A Systematic Review of the Efficacy and Safety of Favipiravir (Avigan) for the Treatment of Novel COVID-19 Infections
Main Article Content
Abstract
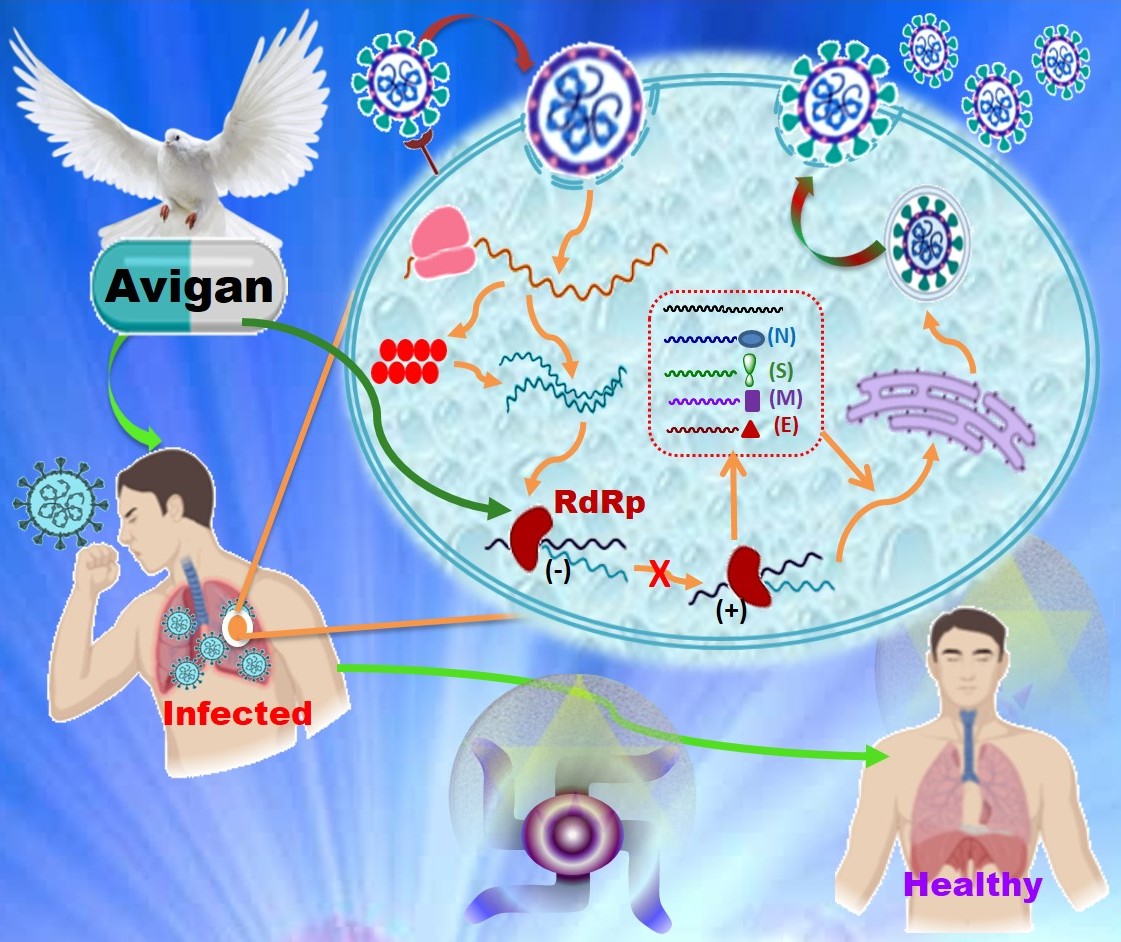
Since the emergence of SARS-CoV-2 infection in Wuhan, China, in December 2019, the spread has caused COVID19 disease in over 8.1 million people and more than 439 thousand deaths across the globe as on 16th June 2020. Absence of any particular cure or vaccine lead the health care system to treat the patients with the existing potential antiviral drugs. Recently, Favipiravir, a potential RdRp inhibitor of SARS-COV-2 sheds the ray of hope for treating COVID-19 patients efficiently.
Article Details
The Medical Research Archives grants authors the right to publish and reproduce the unrevised contribution in whole or in part at any time and in any form for any scholarly non-commercial purpose with the condition that all publications of the contribution include a full citation to the journal as published by the Medical Research Archives.
References
2. Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proceedings of the Japan Academy, Series B. 2017 Aug 2;93(7):449-63.
3. Zhong NS, Zheng BJ, Li YM, Poon LL, Xie ZH, Chan KH, Li PH, Tan SY, Chang Q, Xie JP, Liu XQ. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. The Lancet. 2003 Oct 25;362(9393):1353-8.
4. Drosten C, Günther S, Preiser W, Van Der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, Berger A. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. New England journal of medicine. 2003 May 15;348(20):1967-76.
5. Guan Y, Peiris JS, Zheng B, Poon LL, Chan KH, Zeng FY, Chan CW, Chan MN, Chen JD, Chow KY, Hon CC. Molecular epidemiology of the novel coronavirus that causes severe acute respiratory syndrome. The Lancet. 2004 Jan 10;363(9403):99-104.
6. World Health Organization. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. http://www. who. int/csr/sars/country/table2004_04_21/en/index. html. 2003 Sep.
7. Wang M, Yan M, Xu H, Liang W, Kan B, Zheng B, Chen H, Zheng H, Xu Y, Zhang E, Wang H. SARS-CoV infection in a restaurant from palm civet. Emerging infectious diseases. 2005 Dec;11(12):1860.
8. Wise J. Patient with new strain of coronavirus is treated in intensive care at London hospital.
9. Lung Injury Investigation Committee. Korea Centers for Disease Control and Prevention. Report of the incidence of humidifier disinfectant-associated damages. Seoul: Han Rim Won Publishing. 2014:39.
10. Phan LT, Nguyen TV, Luong QC, Nguyen TV, Nguyen HT, Le HQ, Nguyen TT, Cao TM, Pham QD. Importation and human-to-human transmission of a novel coronavirus in Vietnam. New England Journal of Medicine. 2020 Feb 27;382(9):872-4.
11. Riou J, Althaus CL. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Eurosurveillance. 2020 Jan 30;25(4):2000058.
12. Parry J. China coronavirus: cases surge as official admits human to human transmission.
13. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KS, Lau EH, Wong JY, Xing X. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. New England Journal of Medicine. 2020 Jan 29.
14. Vickers NJ. Animal Communication: When I’m Calling You, Will You Answer Too?. Current Biology. 2017 Jul 24;27(14):R713-5.
15. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 Mar 5.
16. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell research. 2020 Mar;30(3):269-71.
17. Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, Li Y, Hu Z, Zhong W, Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell discovery. 2020 Mar 18;6(1):1-4.
18. Renna M, Schaffner C, Brown K, Shang S, Tamayo MH, Hegyi K, Grimsey NJ, Cusens D, Coulter S, Cooper J, Bowden AR. Azithromycin blocks autophagy and may predispose cystic fibrosis patients to mycobacterial infection. The Journal of clinical investigation. 2011 Aug 1;121(9).
19. Tran DH, Sugamata R, Hirose T, Suzuki S, Noguchi Y, Sugawara A, Ito F, Yamamoto T, Kawachi S, Akagawa KS, Ōmura S. Azithromycin, a 15-membered macrolide antibiotic, inhibits influenza A (H1N1) pdm09 virus infection by interfering with virus internalization process. The Journal of antibiotics. 2019 Oct;72(10):759-68.
20. Guy RK, DiPaola RS, Romanelli F, Dutch RE. Rapid repurposing of drugs for COVID-19. Science. 2020 May 22;368(6493):829-30.
21. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DS, Du B. Clinical characteristics of coronavirus disease 2019 in China. New England journal of medicine. 2020 Apr 30;382(18):1708-20.
22. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 Mar 5.
23. Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. Journal of Advanced Research. 2020 Mar 16.
24. Xia S, Liu M, Wang C, Xu W, Lan Q, Feng S, Qi F, Bao L, Du L, Liu S, Qin C. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell research. 2020 Apr;30(4):343-55.
25. Holmes KV. SARS-associated coronavirus. New England Journal of Medicine. 2003 May 15;348(20):1948-51.
26. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD. A pneumonia outbreak associated with a new coronavirus of probable bat origin. nature. 2020 Mar;579(7798):270-3.
27. Zowalaty ME, Järhalt JD. From SARS to COVID-19: A previously unknown SARS-related coronavirus (SARS-CoV-2) of pandemic potential infecting humans–Call for a One Health approach. One Health. 2020;9(100124):10-16.
28. Bosch BJ, Martina BE, van der Zee R, Lepault J, Haijema BJ, Versluis C, Heck AJ, de Groot R, Osterhaus AD, Rottier PJ. Severe acute respiratory syndrome coronavirus (SARS-CoV) infection inhibition using spike protein heptad repeat-derived peptides. Proceedings of the National Academy of Sciences. 2004 Jun 1;101(22):8455-60.
29. Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, evaluation and treatment coronavirus (COVID-19). InStatpearls [internet] 2020 Mar 8. StatPearls Publishing.
30. Lei J, Kusov Y, Hilgenfeld R. Nsp3 of coronaviruses: Structures and functions of a large multi-domain protein. Antiviral research. 2018 Jan 1;149:58-74.
31. Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proceedings of the Japan Academy, Series B. 2017 Aug 2;93(7):449-63.
32. Sissoko D, Laouenan C, Folkesson E, M’lebing AB, Beavogui AH, Baize S, Camara AM, Maes P, Shepherd S, Danel C, Carazo S. Experimental treatment with favipiravir for Ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLoS medicine. 2016 Mar;13(3).
33. Bai CQ, Mu JS, Kargbo D, Song YB, Niu WK, Nie WM, Kanu A, Liu WW, Wang YP, Dafae F, Yan T. Clinical and virological characteristics of Ebola virus disease patients treated with favipiravir (T-705)—Sierra Leone, 2014. Clinical Infectious Diseases. 2016 Nov 15;63(10):1288-94.
34. Furuta Y, Takahashi K, Kuno-Maekawa M, Sangawa H, Uehara S, Kozaki K, Nomura N, Egawa H, Shiraki K. Mechanism of action of T-705 against influenza virus. Antimicrobial agents and chemotherapy. 2005 Mar 1;49(3):981-6.
35. Baranovich T, Wong SS, Armstrong J, Marjuki H, Webby RJ, Webster RG, Govorkova EA. T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro. Journal of virology. 2013 Apr 1;87(7):3741-51.
36. de Ávila AI, Gallego I, Soria ME, Gregori J, Quer J, Esteban JI, Rice CM, Domingo E, Perales C. Lethal mutagenesis of hepatitis C virus induced by favipiravir. PloS one. 2016;11(10).
37. Arias A, Thorne L, Goodfellow I. Favipiravir elicits antiviral mutagenesis during virus replication in vivo. Elife. 2014 Oct 21;3:e03679.
38. Bocan TM, Basuli F, Stafford RG, Brown JL, Zhang X, Duplantier AJ, Swenson RE. Synthesis of [18 F] Favipiravir and Biodistribution in C3H/HeN Mice as Assessed by Positron Emission Tomography. Scientific reports. 2019 Feb 11;9(1):1-0.
39. Madelain V, Guedj J, Mentré F, Nguyen TH, Jacquot F, Oestereich L, Kadota T, Yamada K, Taburet AM, De Lamballerie X, Raoul H. Favipiravir pharmacokinetics in nonhuman primates and insights for future efficacy studies of hemorrhagic fever viruses. Antimicrobial agents and chemotherapy. 2017 Jan 1;61(1):e01305-16.
40. Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proceedings of the Japan Academy, Series B. 2017 Aug 2;93(7):449-63.
41. Wang Y, Fan G, Salam A, Horby P, Hayden FG, Chen C, Pan J, Zheng J, Lu B, Guo L, Wang C. Comparative effectiveness of combined favipiravir and oseltamivir therapy versus oseltamivir monotherapy in critically ill patients with influenza virus infection. The Journal of Infectious Diseases. 2020 Apr 27;221(10):1688-98.
42. Madelain V, Nguyen TH, Olivo A, De Lamballerie X, Guedj J, Taburet AM, Mentré F. Ebola virus infection: review of the pharmacokinetic and pharmacodynamic properties of drugs considered for testing in human efficacy trials. Clinical pharmacokinetics. 2016 Aug 1;55(8):907-23.
43. Du YX, Chen XP. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019‐nCoV infection. Clinical Pharmacology& Therapeutics. 2020 Apr 4.
44. Zhao Y, Harmatz JS, Epstein CR, Nakagawa Y, Kurosaki C, Nakamura T, Kadota T, Giesing D, Greenblatt DJ. Favipiravir inhibits acetaminophen sulfate formation but minimally affects systemic pharmacokinetics of acetaminophen. British journal of clinical pharmacology. 2015 Nov 1;80(5):1076-85.
45. Obach RS, Huynh P, Allen MC, Beedham C. Human liver aldehyde oxidase: inhibition by 239 drugs. The Journal of Clinical Pharmacology. 2004 Jan;44(1):7-19.
46. Renwick AB, Ball SE, Tredger JM, Price RJ, Walters DG, Kao J, Scatina JA, Lake BG. Inhibition of zaleplon metabolism by cimetidine in the human liver: in vitro studies with subcellular fractions and precision-cut liver slices. Xenobiotica. 2002 Jan 1;32(10):849-62.
47. Dalvie D, Di L. Aldehyde oxidase and its role as a drug metabolizing enzyme. Pharmacology & therapeutics. 2019 May 24.
48. Rochat B, Kosel M, Boss G, Testa B, Gillet M, Baumann P. Stereoselective biotransformation of the selective serotonin reuptake inhibitor citalopram and its demethylated metabolites by monoamine oxidases in human liver. Biochemical pharmacology. 1998 Jul 1;56(1):15-23.
49. Lake BG, Ball SE, Kao J, Renwick AB, Price RJ, Scatina JA. Metabolism of zaleplon by human liver: evidence for involvement of aldehyde oxidase. Xenobiotica. 2002 Jan 1;32(10):835-47.
50. Oliver BG, Robinson P, Peters M, Black J. Viral infections and asthma: an inflammatory interface?.
51. Novel CP. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghualiuxingbingxuezazhi= Zhonghualiuxingbingxuezazhi. 2020 Feb 17;41(2):145.
52. National Institutes of Health. Global Initiative for Asthma. Global strategy for asthma management and prevention. NHLBI/WHO work shop report. 1995.
53. Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, Van Weel C, Zielinski J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. American journal of respiratory and critical care medicine. 2007 Sep 15;176(6):532-55.
54. Yang M, Zhang Y, Chen H, Lin J, Zeng J, Xu Z. Inhaled corticosteroids and risk of upper respiratory tract infection in patients with asthma: a meta-analysis. Infection. 2019 Jun 1;47(3):377-85.
55. Yang M, Chen H, Zhang Y, Du Y, Xu Y, Jiang P, Xu Z. Long-term use of inhaled corticosteroids and risk of upper respiratory tract infection in chronic obstructive pulmonary disease: a meta-analysis. Inhalation toxicology. 2017 Apr 16;29(5):219-26.
56. Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews. 2012 (7).
57. Davies JM, Carroll ML, Li H, Poh AM, Kirkegard D, Towers M, Upham JW. Budesonide and formoterol reduce early innate antiviral immune responses in vitro. PLoS One. 2011;6 (11).
58. Simpson JL, Carroll M, Yang IA, Reynolds PN, Hodge S, James AL, Gibson PG, Upham JW. Reduced antiviral interferon production in poorly controlled asthma is associated with neutrophilic inflammation and high-dose inhaled corticosteroids. Chest. 2016 Mar 1;149(3):704-13.
59. Singanayagam A, Glanville N, Girkin JL, Ching YM, Marcellini A, Porter JD, Toussaint M, Walton RP, Finney LJ, Aniscenko J, Zhu J. Corticosteroid suppression of antiviral immunity increases bacterial loads and mucus production in COPD exacerbations. Nature communications. 2018 Jun 8;9(1):1-6.
60. Irie K, Nakagawa A, Fujita H, Tamura R, Eto M, Ikesue H, Muroi N, Tomii K, Hashida T. Pharmacokinetics of Favipiravir in Critically Ill Patients with COVID‐19. Clinical and Translational Science. 2020 May 31.
61. Jeon S, Ko M, Lee J, Choi I, Byun SY, Park S, Shum D, Kim S. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrobial Agents and Chemotherapy. 2020 May 4.
62. Halpin DM, Singh D, Hadfield RM. Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective. European Respiratory Society. 2020.
63. World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. World Health Organization; 2020.