
Jiang et al. investigated the possible drug metabolisms and assessed drug cytotoxicity using a microwell-based microfluidic chip⁶⁴. It had the advantage of strong integration in a more physiologically appropriate setting and was easy to use. Drug cytotoxicity was assessed using a microfluidic sidewall-attached droplet array, according to Fang et al.⁶⁵. They cultivated cells in a 3D droplet array, which has the benefit of preventing cell adhesion on the chip surface and enabling the execution of several operations on cells within droplets. Additionally, they tested the effectiveness of the anticancer drug doxorubicin, and the results showed that doxorubicin clearly reduces cell viability in a dose-dependent manner. A number of microfluidic chips have been published to assess the cytotoxicity of anticancer medications in various settings⁴⁶,⁶⁷,⁶⁸.
First, a multiple-channel array chip was created, allowing for the management of oxygen tension and the completion of cell-based high throughput toxicity studies for cisplatin and tirapazamine. The outcomes of the experiment demonstrated that cisplatin and tirapazamine exhibit opposing mechanisms of oxygen-dependent cytotoxicity.
By mimicking the composition and operations of human organs, OOC biomimetic systems are proposed as non-traditional models for assessing the efficacy or safety of medications. In vitro co-culture models can mimic the complex interactions between cells in an environment similar to that found in vivo.
By simulating microfluidic dynamics, these devices can also be used in medically appropriate ways. Additional advantages of cell co-cultures on the chip include regulated medication administration, sensor integration on the same platform, microscopic super-resolution analysis, and high-throughput analysis with reduced costs and time⁶⁹. One disadvantage of these platforms is that they are designed for specific uses, which prevents them from being used in a general way⁵⁸.
According to the standard guidelines, it may be crucial to concentrate on simulating several organs in order to identify genuine advantages and link the OOC models with particular local tissue structures and cellular phenotypes in order to eventually replicate human physiology in vivo [126]. As a result, standard open technology platforms can be obtained by assembling adaptable models for fit-for-purpose OOC with technical and biological modules⁷⁰. Chips composed of soft tissue materials are the best options for the toxicity or effectiveness of medications using the OOC method. Glass or PDMS biochips are the best options for creating the right circumstances for a variety of tests that show how sensitive the organs are to medications. These materials provide transparency for optical interrogation and may confer qualities similar to the physiological environment, making it possible to create replicas of body components. They are also very easy to process into complicated shapes⁷¹. The use of these platforms in drug tests and pharmacological screening studies has the benefit of lowering the number of experiments for many organ types, including the heart, kidneys, liver, lungs, and central nervous system⁷⁰ or cancer research⁷¹. Another benefit of LOC microfluidic devices is their ability to evaluate medications with low permeability⁷². Innovative medication delivery methods are still desperately needed, and microfluidics offers a state-of-the-art method for this.
Numerous studies have proven the benefits of LOCs over traditional techniques for the synthesis of sophisticated delivery systems. Fontana et al.⁷³ provided an overview of droplet microfluidic techniques as a potent tool for the creation of monodisperse drug delivery systems, including liposomes, polymersomes, microcapsules, and microspheres. In contrast to conventional (2D, static) pharmaceutical assays, Cavero et al. discussed the advantages of OOCs microdevices for human-predictive biological insights on drug candidates in an Expert Opinion on Drug Safety review paper. In addition to discussing the importance of these OOC platforms for drug research and development, the authors present a wide range of them, including those related to cancer, lung, blood-brain barrier, heart, intestine, kidney, liver, pharmacokinetics, placenta, and vessel-on-chip⁷⁴.
The following provides some clear and particular instances of testing methodologies that are concentrated on one drug–one or multiple organs⁷⁵, multiple drug–one organ systems⁷⁶, and combinations of these. Kim et al.⁷⁷ investigated the pharmacokinetic profile that reduces the nephrotoxicity of gentamicin under dynamic conditions in a kidney-on-a-chip model. Using a specialised OOC, the study aimed to bridge the renal clearance gap between people and animals. Gentamicin was discovered to change cell-cell junctions, increase membrane permeability, and reduce cell viability, particularly when exposed at low levels for an extended period of time. For the assessment of cardiovascular toxicity, artificial endothelialised myocardium and heart-on-a-chip models were created using three-dimensional (3D) bioprinting⁷⁸. After that, the dose-dependent reactions of endothelial cells and cardiomyocytes to doxorubicin exposure were assessed. Using multi-material 3D printing, Lind et al.⁷⁹ created cardiac micro-physiological models with strain sensors integrated into microarchitectures. Over a four-week period, the OOC platform was validated by analysing the mechanical responses of human stem cell-derived laminar cardiac tissues and the effects of isoproterenol and verapamil medications. To replicate the intricacy of in vivo physiology, Phan et al.⁴ suggested a vascularised OOC platform for extensive drug screening. In order to successfully
identify both anti-angiogenic and antitumor medicines, a number of arrays of vascularised micro-tumors were developed and evaluated against up to twelve FDA-approved anti-cancer medications. In a multi-organ human-OOC model system, the impact of hepatic metabolism on off-target cardiotoxicity was examined⁸⁰. To evaluate terfenadine and fexofenadine medications, which may have cardiac side effects reliant on hepatic metabolism, human primary hepatocytes were co-cultured with iPSC-derived cardiomyocytes. In order to study the toxicity of drug metabolites, Theobald et al. reported creating a liver-kidney-on-chip model⁸¹.
The technology used for in vitro drug screening makes it possible to simulate the flow-dependent interaction between several organ-specific cell types. The effectiveness of this OOC method was confirmed by the toxicity assessment of benzo[a]pyrene and aflatoxin B1 medications. To screen for organophagonsic hepatotoxicity, a 3D tetraculture brain-on-chip platform was suggested⁸². By assessing the impact of drugs on barrier integrity, the study demonstrated the great usefulness of such platforms. However, Isoherranen et al.⁸³ examined the growing use of OOCs in quantitative clinical pharmacology evaluation. Advances in the microphysiological system, such OOC technology, are said to have the potential to better personalise treatments, forecast drug effects, and plan preclinical and clinical trials. A evaluation of OOC technology’s use in drug development was conducted relatively recently⁸⁴.
The biomimetic OOC system, which mimics the biology and physiology of human organs, has demonstrated more benefits than conventional methods for medication efficacy and testing. It is explained how a ‘human-on-chip’ system can more accurately assess the toxicity and efficacy of drugs by simulating intricate and dynamic processes such as drug absorption, distribution, metabolism, and excretion. A detailed assessment of the integration of microfluidic LOCs with pharmacological/toxicological experiments and pharmaceutical analysis was recently conducted by Ai et al.⁸⁵. The scientific community’s efforts to create ‘Pharm-LOC’ systems that can handle the entire spectrum of pharmacological advancements, from post-marketing product management to recent drug discovery, were compiled by the authors. Applications including drug separation and analysis on a chip, the creation of novel tools for pharmacological/toxicological models on a chip, and the use of chip-based models for drug safety and efficacy screening were the main topics of their literature review⁸⁵. Several perspectives for the future challenges and breakthroughs related to Pharm-LOC advances, such as automating drug discovery, precision nanomedicine, and personalized therapy, are then highlighted.
However, its poor resemblance to real in vivo tissues and the lack of development of human disease models may be a bottleneck in the therapeutic usage of the OOCs model⁸⁶. One of the new applications of LOC technology that has garnered increased attention is the importance of microfluidics to pharmaceutical science. In response to the need for LOC technologies for pharmaceutical science, Pharm-LOC was created. It represents chip-based platforms for the full chain of pharmacy applications, from drug discovery to post-marketing product management. All aspects of chip-based ideas, procedures, and tools for pharmaceutical production, pharmacological/toxicological testing, and analysis are included in Pharm-LOC. This review focused on the latest advancements in the use of LOC for pharmaceutical testing and analysis. The primary topics include the creation of innovative pharmacological and toxicological models on a chip, the isolation and analysis of drug molecules on a chip, and the use of chip-based models to assess the safety and effectiveness of drugs. We also attempt to provide an overview and outlook on the difficulties and potential innovations of Pharm-LOC development.
8. Advantages, Disadvantages and Limitations of the Nanoscale Sensor Chips
Small fluid volumes in LOC devices, lower reaction times, costs, and reagent amounts, which is advantageous when handling clinical samples, particularly in underdeveloped nations where low sample concentrations result in less chemical waste being produced. Thus, these small systems can also aid in large-scale manufacturing. But as a developing area, LOC has drawbacks at the microscale level, where capillary forces, surface roughness, and material-to-material chemical interactions become more noticeable. This could result in experimental issues that are not possible with conventional lab apparatus. A low signal-to-noise ratio may result from the detecting principles with microscale dynamics¹⁹,³²,⁸⁷. But by saving thousands of dollars on diagnostic equipment, LOC has gradually expanded its global presence.
Comparing LOC technology to conventional laboratory techniques reveals a number of benefits:
-
Reduced Sample and Reagent Volumes: The sample and reagent volumes used by LOC devices are usually very small, ranging from microlitres to nanolitres. When handling hazardous materials, this volume decrease results in lower costs, less waste production, and increased safety.
-
Faster Analysis: The time needed for sample preparation, reaction, and analysis is substantially reduced by LOC devices, which integrate and automate several laboratory procedures on a single chip. High-throughput screening and quick diagnostic tests are made possible by this acceleration.
-
Enhanced Sensitivity and Specificity: Improved sensitivity and specificity in sample analysis are the results of precise fluid flow control and miniaturisation of LOC devices. The efficiency of biological reactions and the limit of detection are improved by the high surface-to-volume ratio in microfluidic channels.
-
Portability and Point-of-Care Use: LOC devices require little instrumentation to operate and are small and portable. The portability makes it possible to use LOC devices in POC settings like clinics, emergency rooms, or distant sites by putting laboratory-grade analysis closer to the patient or sample source.
9. Biological, technical and clinical challenges and future outlook
LOC technology has many benefits, but before it can be widely used, a few issues must be resolved. Integrating several functions on a single chip while preserving the resilience and dependability of device is one of the primary problems. The mass production and commercialisation of LOC devices depend on the creation of standardised manufacturing procedures and quality control techniques³⁰.
In order to enhance the functionality and performance of LOC devices, future LOC technology research will concentrate on creating new materials and fabrication processes. The sensitivity and multiplexing capabilities of LOC devices will be improved by integrating cutting-edge sensing and detecting techniques, such as optical detection or sensors based on nanomaterials. Furthermore, automated analysis and interpretation of the complex data produced by LOC devices will be made possible by the integration of machine learning and artificial intelligence technologies⁵².
10. Conclusions
The high surface area to volume ratio of LOC devices makes them particularly advantageous for POC biosensing. Significant academic and industrial research is being conducted owing to the urgent need for POC devices to develop LOC based biosensors using proteins, cells, nucleic acids, and metabolites as analytes. The working of LOC devices
advancements in various components, and a preview of specific applications were all covered in this review. It is determined that the use of LOC devices will move quickly from POC research to proven clinical applications. It is strongly advised that a comprehensive validation method be used in the early phases of development in order to expedite the introduction of the product in clinical settings. In this review, the evolution of LOC technology for toxic drug detection and healthcare transformation are covered. It also discussed the possibility for commercialisation and miniaturisation of LOC systems.
Acknowledgements:
The author IT is grateful to IOE incentive grant for faculty (Scheme Number-6031), BHU.
Author contributions:
AKS: Methodology, Conceptualization, Visualization, Writing – original draft; Writing – Review & Editing, Data curation, Investigation; Formal analysis;
IT: Supervision, Validation.
Conflicts of interest:
The authors declare that they have no known competing financial interests, personal relationships that could have appeared to influence the work reported in this paper.
References
1. Esch E, Bahinski A, Huh D. Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov. 2015;14:248-260.
2. UNODC U. Executive Summary, Conclusions and Policy Implications [Internet]. Bookl 1 Vienna, Austria United Nations. Published online 2018:1-34.
3. Wei F, Patel P, Liao W, et al. Electrochemical sensor for multiplex biomarkers detection. Clin Cancer Res. 2009;15(13):4446-4452. doi:10.1158/1078-0432.CCR-09-0050
4. Phan DTT, Wang X, Craver BM, et al. A vascularized and perfused organ-on-a-chip platform for large-scale drug screening applications. Lab Chip. 2017;17(3):511-520. doi:10.1039/c6lc01422d
5. Chen H, Zheng J, Zhang X, Luo M, Wang Z, Qiao X. Surface desorption atmospheric pressure chemical ionization mass spectrometry for direct ambient sample analysis without toxic chemical contamination. J Mass Spectrom. 2007;42:1045-1056.
6. Leary PE, Kammrath BW, Lattman KJ, Beals GL. Deploying Portable Gas Chromatography–Mass Spectrometry (GC-MS) to Military Users for the Identification of Toxic Chemical Agents in Theater. Appl Spectrosc. 2019;73(8):841-858. doi:10.1177/0003702819849499
7. Darwish IA. Immunoassay Methods and their Applications in Pharmaceutical Analysis: Basic Methodology and Recent Advances. Int J Biomed Sci. 2006;2(3):217-235. doi:10.59566/ijbs.2006.2217
8. Parameswara Rao K, Rao MC. Spectrophotometric methods in the analysis of drugs in pure and dosage forms. Int J Chem Sci. 2016;14(4):2389-2396.
9. Gummadi S, Kommoju M. Colorimetric Approaches To Drug Analysis And Applications – A Review. Am J PharmTech Res. 2019;9(1):14-37. doi:10.46624/ajptr.2019.v9.i1.002
10. Neužil P, Giselbrecht S, Länge K, Huang TJ, Manz A. Revisiting lab-on-a-chip technology for drug discovery. Nat Rev Drug Discov. 2012;11(8): 620-632. doi:10.1038/nrd3799
11. Staicu CE, Jipa F, Axente E, Radu M, Radu BM, Sima F. Lab-on-a-chip platforms as tools for drug screening in neuropathologies associated with blood–brain barrier alterations. Biomolecules. 2021;11(6). doi:10.3390/biom11060916
12. Kuru Cİ, Ulucan-Karnak F, Akgöl S. Lab-on-a-chip sensors: recent trends and future applications. Fundam Sens Technol Princ Nov Des. Published online January 1, 2023:65-98. doi:10.1016/B978-0-323-88431-0.00012-0
13. Ricotta V, Yu Y, Clayton N, et al. A chip-based potentiometric sensor for a Zika virus diagnostic using 3D surface molecular imprinting. Analyst. 2019;144(14):4266-4280. doi:10.1039/c9an00580c
14. Lim YC, Kouzani AZ, Duan W. Lab-on-a-chip: a component view. Microsyst Technol. 2010; 16:1995-2015.
15. Denizli A. Molecular imprinting-based sensors : Lab-on-chip integration and biomedical applications. J Pharm Biomed Anal. 2023;225 (December 2022).
16. Mark D, Haeberle S, Roth G, Stetten F Von, Zengerle R. Microfluidic lab-on-a-chip platforms: Requirements, characteristics and applications. Chem Soc Rev. 2010;39(3):1153-1182.
doi:10.1039/b820557b
17. Romao VC, Martins SAM, Germano J, Cardoso FA, Cardoso S, Freitas PP. Lab-on-Chip Devices: Gaining Ground Losing Size. ACS Nano. 2017;11(11):10659-10664.
18. Fu YQ, Luo JK, Nguyen NT, et al. Advances in piezoelectric thin films for acoustic biosensors, acoustofluidics and lab-on-chip applications. Prog Mater Sci. 2017;89:31-91. doi:10.1016/J.PMATSC I.2017.04.006
19. Sengupta P, Khanra K, Chowdhury AR, Datta P. Lab-on-a-Chip Sensing Devices for Biomedical Applications. Elsevier Ltd; 2019. doi:10.1016/B978-0-08-102420-1.00004-2
20. Ai Y, Zhang F, Wang C, Xie R, Liang Q. Recent progress in lab-on-a-chip for pharmaceutical analysis and pharmacological/toxicological test. TrAC – Trends Anal Chem. 2019;117:215-230. doi:10.1016/j.trac.2019.06.026
21. Huang XJ, Choi YK. Chemical sensors based on nanostructured materials. Sensors Actuators B Chem. 2007;122(2):659-671. doi:10.1016/J.SNB.2 006.06.022
22. El-Ansary A, Faddah LM. Nanoparticles as biochemical sensors. Nanotechnol Sci Appl. 2010;3(1):65-76. doi:10.2147/NSA.S8199
23. Kim Y, Jeon Y, Na M, Hwang SJ, Yoon Y. Recent Trends in Chemical Sensors for Detecting Toxic Materials. Sensors. 2024;24(2). doi:10.3390/s 24020431
24. Cui F, Zhou Z, Zhou HS. Review—Measurement and Analysis of Cancer Biomarkers Based on Electrochemical Biosensors. J Electrochem Soc. 2020;167(3):037525. doi:10.1149/2.0252003jes
25. Chen YT, Lee YC, Lai YH, et al. Review of Integrated Optical Biosensors for Point-of-Care Applications. Biosensors. 2020;10(12):1-22. doi:10.3390/BIOS10120209
26. Azizipour N, Avazpour R, Rosenzweig DH, Sawan M. Evolution of Biochip Technology: A Review from Lab-on-a-Chip to Organ-on-a-Chip. Micromachines. 2020;11(6):599.
27. Hong CC, Lin CC, Hong CL, Lin ZX, Chung MH, Hsieh PW. Handheld analyzer with on-chip molecularly-imprinted biosensors for electrical detection of propofol in plasma samples. Biosens Bioelectron. 2016;86:623-629. doi:10.1016/J.BIO S.2016.07.032
28. Ugolini GS, Cruz-Moreira D, Visone R, Redaelli A, Rasponi M. Microfabricated physiological models for in vitro drug screening applications. Micromachines. 2016;7(12). doi:10.3390/mi7120233
29. Wang H, Zhu W, Xu C, Su W, Li Z. Engineering organoids-on-chips for drug testing and evaluation. Metabolism. 2025;162:156065. doi:10.1016/J.METABOL.2024.156065
30. Wang H, Zhu W, Xu C, Su W, Li Z. Engineering organoids-on-chips for drug testing and evaluation. Metabolism. 2025;162(July 2024): 156065. doi:10.1016/j.metabol.2024.156065
31. Guber AE, Heckele M, Herrmann D, et al. Microfluidic lab-on-a-chip systems based on polymers—fabrication and application. Chem Eng J. 2004;101(1-3):447-453. doi:10.1016/J.CEJ.2004.0 1.016
32. Yilmaz B, Yilmaz F. Lab-on-a-Chip Technology and Its Applications. Omi Technol Bio-engineering Towar Improv Qual Life. 2018;1:145-153. doi:10.1016/B978-0-12-804659-3.00008-7
33. Whitesides G. The origins and the future of microfluidics. Nature. 2006;442:368-373.
34. Jovanovich S, Bogdan G, Belcinski R, et al. Developmental validation of a fully integrated sample-to-profile rapid human identification system for processing single-source reference buccal samples. Forensic Sci Int Genet. 2015;16:181-194. doi:10.1016/J.FSIGEN.2014.12.004
35. Hopwood AJ, Hurth C, Yang J, et al. Integrated Microfluidic System for Rapid Forensic DNA Analysis: Sample Collection to DNA Profile. Anal Chem. 2010;82(16):6991-6999.
36. Lee NY. Recent progress in lab-on-a-chip technology and its potential application to clinical diagnoses. Int Neurourol J. 2013;17(1):2-10. doi:10.5213/inj.2013.17.1.2
37. Ghallab YH, Ismail Y. CMOS Circuits and Systems for Lab‐on‐a‐Chip Applications. Intech Open. Published online 2016.
https://www.intechopen.com/books/advanced-biometric-technologies/liveness-detection-in-biometrics
38. Nielsen JB, Hanson RL, Almughamsi HM, Pang C, Fish TR, Woolley AT. Microfluidics: innovations in materials and their fabrication and functionalization. Anal Chem. 2020;92(1):150-168. doi:10.1021/acs.analchem.9b04986
39. Dkhar DS, Kumari R, Malode SJ, Shetti NP, Chandra P. Integrated lab-on-a-chip devices: Fabrication methodologies, transduction system for sensing purposes. J Pharm Biomed Anal. 2023;223(October 2022):115120. doi:10.1016/j.jp ba.2022.115120
40. Xu M, Obodo D, Yadavalli VK. The design, fabrication, and applications of flexible biosensing devices. Biosens Bioelectron. 2019;124-125:96-114. doi:10.1016/J.BIOS.2018.10.019
41. Singh AK, Jaiswal N, Tiwari I, Ahmad M, Silva SRP. Electrochemical biosensors based on in situ grown carbon nanotubes on gold microelectrode array fabricated on glass substrate for glucose determination. Microchim Acta. 2023;190(2). doi:10.1007/s00604-022-05626-6
42. Saylan Y, Denizli A. Molecularly imprinted polymer-based microfluidic systems for point-of-care applications. Micromachines. 2019;10(11). doi:10.3390/mi10110766
43. Luka G, Ahmadi A, Najjaran H, et al. Microfluidics integrated biosensors: A leading technology towards lab-on-A-chip and sensing applications. Sensors (Switzerland). 2015;15(12): 30011-30031. doi:10.3390/s151229783
44. Wang Z, Han T, Jeon TJ, Park S, Kim SM. Rapid detection and quantification of bacteria using an integrated micro/nanofluidic device. Sensors Actuators B Chem. 2013;178:683-688. doi:10.1016/J.SNB.2013.01.017
45. Liu KK, Wu RG, Chuang YJ, Khoo HS, Huang SH, Tseng FG. Microfluidic systems for biosensing. Sensors. 2010;10(7):6623-6661. doi:10.3390/s100706623
46. Breslauer DN, Lee PJ, Lee LP. Microfluidics-based systems biology. Mol Biosyst. 2006;2(2):97-112. doi:10.1039/b515632g
47. Weibel DB, Whitesides GM. Applications of microfluidics in chemical biology. Curr Opin Chem Biol. 2006;10(6):584-591. doi:10.1016/J.CBPA.200 6.10.016
48. Hong J, Edel JB, deMello AJ. Micro- and nanofluidic systems for high-throughput biological screening. Drug Discov Today. 2009;14(3-4):134-146. doi:10.1016/J.DRUDIS.2008.10.001
49. Lafleur JP, Jönsson A, Senkbeil S, Kutter JP. Recent advances in lab-on-a-chip for biosensing applications. Biosens Bioelectron. 2016;76:213-233. doi:10.1016/J.BIOS.2015.08.003
50. Jr. NSL, Bocková M, Adam P, Homola J. Biosensor Enhancement Using Grooved Micromixers: Part II, Experimental Studies. Anal Chem. 2015;87(11):5524-5530.
51. Jr. NSL, Homola J. Biosensor Enhancement Using Grooved Micromixers: Part I, Numerical Studies. Anal Chem. 2015;87(11):5516-5523.
52. Hub L, Systems CD. Lab-on-a-Chip : Miniaturizing Laboratory Processes. Lab-on-a-Chip: Miniaturizing and Automating Analysis for Lab and Field.
53. Pol R, Céspedes F, Gabriel D, Baeza M. Microfluidic lab-on-a-chip platforms for environmental monitoring. TrAC Trends Anal Chem. 2017;95:62-68. doi:10.1016/J.TRAC.2017.08.001
54. Jiang L, Li S, Zheng J, Li Y, Huang H. Recent progress in microfluidic models of the blood-brain barrier. Micromachines. 2019;10(6):1-20. doi:10.3390/mi10060375
55. Dhiman N, Kingshott P, Sumer H, Sharma CS, Rath SN. On-chip anticancer drug screening – Recent progress in microfluidic platforms to address challenges in chemotherapy. Biosens Bioelectron. 2019;137:236-254. doi:10.1016/J.BIOS.2019.02.070
56. Ishida S. Organs-on-a-chip: Current applications and consideration points for in vitro ADME-Tox studies. Drug Metab Pharmacokinet. 2018;33(1):49-54. doi:10.1016/J.DMPK.2018.01.003
57. Mitxelena-Iribarren O, Zabalo J, Arana S, Mujika M. Improved microfluidic platform for simultaneous multiple drug screening towards personalized treatment. Biosens Bioelectron. 2019; 123:237-243. doi:10.1016/J.BIOS.2018.09.001
58. Musteata FM. Pharmacokinetic Applications of Microdevices and Microsampling Techniques. Bioanalysis. 2009;1(1):171-185.
59. Sontheimer-Phelps A, Hassell BA, Ingber D. Modelling cancer in microfluidic human organs-on-chips. Nat Rev Cancer. 2019;19:65-81.
60. Li L, Li Y, Shao Z, Luo G, Ding M, Liang Q. Simultaneous Assay of Oxygen-Dependent Cytotoxicity and Genotoxicity of Anticancer Drugs on an Integrated Microchip. Anal Chem. 2018;90 (20):11899-11907.
61. Benam K, Villenave R, Lucchesi C, et. al. Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat Methods. 2016;13:151-157.
62. Low LA, Tagle DA. Tissue chips-innovative tools for drug development and disease modeling. Lab Chip. 2017;17(18):3026-3036. doi:10.1039/c 7lc00462a
63. Ewart L, Fabre K, Chakilam A, et al. Navigating tissue chips from development to dissemination: A pharmaceutical industry perspective. Exp Biol Med. 2017;242(16):1579-1585. doi:10.1177/15353 70217715441
64. Chen Y, Gao D, Liu H, Lin S, Jiang Y. Drug cytotoxicity and signaling pathway analysis with three-dimensional tumor spheroids in a microwell-based microfluidic chip for drug screening. Anal Chim Acta. 2015;898:85-92. doi:10.1016/J.ACA.2 015.10.006
65. Shi-Ping Zhao, Ma Y, Lou Q, Zhu H, Bo Yang, Fang Q. Three-Dimensional Cell Culture and Drug Testing in a Microfluidic Sidewall-Attached Droplet Array. Anal Chem. 2017;89(19):10153-10157.
66. Wang Z, Liu Z, Li L, Al. E. Investigation into the hypoxia-dependent cytotoxicity of anticancer drugs under oxygen gradient in a microfluidic device. Microfluid Nanofluid. 2015;19:1271-1279.
67. Orbach SM, Less RR, Anjaney Kothari PR. In Vitro Intestinal and Liver Models for Toxicity Testing. ACS Biomater Sci Eng. 2017;9(3):1898-1910.
68. Dong R, Liu Y, Mou L, Deng J, Jiang X. Microfluidics-Based Biomaterials and Biodevices. Adv Mater. 2019;31(45):1-18. doi:10.1002/adma.2 01805033
69. Oddo A, Peng B, Tong Z, et al. Advances in Microfluidic Blood–Brain Barrier (BBB) Models. Trends Biotechnol. 2019;37(12):1295-1314. doi:10.1016/j.t ibtech.2019.04.006
70. Mastrangeli M, Millet S, Mummery C, et al. Building blocks for a European organ-on-chip roadmap. ALTEX. 2019;36(3):481-492. doi:10.1457 3/ALTEX.1905221
71. Mastrangeli M, Millet S, van den Eijnden-Van Raaij J. Organ-on-chip in development: Towards a roadmap for organs-on-chip. ALTEX. 2019;36(4): 650-668. doi:10.14573/altex.1908271
72. Chirra HD, Shao L, Ciaccio N, et al. Planar Microdevices for Enhanced In Vivo Retention and Oral Bioavailability of Poorly Permeable Drugs. Adv Healthc Mater. 2014;3(10):1648-1654. doi:10.1002/a dhm.201300676
73. Fontana F, Ferreira MPA, Correia A, Hirvonen J, Santos HA. Microfluidics as a cutting-edge technique for drug delivery applications. J Drug Deliv Sci Technol. 2016;34:76-87. doi:10.1016/J.JD DST.2016.01.010
74. Cavero I, Guillon JM, Holzgrefe HH. Human organotypic bioconstructs from organ-on-chip devices for human-predictive biological insights on drug candidates. Expert Opin Drug Saf. 2019;18 (8):651-677. doi:10.1080/14740338.2019.1634689
75. Sophia M. Orbach, Less RR, Anjaney Kothari PR. In Vitro Intestinal and Liver Models for Toxicity Testing. ACS Biomater Sci Eng. 2017;9(3):1898-1910.
76. Bein A, Shin W, Jalili-Firoozinezhad S, et al. Microfluidic Organ-on-a-Chip Models of Human Intestine. Cmgh. 2018;5(4):659-668. doi:10.1016/j.jc mgh.2017.12.010
77. Kim S, LesherPerez SC a., Kim BC hou. C, et al. Pharmacokinetic profile that reduces nephrotoxicity of gentamicin in a perfused kidney-on-a-chip. Biofabrication. 2016;8(1):015021.
doi:10.1088/1758-5090/8/1/015021
78. Zhang YS, Arneri A, Bersini S, et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials. 2016;110:45-59. doi:10.1016/J.BIOM ATERIALS.2016.09.003
79. Lind JU, Busbee TA, Valentine AD, et al. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat Mater. 2017;16(3):303-308. doi:10.1038/nmat4782
80. Oleaga C, Riu A, Rothemund S, et al. Investigation of the effect of hepatic metabolism on off-target cardiotoxicity in a multi-organ human-on-a-chip system. Biomaterials. 2018;182:176-190. doi:10.1016/J.BIOMATERIALS.2018.07.062
81. Theobald J, Ghanem A, Wallisch P, et al. Liver-Kidney-on-Chip to Study Toxicity of Drug Metabolites. ACS Biomater Sci Eng. 2018;4(1):78-89. doi:10.1021/acsbiomaterials.7b00417
82. Koo Y, Hawkins BT, Yun Y. Three-dimensional (3D) tetra-culture brain on chip platform for organophosphate toxicity screening. Sci Rep. 2018;8(1):1-7. doi:10.1038/s41598-018-20876-2
83. Isoherranen N, Madabushi R, Huang S-M. Emerging Role of Organ-on-a-Chip Technologies in Quantitative Clinical Pharmacology Evaluation. Clin Transl Sci. 2019;12:113-121.
84. Zhu J. Application of Organ-on-Chip in Drug Discovery. J Biosci Med. 2020;08(03):119-134. doi:10.4236/jbm.2020.83011
85. Ai Y, Zhang F, Wang C, Xie R, Liang Q. Recent progress in lab-on-a-chip for pharmaceutical analysis and pharmacological/toxicological test. TrAC Trends Anal Chem. 2019;117:215-230. doi:10.1016/J.TRAC.2019.06.026
86. Weinhart M, Hocke A, Hippenstiel S, Kurreck J, Hedtrich S. 3D organ models—Revolution in pharmacological research? Pharmacol Res. 2019; 139:446-451. doi:10.1016/J.PHRS.2018.11.002
87. Dkhar DS, Kumari R, Malode SJ, Shetti NP, Chandra P. Integrated lab-on-a-chip devices: Fabrication methodologies, transduction system for sensing purposes. J Pharm Biomed Anal. 2023; 223:115120. doi:10.1016/J.JPBA.2022.115120

