Micronutrient Supplementation in Cancer Malnutrition Management
A Different Approach to Malnutrition-Related Appetite and Weight Loss in Cancer Patients: Is Saturation Enough at The Cell Level?
1Mahmut Ilker Yilmaz, 2Mustafa Ozturk, 3Zeynep Demir, 1Muhammet Fatih Demir, 4Beste Alimert Altunors, 5Vahit Arslan, Engin Onalan, 6Murat Aydin, 7Fatih Muhammed Kaya, 8Yaprak Demir, 9Koray Kocak, 10Mahmut Can Yagmurdur, 11Emin Ozgur Akgul, 12Sinem Tuncer
- Epigenetic Health Solutions, Unit of Nephrology, Ankara, Turkey
- Private Medistate Hospital, Department of Oncology, Istanbul, Turkey
- Wake Forest Institute for Regenerative Medicine, Winston-Salem, NY, USA
- Epigenetic Health Solutions, Unit of Nephrology, Ankara, Turkey
- Private Losante Hospital, Department of Emergency Medicine, Cankaya, Ankara,Turkey
- Private Mim Hospital, Department of Emergency Medicine, Cankaya, Ankara,Turkey
- Private Anadolu Hospital, Department of Biochemistry, Eskisehir, Turkey
- Igdir Hospital, Department of Physical Therapy and Rehabilitation, Igdir, Turkey
- Arztekammer Berlin, Germany
- Edumed Academy, Ankara, Turkey
- Phytocenter, Research Center, Ankara, Turkey
- Public Health Institution of Türkiye, Ankara Turkey
OPEN ACCESS
PUBLISHED:31 March 2025
CITATION:YILMAZ, Mahmut Ilker et al. A Different Approach to Malnutrition-Related Appetite and Weight Loss in Cancer Patients: Is Saturation Enough at The Cell Level?. Medical Research Archives. Available at: <https://esmed.org/MRA/mra/article/view/6419>.
COPYRIGHT: © 2025 European Society of Medicine. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.18103/mra.v13i3.6419.
ISSN: 2375-1924
Abstract
Background: Cancer is one of the most significant, common, and dangerous diseases of our age. Genetic and epigenetic factors play an important role in the disease process. Three key mechanisms dominate the pathogenesis of chronic diseases: inflammation, oxidative stress, and endothelial dysfunction. The proper functioning of these mechanisms is vital for maintaining health. In this context, three micronutrients stand out in operating these mechanisms. These are vitamins, trace elements and minerals. These nutrients are primarily obtained through the consumption of macromolecules such as carbohydrates, proteins, fats, vegetables, and fruits. While the disease, cancer patients may experience loss of appetite, nausea, and vomiting, leading to weight loss due to malnutrition. This malnutrition can result from chemotherapy, radiotherapy, or cancer-related factors. This seriously impairs the quality of life of cancer patients and leads to death in a short time. Aim: This study aims to evaluate whether targeted micronutrient supplementation—via three functional food supplements—can effectively correct malnutrition-related appetite loss and weight reduction in cancer patients. Specifically, the investigation focuses on determining if enhancing cellular saturation with key micronutrients can modulate critical cellular mechanisms, including inflammation, oxidative stress, and endothelial dysfunction, thereby improving nutritional status, promoting weight gain, and ultimately enhancing the quality of life and survival outcomes in patients with stage IV metastatic cancers. Methods: The aim of this study is to investigate the effects of 3 functional food supplements on 98 people with stomach (n=18), breast (n=18), colon (n=18), lung (n=24) and pancreatic cancer (n=20) who had Stage 4 metastasis and completed chemotherapy and radiotherapy treatments adult individuals. The 52-week study was completed with 51 men and 47 adult women. The median age of the participants was 61 (26-87) years. The initial weight of the patients included in the study was between 31-44 kg. In this study, Morinda citrifolia (anti-atherosclerotic liquid- AAL) (3 mL once per day orally) Omega-3 (anti-inflammatory capsules- AIC) (3 capsules once per day orally) extract with Alaskan blueberry and 21 different red purple fruit vegetables (antioxidant liquid- AOL) (30 mL once per day orally) have been used. Results: With 52 weeks of follow-up, 74 of the patients included in the study were still alive at the end of the first year. The body weights of 74 surviving patients were between 48-76 kg. The patients received vitamins, minerals and trace elements that will ensure the correct functioning of the three mechanisms with the 3 products they purchased, thus preventing malnutrition and causing a significant increase in their appetite and weight gain. Conclusions: Malnutrition, which is an important factor in the ongoing decrease in appetite and weight loss in cancer patients, is corrected with micronutrition (vitamins, minerals and trace elements) at the cellular level, and a significant improvement in appetite and weight gain prolongs the person’s survival and increases the quality of life.
Keywords
malnutrition, cancer, appetite loss, weight loss, micronutrients, supplementation
Introduction
Cancer remains a leading cause of morbidity and mortality worldwide, with approximately one in five people diagnosed with cancer before the age of 75 and nearly half of these cases resulting in death. Despite significant advances in early detection and treatment, the multifactorial etiology of cancer, which includes both genetic and epigenetic determinants, continues to complicate its management. In recent years, it has become increasingly recognized that lifestyle factors, particularly diet, are important not only in the prevention but also in the regression of malignancies.
Malnutrition is an important and often underestimated challenge in cancer. Cancer patients, due to both the disease process and the adverse effects of treatments such as chemotherapy and radiotherapy, often experience weight loss, muscle wasting and a decline in their overall nutritional status. These nutritional deficiencies are associated with a lower body mass index, sarcopenia, and reduced tolerance to treatment, and thus contribute to increased toxicity of treatment and poorer clinical outcomes.
More importantly, malnutrition exacerbates the pathophysiological mechanisms underlying cancer, including chronic inflammation, oxidative stress and endothelial dysfunction, each of which plays an important role in cancer progression.
Micronutrients, including vitamins, minerals, and trace elements, are essential for maintaining cellular integrity and regulating these critical pathways. Emerging evidence suggests that targeted nutritional interventions may help restore cellular homeostasis by mitigating inflammation, reducing oxidative stress, and improving endothelial function. Such interventions are particularly relevant for cancer patients, whose nutritional challenges extend beyond mere caloric deficiency to encompass complex metabolic and molecular derangements. This study explores a novel approach aimed at counteracting malnutrition-related appetite loss and weight reduction by administering three functional food supplements designed to optimize these cellular mechanisms. By investigating the effects of these supplements on inflammatory markers, oxidative stress indices, and endothelial function, the study seeks to determine whether enhancing micronutrient saturation at the cellular level can improve nutritional status, increase appetite, promote weight gain, and ultimately enhance the quality of life and survival in cancer patients.
Material and Methods
Study Design and Participants
This quasi-experimental pre–posttest study was conducted at the Epigenetic Health Center Outpatient Clinics in Ankara, Turkiye, from December 1, 2018, to June 1, 2021. The study design adhered to the TREND statement checklist for nonrandomized evaluations of behavioral and public health interventions. Participants were selected based on the following inclusion criteria: individuals older than 18 years with systolic blood pressure ≤140 mmHg and/or diastolic blood pressure ≤90 mmHg, and a normal estimated glomerular filtration rate (eGFR ≥90 mL/min). Patients were excluded if they had a history of treatment with angiotensin converting enzyme inhibitors or angiotensin receptor blockers, obesity (BMI >30 kg/m²), dyslipidemia (total cholesterol >280 mg/dL and/or fasting triglycerides >180 mg/dL), renal failure (eGFR <90 mL/min), nephrotic syndrome (urinary protein excretion >3000 mg/day), or a history of cardiovascular disease (evidenced by abnormal electrocardiogram, smoking, or recent/current use of statins).
Out of 261 patients who met the inclusion criteria, 98 patients (predominantly male, 94 M, with a mean age of 58 ± 14 years) with chronic diseases were enrolled in the study. These 98 patients; diagnosed with Stage 4 metastatic cancers—including stomach (n=18), breast (n=18), colon (n=18), lung (n=24), and pancreatic cancer (n=20)—who had completed chemotherapy and radiotherapy treatments. The study was conducted over a 52-week period and comprised 51 men and 47 women, with a median age of 61 years (range 26–87 years) and an initial body weight between 31 and 44 kg.
Baseline Evaluation
At baseline, each patient underwent a comprehensive evaluation that included a standard physical examination, chest X-ray, baseline electrocardiogram, and two-dimensional echocardiography. Routine clinical laboratory tests were performed, which included assessments of liver and kidney function and 24-hour urinary protein measurements. Arterial blood pressure was recorded in the right arm using a mercury sphygmomanometer in a resting condition (three separate measurements in the morning with the mean value calculated).
Measurements
Blood chemistry: Morning blood samples were collected from patients after 12 hours of fasting. Subjects were asked to refrain from physical activity for at least 30 minutes prior to the blood draw. In addition to routine clinical laboratory tests, serum ADMA, MDA, CuZn-SOD, GSH-Px, hsCRP and PTX3 concentrations and basal insulin levels were analyzed from all patients. After the intervention period, blood samples were obtained for the measurement of serum ADMA, MDA, CuZn-SOD, GSH-Px, hsCRP and PTX3 concentration. The measurement of total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL) cholesterol and fasting plasma glucose (FPG) was performed by enzymatic colorimetric method with Olympus AU 600 auto analyzer using reagents from Olympus Diagnostics, GmbH (Hamburg, Germany). Low-density lipoprotein (LDL) cholesterol was calculated by Friedewald’s formula.
Serum basal insulin values were determined by the coated tube method (DPC-USA). In particular, insulin resistances index Homeostasis Model Assessment-Insulin resistance (HOMA-IR) was computed with the formula: (HOMA-IR) = FPG (mg/dl) x immunoreactive insulin (IRI) (µIU/ml)/405. All samples were run in triplicates.
ADMA measurements: Measurements of serum ADMA were done using high performance liquid chromatography (HPLC), as described by Chen et al. In brief, 20 mg of 5-sulfosalisilic acid (5-SSA) was added to 1 ml serum and the mixture was left in an ice-bath for 10 min. The precipitated protein was removed by centrifugation at 2000 g for 10 min. Ten micro liters of the supernatant which was filtered through a 0.2 µm filter was mixed with 100 µl of derivatization reagent (prepared by dissolving 10 mg o-phtaldialdehyde in 0.5 ml of methanol, 2 ml of 0.4 M borate buffer (pH 10.0) and 30 µl of 2-mercaptoethanol) and then injected into the chromatographic system. Separation of ADMA was achieved with a 150×4 mm I.D. Nova-pak C18 column with a particle size of 5 µm (Waters, Millipore, Milford, MA, USA) using 50 mM sodium acetate (pH 6.8), methanol and tetrahydrofurane as mobile phase (A, 82:17:1; B, 22:77:1) at a flowrate of 1.0 ml/min. The area of peak detected by the fluorescent detector (Ex: 338 nm) was used as quantification. The variability of the method was less than 7%, and the detection limit of the assay was 0.01 µM.
High sensitive C reactive protein (hsCRP) assessment: Briefly, serum samples were diluted with a ratio of 1/101 with the diluent’s solution. Calibrators, kit controls and serum samples were all added on each micro well with an incubation period of 30 minutes. After 3 washing intervals 100 µL enzyme conjugate (peroxidase labeled anti-CRP) was added on each micro well for additional 15 minutes incubation in room temperature in dark. The reaction was stopped with a stop solution and photometric measurement was performed at the 450 nm wavelength.
Plasma PTX-3 measurements: Plasma PTX 3 concentration was measured posteriori from frozen samples by using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Perseus Proteomics Inc, Japan).
Erythrocyte antioxidant capacity: Blood samples were drawn after overnight fasting from the antecubital vein and collected in heparinized polypropylene tubes. Plasma and erythrocytes were separated and used for measuring trace elements and antioxidant enzymes. Erythrocyte CuZn-SOD and GSH-Px activity was measured in a UV-VIS Recording Spectrophotometer (UV-2100S; Shimadzu Co., Kyoto, Japan) as previously described by Aydin et al. Erythrocyte zinc (Zn), copper (Cu), and iron (Fe) levels were measured by flame atomic absorption spectrophotometry using a Varian atomic absorption spectrophotometer (30/40 model; Varian Techtron Pty Ltd., Victoria, Australia). The wavelengths used were as follows: 213.9-nm wavelengths for Zn, 324.7-nm wavelengths for Cu, and 248.3-nm wavelengths for Fe. Results were expressed as units per milliliter for CuZn-SOD and GSH-Px and as micrograms per milliliter for Zn, Fe, and Cu.
Erythrocyte MDA level measurement: Erythrocyte MDA levels were determined on erythrocyte lysate obtained after centrifugation and in accordance with the method described by Jain. After the reaction of thiobarbituric acid with MDA, the reaction product was measured spectrophotometrically at 532 nm. Tetrametoxypropane solution was used as standard. MDA levels of erythrocyte were expressed as nanomoles per milliliter.
Assessment of endothelial dysfunction: The determination of endothelial dysfunction was performed according to the method described by Celemajer et al. Measurements were made by a single observer using an ATL 5000 ultrasound system (Advanced Technology Laboratories Inc., Bothell, WA., USA) with a 12-Mhz probe. All vasoactive medications were withheld for 24 hours before the procedure. The subjects remained at rest in the supine position for at least 15 min before the examination started. Subject’s right arm was comfortably immobilized in the extended position to allow consistent recording of the brachial artery 2–4 cm above the antecubital fossa. Three adjacent measurements of end-diastolic brachial artery diameter were made from single 2-D frames. All ultrasound images were recorded on S-VHS videotape for subsequent blinded analysis. The maximum FMD diameters were calculated as the average of the three consecutive maximum diameter measurements after hyperemia. The FMD levels were then calculated as the percent change in diameter compared with baseline resting diameters.
Intervention Protocol
Following baseline measurements, an open-label intervention was initiated immediately. Participants, who were diagnosed with Stage 4 metastatic cancers (stomach, breast, colon, lung, or pancreatic) and had completed chemotherapy and radiotherapy, received three functional food supplements daily for 52 weeks:
- Anti-atherosclerotic liquid (AAL): 3 mL of Morinda citrifolia extract administered orally once per day.
- Anti-inflammatory capsules (AIC): 3 capsules of omega-3 administered orally once per day.
- Antioxidant liquid (AOL): 30 mL of an extract containing Alaskan blueberry and 21 different red-purple fruit and vegetables administered orally once per day.
During the study, serum creatinine and potassium levels were monitored biweekly. The dosages of the supplements were titrated to maintain serum potassium concentrations below 5.5 mEq/L. All patients continued to receive their current standard treatments for their underlying diseases, and no additional dietary or vitamin supplements were used.
Endpoints
The primary endpoint was FMD percentage change in cohort at the 12th week of the study. Secondary endpoints included status of the antioxidant parameters, inflammatory marker (hsCRP), endothelial biomarkers (ADMA, HOMA), and serum lipid profile.
Statistical Methods
With a study population of 98 patients and a standard deviation of the difference of FMD change after therapies of 0.50, our study has a 90% power to detect as statistically significant with a p value<0.001 a FMD change of 0.2% or greater. Non-normally distributed variables were expressed as median (range) and normally distributed variables as mean ± SD. A p value <0.05 was statistically significant. Kolmogorov Smirnov test was used for analysis distribution of data. One Way ANOVA, student t test and paired sample t test was used for comparing numeric data. Comparisons between groups of nominal variables were performed with the Chi-square test. Pearson’s correlation analysis was used to determine correlations between two variables. Multiple regression analysis was applied to identify the independent correlates of flow mediated dilatation. Multiple regression models were built by including all significant univariate correlates of the outcome measures (FMD changes). The models had sufficient power to test the independent association of FMD with relevant correlates, i.e. at least 10 observations per covariate in the same models. All statistical analyses were performed by using SPSS 21.0 (SPSS Inc., Chicago, IL, USA) statistical package.
Results
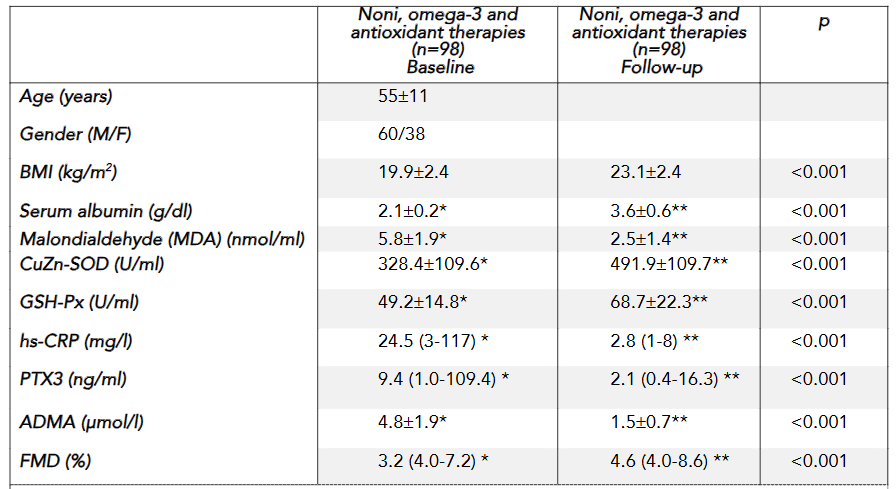
At the end of the 52-week follow-up period, 74 out of the 98 patients (approximately 75.5%) were still alive. Among these survivors, body weights increased markedly, ranging from 48 to 76 kg compared to the initial weight range of 31 to 44 kg. This improvement in nutritional status was accompanied by several significant biochemical and physiological changes.
Biochemical and Cellular Improvements
Following the administration of the three functional supplements—anti-atherosclerotic liquid (AAL), anti-inflammatory capsules (AIC), and antioxidant liquid (AOL)—we observed statistically significant reductions in serum markers associated with inflammation and oxidative stress. Specifically, there were notable decreases in serum high-sensitivity C-reactive protein (hsCRP) and pentraxin-3 (PTX3) levels, indicating reduced inflammation, while serum malondialdehyde (MDA) levels were significantly lowered, reflecting decreased oxidative stress. In addition, serum asymmetrical dimethylarginine (ADMA) levels, an indicator of endothelial dysfunction, decreased notably. These biochemical improvements were paralleled by enhanced endothelial function, as evidenced by an increase in flow-mediated dilation (FMD), suggesting that the intervention effectively improved vascular responsiveness.
Correlations Between Clinical and Biochemical Parameters
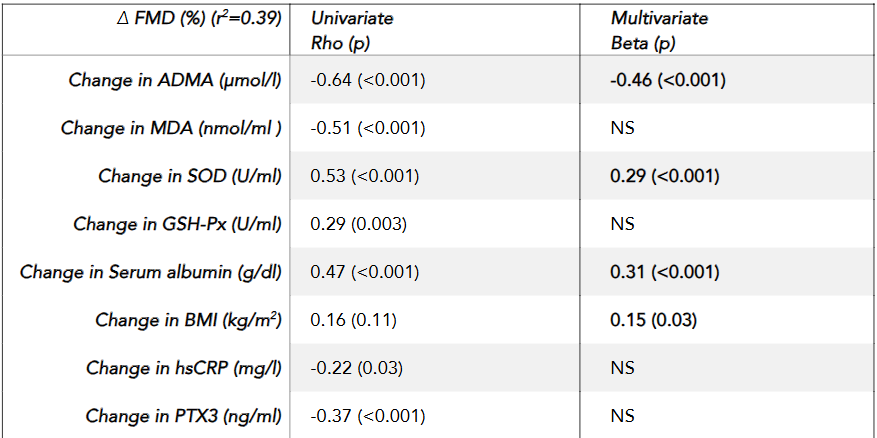
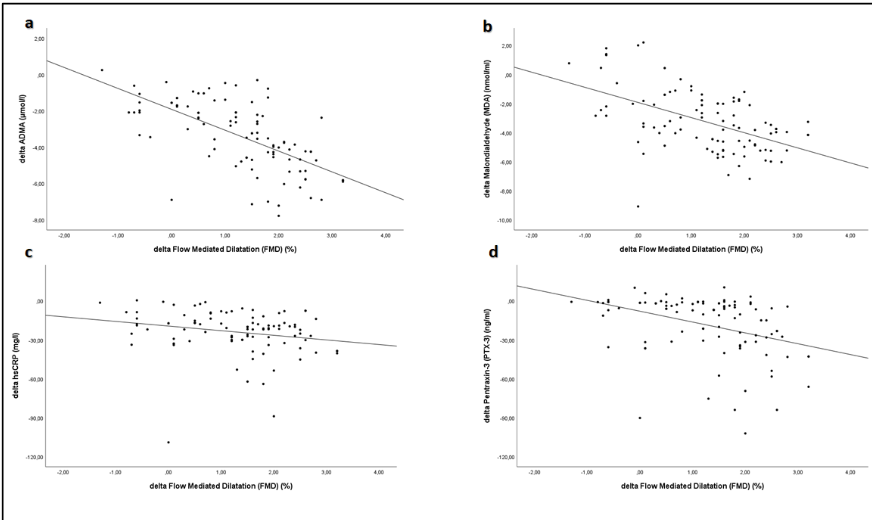
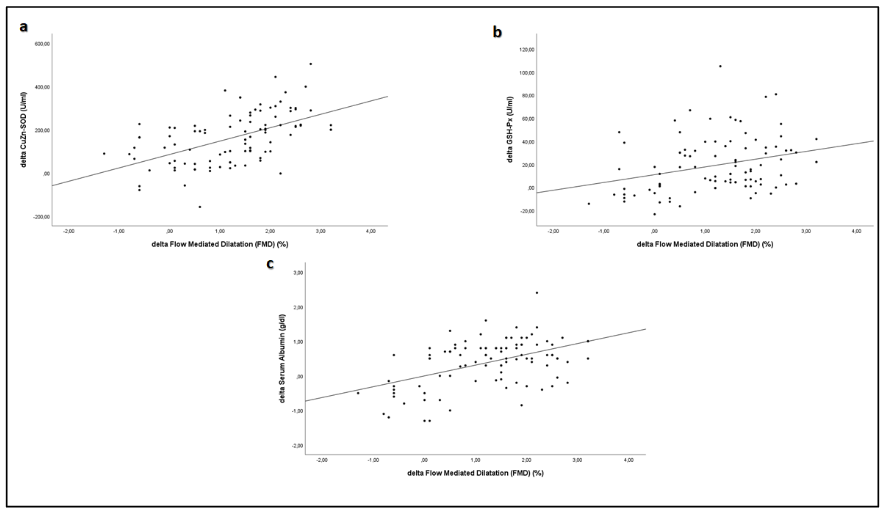
Univariate correlation analysis revealed that the percentage increase in FMD was significantly negatively correlated with reductions in serum ADMA (rho = -0.64, p < 0.001), MDA (rho = -0.51, p < 0.001), PTX3 (rho = -0.37, p < 0.001), and hsCRP (rho = -0.22, p = 0.03), while a significant positive relationship was observed between the increase in FMD and elevations in antioxidant enzymes CuZn-SOD (rho = 0.48, p < 0.001), glutathione peroxidase (GSH-Px) (rho = 0.29, p = 0.003), and serum albumin levels (rho = 0.47, p < 0.001). Multivariate regression analysis further demonstrated that changes in FMD were independently associated with reductions in ADMA (Beta = -0.46, p < 0.001), increases in CuZn-SOD (Beta = 0.29, p < 0.001), elevations in serum albumin (Beta = 0.31, p < 0.001), and increases in BMI (Beta = 0.15, p = 0.03). These findings suggest that the improvement in vascular function is closely linked to the attenuation of inflammatory and oxidative stress pathways as well as improved nutritional status.
Clinically, the intervention resulted in a significant increase in body mass index (BMI), with mean values rising from 19.9 ± 2.4 kg/m² to 23.1 ± 2.4 kg/m², and an increase in serum albumin levels from 2.1 ± 0.2 g/dl to 3.6 ± 0.6 g/dl, indicating an improved nutritional status and protein reserve—a key prognostic indicator in cancer patients. The extended results demonstrate that targeted micronutrient supplementation not only addresses biochemical imbalances by reducing inflammation and oxidative stress but also translates into tangible clinical benefits, such as weight gain and improved endothelial function. These changes collectively contribute to enhanced quality of life and may have implications for survival in patients with advanced metastatic cancers.
Discussion
Cancer cachexia affects approximately 70% of cancer patients and is responsible for up to 22% of cancer deaths. Malnutrition is estimated to affect between 15% and 80% of cancer patients, emphasizing its widespread impact. Malnutrition is common in most cancer patients and is a major cause of illness and death in advanced stages of the disease. Research from Germany, France, Spain, and Brazil found that malnutrition rates in cancer patients range from 25% to over 70%, depending on nutritional assessments. In fact, cancer patients are among the most malnourished groups of all patients.
Malnutrition is a common and serious complication in cancer patients, often resulting from inadequate food intake, weight loss, metabolic derangements, and decreased physical activity. These issues frequently arise after surgery, radiotherapy, and chemotherapy, exacerbated by treatment-related side effects such as nausea, vomiting, diarrhea, and mucositis. Studies indicate that weight loss in cancer patients ranges from 31% to 87%, and as weight loss progresses, median survival significantly decreases. Even before initiating cancer treatment, patients may experience profound metabolic and physiological changes, increasing their demand for both macro- and micronutrients. Malnutrition can be the first clinical sign of cancer and is strongly associated with increased mortality risk. Additionally, it has been linked to reduced treatment efficacy, lower quality of life, and poorer overall survival outcomes. One of the primary consequences of progressive weight loss and nutritional decline in cancer patients is a significant reduction in survival rates.
Given its profound impact, malnutrition also influences clinical decision-making regarding tumor resection, a potentially curative intervention in cancer treatment. Moreover, malnutrition has been shown to lower response rates to chemotherapy and increase the risk of chemotherapy-induced toxicity. Cancer treatments, including chemotherapy and radiotherapy, often induce symptoms that further impair food intake and quality of life, compounding the nutritional challenges faced by patients.
In our study, we demonstrated that a three-month regimen of AAL, AIC, and AOL significantly improved nutritional markers in cancer patients. Specifically, we observed statistically significant increases in serum levels of vitamin B12, vitamin D, folic acid, hemoglobin, HDL cholesterol, serum albumin, and magnesium. Concurrently, we recorded notable decreases in total cholesterol, triglycerides, LDL cholesterol, HOMA, and HbA1c values. These findings highlight the potential role of targeted nutritional supplementation in improving metabolic and biochemical parameters in cancer patients.
Preventing and Managing Cancer-Associated Malnutrition and Cachexia
Cachexia, a specific form of cancer-related malnutrition, is characterized by progressive and unintended weight loss, lean body mass reduction, and muscle wasting. Malnutrition, particularly cancer cachexia, should be prevented and closely monitored from the time of initial cancer diagnosis. Patients experiencing weight loss often have significantly decreased nutritional intake, further exacerbating their condition. This study is the first to demonstrate a significant increase in BMI (from 19.9 ± 2.4 to 23.1 ± 2.4 kg/m²) following a three-month supplementation with AAL, AIC, and AOL in cancer patients. Notably, no adverse effects were observed, and all patients tolerated the interventions well.
Preventive strategies for cancer cachexia are crucial, as cachexia remains one of the leading causes of cancer-related mortality. The prevention of cachexia observed in our study may be attributed to the suppression of systemic inflammation and oxidative stress, which are key pathophysiological drivers of cancer cachexia. Oxidative stress is a common disorder in most cancer types. Antioxidants are one of the most common types of dietary supplements and may help protect against the harmful effects of chemotherapy. Yilmaz et al. have demonstrated that effective correction of 3 important pathways in the occurrence of chronic diseases using 3 different products was effective in cancer patients and chronic diseases.
Inflammation plays a key role in the pathogenesis of cachexia. An imbalance between pro-inflammatory cytokines (such as tumor necrosis factor-α [TNF-α], IL-1, IL-6, interferon-γ [IFN-γ]) and anti-inflammatory cytokines (such as IL-4, IL-12, IL-15) is believed to contribute to the progression of cachexia. In this study, we demonstrated increases in serum CuZn-SOD (U/ml) (328.4 ± 109.5 to 491.9 ± 109.7), GSH-Px (U/ml) (49.2 ± 14.8 to 68.7 ± 22.3), and FMD (%) (3.2 to 4.6) values, along with decreases in serum MDA (nmol/ml) (5.8 ± 1.9 to 2.5 ± 1.4), hs-CRP (mg/l) (24.5 to 2.8), PTX3 (ng/ml) (9.4 to 2.1), and ADMA (µmol/l) (4.8 ± 1.9 to 1.5). These findings show that the use of AAL, AIC, and AOL significantly reduces inflammation and oxidative stress and has beneficial effects on endothelial dysfunction in terminally ill cancer patients. Avoiding these mechanisms may prevent cancer cachexia. These micronutrients should always be considered in nutritional care of terminally ill cancer patients with the aim of palliation, which supports nutritional status, body composition, and quality of life.
Our analyses suggest that the three investigated mechanisms—improved nutritional status, reduced inflammatory/oxidative stress biomarkers, and enhanced endothelial function—are interlinked and may collectively contribute to extending the lifespan of patients. By addressing the malnutrition-induced triggers of inflammation and atherosclerosis, the nutrients administered in this study appear to hold promise in slowing disease progression and potentially enhancing survival outcomes in this high-risk patient population.
While these findings are promising, the study’s observational design and sample size limit the strength of the conclusions. There is a clear need for larger, randomized controlled trials to further analyze the long-term impact of these supplements on life expectancy. Future studies should also investigate the sustainability of the observed benefits and clarify the precise biological mechanisms by which nutritional interventions modulate inflammation and vascular health in advanced cancer patients.
Conclusion
This study demonstrates that targeted micronutrient supplementation with three functional food supplements – Anti-Atherosclerotic Liquid (AAL), Anti-Inflammatory Capsules (AIC) and Antioxidant Liquid (AOL) – can effectively address malnutrition-related appetite loss and weight loss in patients with advanced metastatic cancer. The intervention was associated with significant weight gain, higher body mass index and serum albumin levels, as well as significantly lower serum markers of inflammatory (hsCRP and PTX3), oxidative stress (MDA) and endothelial dysfunction (ADMA). These biochemical improvements were accompanied by improved endothelial function as evidenced by increased flow-mediated dilatation (FMD), indicating a restoration of cellular homeostasis. The significant correlations observed between improved FMD and both reduced markers of inflammation/oxidative stress and increased levels of antioxidant enzymes further highlight the critical role of micronutrient saturation at the cellular level in counteracting the deleterious effects of cancer cachexia. Collectively, these findings suggest that this novel nutritional strategy may not only improve nutritional status and quality of life but also prolong survival in terminal cancer patients. Future research should confirm these findings in larger, controlled trials and explore integrating micronutrient-based interventions into standard oncology care.
Conflict of Interest:
None
Funding Statement:
None.
Acknowledgements:
None.
References:
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: An overview. Int J Cancer. Apr 5 2021;doi:10.1002/ijc.33588
- Taylor SR, Falcone JN, Cantley LC, Goncalves MD. Developing dietary interventions as therapy for cancer. Nat Rev Cancer. Aug 2022;22(8):452-466. doi:10.1038/s41568-022-00485-y
- Song M, Giovannucci E. Preventable Incidence and Mortality of Carcinoma Associated With Lifestyle Factors Among White Adults in the United States. JAMA Oncol. Sep 1 2016;2(9):1154-61. doi:10.1001/jamaoncol.2016.0843
- Tajan M, Vousden KH. Dietary Approaches to Cancer Therapy. Cancer Cell. Jun 8 2020;37(6):767-785. doi:10.1016/j.ccell.2020.04.005
- Kanarek N, Petrova B, Sabatini DM. Dietary modifications for enhanced cancer therapy. Nature. Mar 2020;579(7800):507-517. doi:10.1038/s41586-020-2124-0
- Antoun S, Baracos VE, Birdsell L, Escudier B, Sawyer MB. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann Oncol. Aug 2010;21(8):1594-1598. doi:10.1093/annonc/mdp605
- Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. Jul 1 2012;118(13):3377-86. doi:10.1002/cncr.26646
- Sánchez-Lara K, Turcott JG, Juárez E, et al. Association of nutrition parameters including bioelectrical impedance and systemic inflammatory response with quality of life and prognosis in patients with advanced non-small-cell lung cancer: a prospective study. Nutr Cancer. 2012;64(4):526-34. doi:10.1080/01635581.2012.668744
- Zhang X, Edwards BJ. Malnutrition in Older Adults with Cancer. Curr Oncol Rep. Jul 29 2019;21(9):80. doi:10.1007/s11912-019-0829-8
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. Feb 17 2001;357(9255):539-45. doi:10.1016/s0140-6736(00)04046-0
- Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative Stress in Cancer. Cancer Cell. Aug 10 2020;38(2):167-197. doi:10.1016/j.ccell.2020.06.001
- Richards J, Arensberg MB, Thomas S, Kerr KW, Hegazi R, Bastasch M. Impact of Early Incorporation of Nutrition Interventions as a Component of Cancer Therapy in Adults: A Review. Nutrients. Nov 5 2020;12(11)doi:10.3390/nu12113403
- Yilmaz MI, Romano M, Basarali MK, et al. The Effect of Corrected Inflammation, Oxidative Stress and Endothelial Dysfunction on Fmd Levels in Patients with Selected Chronic Diseases: A Quasi-Experimental Study. Sci Rep. Jun 2 2020;10(1):9018. doi:10.1038/s41598-020-65528-6
- Terwoord JD, Beyer AM, Gutterman DD. Endothelial dysfunction as a complication of anti-cancer therapy. Pharmacol Ther. Sep 2022;237:108116. doi:10.1016/j.pharmthera.2022.108116
- Barnighausen T, Rottingen JA, Rockers P, Shemilt I, Tugwell P. Quasi-experimental study designs series-paper 1: introduction: two historical lineages. J Clin Epidemiol. Sep 2017;89:4-11. doi:10.1016/j.jclinepi.2017.02.020
- Des Jarlais DC, Lyles C, Crepaz N, Group T. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. American journal of public health. 2004;94(3):361-366.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. Jun 1972;18(6):499-502.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. Jul 1985;28(7):412-9. doi:10.1007/bf00280883
- Chen BM, Xia LW, Zhao RQ. Determination of N(G),N(G)-dimethylarginine in human plasma by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. May 9 1997;692(2):467-71. doi:10.1016/s0378-4347(96)00531-2
- Aydin A, Orhan H, Sayal A, Ozata M, Sahin G, Isimer A. Oxidative stress and nitric oxide related parameters in type II diabetes mellitus: effects of glycemic control. Clin Biochem. Feb 2001;34(1):65-70. doi:10.1016/s0009-9120(00)00199-5
- Jain SK. Hyperglycemia can cause membrane lipid peroxidation and osmotic fragility in human red blood cells. J Biol Chem. Dec 15 1989;264(35):21340-5.
- Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. Nov 7 1992;340(8828):1111-5. doi:10.1016/0140-6736(92)93147-f
- von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle. Sep 2010;1(1):1-5. doi:10.1007/s13539-010-0002-6
- Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. Oct 1980;69(4):491-7. doi:10.1016/s0149-2918(05)80001-3
- Laviano A, Meguid MM. Nutritional issues in cancer management. Nutrition. May 1996;12(5):358-71. doi:10.1016/s0899-9007(96)80061-x
- Nitenberg G, Raynard B. Nutritional support of the cancer patient: issues and dilemmas. Crit Rev Oncol Hematol. Jun 2000;34(3):137-68. doi:10.1016/s1040-8428(00)00048-2
- Elia M, Van Bokhorst-de van der Schueren MA, Garvey J, et al. Enteral (oral or tube administration) nutritional support and eicosapentaenoic acid in patients with cancer: a systematic review. Int J Oncol. Jan 2006;28(1):5-23. doi:10.3892/ijo.28.1.5
- Camilo ME. Disease-related Malnutrition: An Evidence-based Approach to Treatment: Rebecca J. Stratton, Ceri J. Green, M. Elia (eds.) CABI Publishing, Wallingford, UK 2003. Clinical Nutrition. 2003;22(6):585. doi:10.1016/j.clnu.2003.08.003
- Stratton RJ, Green CJ, Elia M. Disease-related malnutrition: an evidence-based approach to treatment. Cabi; 2003.
- Maasberg S, Knappe-Drzikova B, Vonderbeck D, et al. Malnutrition Predicts Clinical Outcome in Patients with Neuroendocrine Neoplasia. Neuroendocrinology. 2017;104(1):11-25. doi:10.1159/000442983
- Attar A, Malka D, Sabaté JM, et al. Malnutrition is high and underestimated during chemotherapy in gastrointestinal cancer: an AGEO prospective cross-sectional multicenter study. Nutr Cancer. 2012;64(4):535-42. doi:10.1080/01635581.2012.670743
- Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J Parenter Enteral Nutr. Feb 2014;38(2):196-204. doi:10.1177/0148607113502674
- Pressoir M, Desné S, Berchery D, et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br J Cancer. Mar 16 2010;102(6):966-71. doi:10.1038/sj.bjc.6605578
- Planas M, Álvarez-Hernández J, León-Sanz M, Celaya-Pérez S, Araujo K, García de Lorenzo A. Prevalence of hospital malnutrition in cancer patients: a sub-analysis of the PREDyCES® study. Support Care Cancer. Jan 2016;24(1):429-435. doi:10.1007/s00520-015-2813-7
- Silva FR, de Oliveira MG, Souza AS, Figueroa JN, Santos CS. Factors associated with malnutrition in hospitalized cancer patients: a cross-sectional study. Nutr J. Dec 10 2015;14:123. doi:10.1186/s12937-015-0113-1
- Ryan AM, Power DG, Daly L, Cushen SJ, Ní Bhuachalla É, Prado CM. Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc. May 2016;75(2):199-211. doi:10.1017/s002966511500419x
- Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. Feb 2013;10(2):90-9. doi:10.1038/nrclinonc.2012.209
- Fearon KC, Voss AC, Hustead DS. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr. Jun 2006;83(6):1345-50. doi:10.1093/ajcn/83.6.1345
- Meguid MM, Mughal MM, Debonis D, Meguid V, Terz JJ. Influence of nutritional status on the resumption of adequate food intake in patients recovering from colorectal cancer operations. Surg Clin North Am. Dec 1986;66(6):1167-76. doi:10.1016/s0039-6109(16)44080-6
- Deeg HJ, Seidel K, Bruemmer B, Pepe MS, Appelbaum FR. Impact of patient weight on non-relapse mortality after marrow transplantation. Bone Marrow Transplant. Mar 1995;15(3):461-8.
- Rey-Ferro M, Castaño R, Orozco O, Serna A, Moreno A. Nutritional and immunologic evaluation of patients with gastric cancer before and after surgery. Nutrition. Oct 1997;13(10):878-81. doi:10.1016/s0899-9007(97)00269-4
- Dickson TM, Kusnierz-Glaz CR, Blume KG, et al. Impact of admission body weight and chemotherapy dose adjustment on the outcome of autologous bone marrow transplantation. Biol Blood Marrow Transplant. 1999;5(5):299-305. doi:10.1016/s1083-8791(99)70005-4
- Persson C, Sjödén PO, Glimelius B. The Swedish version of the patient-generated subjective global assessment of nutritional status: gastrointestinal vs urological cancers. Clin Nutr. Apr 1999;18(2):71-7. doi:10.1016/s0261-5614(99)80054-5
- Capra S, Ferguson M, Ried K. Cancer: impact of nutrition intervention outcome–nutrition issues for patients. Nutrition. Sep 2001;17(9):769-72. doi:10.1016/s0899-9007(01)00632-3
- Andreyev HJ, Norman AR, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer. Mar 1998;34(4):503-9. doi:10.1016/s0959-8049(97)10090-9
- Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. Dec 22 2010;9:69. doi:10.1186/1475-2891-9-69
- Santarpia L, Contaldo F, Pasanisi F. Nutritional screening and early treatment of malnutrition in cancer patients. J Cachexia Sarcopenia Muscle. Mar 2011;2(1):27-35. doi:10.1007/s13539-011-0022-x
- Rickard KA, Detamore CM, Coates TD, et al. Effect of nutrition staging on treatment delays and outcome in Stage IV neuroblastoma. Cancer. Aug 15 1983;52(4):587-98. doi:10.1002/1097-0142(19830815)52:4<587::aid-cncr2820520402>3.0.co;2-t
- Bozzetti F. Nutritional support of the oncology patient. Crit Rev Oncol Hematol. Aug 2013;87(2):172-200. doi:10.1016/j.critrevonc.2013.03.006
- Langer CJ, Hoffman JP, Ottery FD. Clinical significance of weight loss in cancer patients: rationale for the use of anabolic agents in the treatment of cancer-related cachexia. Nutrition. 2001;17(1):S1-S21.
- Tisdale MJ. Cancer anorexia and cachexia. Nutrition. 2001;17(5):438-442.
- Tisdale MJ. Cancer cachexia. Langenbeck’s Archives of Surgery. 2004;389:299-305.
- Bozzetti F. The patient with incurable aphagic cancer: to feed or not to feed? Nutrition. Jul-Aug 2001;17(7-8):676-7. doi:10.1016/s0899-9007(01)00603-7
- Barber MD, Ross JA, Fearon KC. Cancer cachexia. Surg Oncol. Nov 1999;8(3):133-41. doi:10.1016/s0960-7404(99)00045-6
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. May 2011;12(5):489-95. doi:10.1016/s1470-2045(10)70218-7
- Argiles JM, Lopez-Soriano FJ, Busquets S. Counteracting inflammation: a promising therapy in cachexia. Crit Rev Oncog. 2012;17(3):253-62. doi:10.1615/critrevoncog.v17.i3.30
- Porporato PE. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis. Feb 22 2016;5(2):e200. doi:10.1038/oncsis.2016.3
- Brown NS, Bicknell R. Hypoxia and oxidative stress in breast cancer. Oxidative stress: its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res. 2001;3(5):323-7. doi:10.1186/bcr315
- Oliveira CP, Kassab P, Lopasso FP, et al. Protective effect of ascorbic acid in experimental gastric cancer: reduction of oxidative stress. World J Gastroenterol. Mar 2003;9(3):446-8. doi:10.3748/wjg.v9.i3.446
- Azad N, Rojanasakul Y, Vallyathan V. Inflammation and lung cancer: roles of reactive oxygen/nitrogen species. J Toxicol Environ Health B Crit Rev. Jan 2008;11(1):1-15. doi:10.1080/10937400701436460
- Siddiqui R, Pandya D, Harvey K, Zaloga GP. Nutrition modulation of cachexia/proteolysis. Nutr Clin Pract. Apr 2006;21(2):155-67. doi:10.1177/0115426506021002155
- Saini A, Al-Shanti N, Stewart CE. Waste management – cytokines, growth factors and cachexia. Cytokine Growth Factor Rev. Dec 2006;17(6):475-86. doi:10.1016/j.cytogfr.2006.09.006
- Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. European journal of oncology nursing. 2005;9:S51-S63.
- Kabata P, Jastrzębski T, Kąkol M, et al. Preoperative nutritional support in cancer patients with no clinical signs of malnutrition—prospective randomized controlled trial. Supportive Care in Cancer. 2015;23:365-370.

