Impact of diet and exercise training on physical performance and body composition in male rats: influence of ad libitum feeding and mild caloric restriction
Main Article Content
Abstract
Background: Improvements in body composition have been reported when men and women switch from consuming a Western Diet (WD) to a purified and “clean” diet (PD) known as a "Daniel Fast.” While no laboratory studies have determined physical performance improvements following the Daniel Fast, numerous anecdotal reports of improved physical capacity and well-being have been noted.
Methods: In the present study, Long-Evans rats (N=28) were assigned to be exercise trained (+E) by running on a treadmill three days per week or to act as sedentary controls. After baseline physical performance was evaluated by recording run time to exhaustion, half of the animals in each group were fed for 12 months either a PD formulated to mimic the Daniel Fast, or a WD. Food was provided ad libitum during months 1-3 and was restricted to 90% of animals’ daily needs during months 4-12. Physical performance was evaluated again at the end of months 3, 6, 9, and 12, as was body composition and bone mineral density using dual energy x-ray absorptiometry.
Results: As expected, exercise resulted in an increase in run time as compared to the non-exercise control animals (p<0.05). Dietary intake did not influence run time. Body composition was largely impacted by diet composition, with the PD resulting in less total mass and fat mass as compared to the WD (p<0.05), with only small differences noted in lean mass between diet groups. Bone mineral density increased in a linear fashion from months 3-12, with no differences noted based on diet or exercise training.
Conclusion: A WD, with and without exercise, results in increased total and fat mass in male rats. A PD allows animals to maintain a relatively low body fat, without leading to adverse outcomes pertaining to lean body mass or bone mineral density.

1. Introduction
It is well known that body composition can be influenced by dietary modification, sometimes involving the restriction of certain foods and/or the reduction in total calories. Veganism is a dietary plan that eliminates all animal products and has been linked to a variety of favorable health outcomes.1 While typical caloric restriction plans often reduce calories by 20-30% of normal intake to promote health benefits,2 veganism allows for free access to food, which is sometimes met with more favorable compliance as compared to caloric restriction plans.
The Daniel Fast (PD) is a biblically-inspired fasting plan that has been investigated in human subjects. While it resembles a vegan diet plan, it is much more stringent and places restrictions on the type of food that is allowed,3,4 with choices mainly limited to whole grains, fruits, vegetables, legumes, nuts, seeds, and plant-based oils. No alcohol, sweeteners, or refined foods are allowed with this plan. Due to the limitations, the PD has an abundance of dietary fiber and plant-derived fatty acids, as well as a high amount of antioxidants.
In our human studies with the Daniel Fast, we have observed a variety of health-specific outcomes, including a reduction in body mass and body fat.3,4,5 While we have received anecdotal reports from research participants of improved levels of energy and overall mood,3,4,5 objective physical performance measures have not been investigated in a controlled study. It is possible that a purified diet similar to the PD plan, with and without regular exercise, may yield favorable changes in both body mass/fat and physical performance over time.
The present study sought to determine the independent and combined influences of dietary composition (under conditions of ad libitum intake and mild caloric restriction) and exercise training on functional capacity and body composition in male rats.
2. Materials and Methods
Male Long-Evans rats (N=28) were purchased from Harlan Laboratories, Inc. (Indianapolis, IN) at the age of 3-4 weeks. Upon arrival, all rats were individually housed in standard shoebox caging in a climate controlled room (21ºC) employing a standard 12:12-h light-dark cycle (lights on 0800 hr). They were initially fed a standard rat chow (Harlan 1018) with ad libitum water, and then transitioned to the assigned diet over a two week period by gradually replacing the standard chow diet with an increasing proportion of the experimental diet. Rats were familiarized with the treadmill on three separate days (i.e., walking on the treadmill for 5 minutes at 15-20 m∙min-1) and the 12:12-h light-dark cycle was progressively shifted to lights on at 0300 hr. All housing and experimental procedures were approved by The University of Memphis Institutional Animal Care and Use Committee (Approval # 0734) and were in accordance with the 8th edition of the Guide for the Care and Use of Laboratory Animals.6 Animals were used for this investigation, as we knew that we could fully control the food and exercise patterns, which would not be possible in human subjects, in particular over such a long period of time. Moreover, we also collected tissues (e.g., skeletal muscle, brain) from the animals for biochemical analyses which are not presented here—something that could not have been done if using human subjects.
2.1 Functional Capacity Assessment
Treadmill running time was determined for all animals after the two-week acclimation period following established procedures.7,8 Animals began the test by running on a motorized treadmill (Exer-6M Treadmill, Columbus Instruments, Columbus, OH) at a speed of 20 m∙min-1 without incline for 15 minutes. Speed increased by 5 m∙min-1 every 15 minutes to a maximum speed of 35 m∙min-1. A mild electrical shock (frequency current at 3.0 hz at 1.60 mA with a voltage of a 115) was provided when the animals could not maintain pace with the treadmill belt. Fatigue was considered to occur when an animal started to lower its hindquarters and raise its snout, resulting in a significantly altered gait, to the point of not being able to remain on the treadmill. When this degree of fatigue was noted and the animal had difficulty remaining on the treadmill belt (regardless of the delivery of the electrical shock), the animal was taken off the treadmill and run time was recorded to the nearest second. Testing was performed during the light phase, but during the later hours (between 0900 – 0200 hr) or just before onset of the dark phase. The initial test result was considered as the baseline (month 0). All rats repeated the same treadmill test at the end of months 3, 6, 9, and 12 of the intervention.
2.2 Anthropometric Assessments
Body mass was measured daily using a Mettler Toledo PG2002-S balance equipped with dynamic weighing. At the end of months 3, 6, 9, and 12 of the intervention, all animals underwent a dual energy x-ray absorptiometry (DXA) exam using a Hologic device (Discovery QDR series, Hologic Inc., Bedford, MA). The scans were performed one week following the treadmill test. The rats were anesthetized using isoflurane for a total duration of approximately 10 minutes while the scan was performed. All experimental animals were scanned at least twice during each assessment period. If the first two scans provided percent body fat data that varied by more than 1.5%, a third scan was performed. The two scans that were closest were then averaged and the mean value of these two scans was included in the data analysis. The DXA exam provided data specific to lean body mass, fat mass, percent body fat, and bone mineral density.
Table 1. Dietary composition of the Western Diet and Daniel Fast (purified) Diet
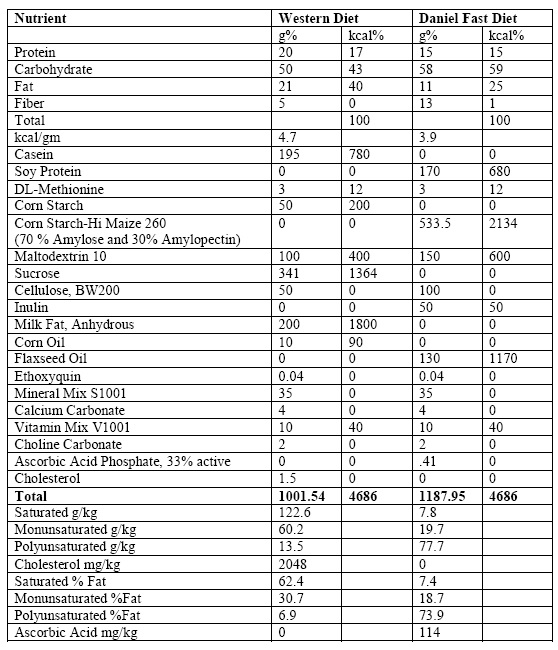
2.3 Dietary and Exercise Interventions
The rats were randomly assigned to one of four intervention groups: Western Diet with exercise (WD+E; n=7); Western Diet without exercise (WD; n=7); Purified Diet with exercise (PD+E; n=7); Purified Diet without exercise (PD; n=7). Both diets (provided in pellet form) were purchased from Research Diets, Inc. (New Brunswick, NJ). The WD was formulated to mimic a typical human WD, containing 17% protein, 43% carbohydrates, and 40% fat. The PD diet included 15% protein, 60% carbohydrates, and 25% fat. The types and quantities of macronutrient sources in the PD were based on the diet plans of the human subjects in our prior studies of the Daniel Fast.3,4,5 The resulting PD mimics what our human subjects consumed in terms of macronutrient types and percentages, fiber, and micronutrients (i.e., antioxidants). The specific nutrient composition of the WD and PD are provided in Table 1.
The dietary intervention period was 12 months in duration, beginning after the two week acclimation period and transition to the assigned diets. During months 1-3, the rats were weighed daily and the diets were provided ad libitum. During months 4-12, the rats were weighed every day and provided a quantity of food that maintained them at 90% of their free-feeding body mass measured just prior to food restriction. Rats in the exercise groups were automatically given 2.5 g more of their respective diet each day than rats that did not exercise to account for their greater energy expenditure. Overall, rats on the WD typically received less per day than rats given the PD, as the density of the WD yielded greater kcal/gram. Unfortunately, we did not determine caloric consumption for each rat during the study period, which may be considered a limitation of this work. Water was provided ad libitum throughout the study.
Equal numbers of rats in each diet group were randomly assigned either exercise or no exercise. Animals in the no exercise group were placed on the treadmill three days per week for a period of 5 minutes while it was turned off. Animals in the exercise groups (+E) performed endurance exercise on a motorized treadmill three days per week (i.e., Monday-Wednesday-Friday) for the 12-month intervention. The speed and duration was progressively increased. Specifically, the animals began training at 20 m∙min-1 for 15 min∙day-1 (week 1), progressed to 25 m∙min-1 for 30 min∙day-1 (week 2), and 25 m∙min-1 for 35 min∙day-1 (weeks 3-52). This progressive increase in intensity and duration of exercise is typical for animal training studies.9 The exercise training was performed in the morning to early afternoon hours.
2.4 Statistical Analysis
Data were analyzed using a 4 (group) x 5 (time) analysis of variance (ANOVA). Tukey post hoc tests and simple contrasts were used to determine post-hoc significance. All analyses were performed using JMP statistical software (JMP Pro, 14.0. SAS Institute; Cary, NC). Statistical significance was set at p≤ 0.05. All data are expressed as the mean ± SD.
3. Results
3.1 Physical Performance
We observed an effect (p<0.0001) for treadmill run time, with both exercise groups having higher run times than the animals that did not exercise (p<0.05). Dietary intake did not have a significant effect on run time, as exercised animals in both the PD and WD groups demonstrated similar run times, while non-exercised animals in the PD and WD groups also demonstrated similar run times. A time effect was also observed (p=0.004), with treadmill run times increasing from baseline to month 3 in the exercise groups, and decreasing from baseline to month 3 in the non-exercise groups; with a continuation of these changes from months 3-12. We also observed an interaction effect (p<0.001), with animals in both exercise groups demonstrating significantly elevated run times relative to baseline at months 3-12. Data for treadmill run time are shown in Figure 1.
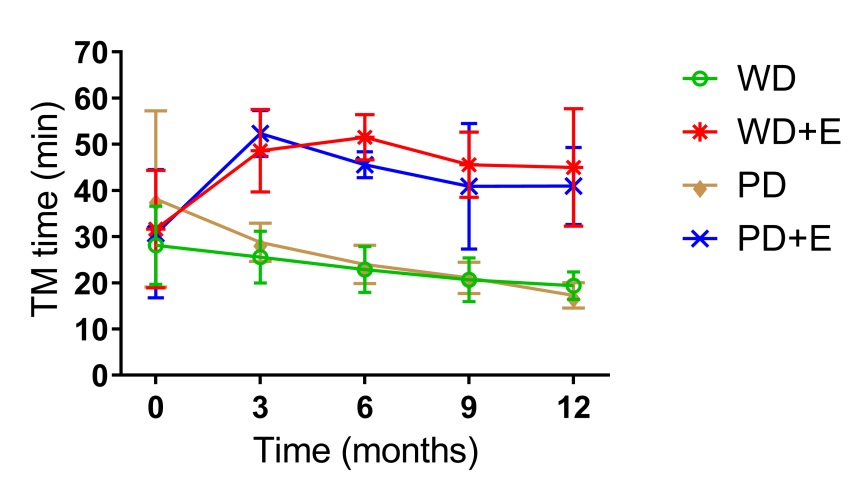
Figure 1. Treadmill run time to exhaustion of rats assigned to a Western Diet or a purified diet for 12 months
Group effect (p<0.0001); both exercise groups having higher run times than the animals that did not exercise (p<0.05).
Time effect (p=0.004); treadmill run times increasing from baseline to month 3 in the exercise groups, and decreasing from baseline to month 3 in the non-exercise groups.
Interaction effect (p<0.001); animals in both exercise groups demonstrating significantly elevated run times relative to baseline at months 3-12.
3.2 Anthropometric Data
Baseline mean body mass values were very similar and as follows: WD: 187g; WD+E: 187g; PD: 185g; PD+E: 193g. Significant group (p<0.0001) and time (p<0.0001) effects were observed for total body mass (Figure 2A). There was a noticeable difference in body mass, particularly in the exercised WD rodents, relative to their non-exercised WD counterparts at 3 months. Measurements of lean body mass showed a significant group effect (p<0.0001), whereby WD animals trended toward greater lean mass beginning at 6 months, regardless of exercise intervention, compared to both PD groups (Figure 2B). Additionally, group (p<0.0001) and time (p<0.0001) effects were observed in measurements of fat mass (Figure 2C). A significant interaction effect (p<0.007) was also observed, indicating that the PD animals had sustained reduced fat mass over time, from month 6-12. Lastly, we noted significant group (p<0.05) and time (p<0.0001) effects in measures of BMD (Figure 2D), suggesting that growth was relatively normal despite the vegan diet composition and calorie restriction.
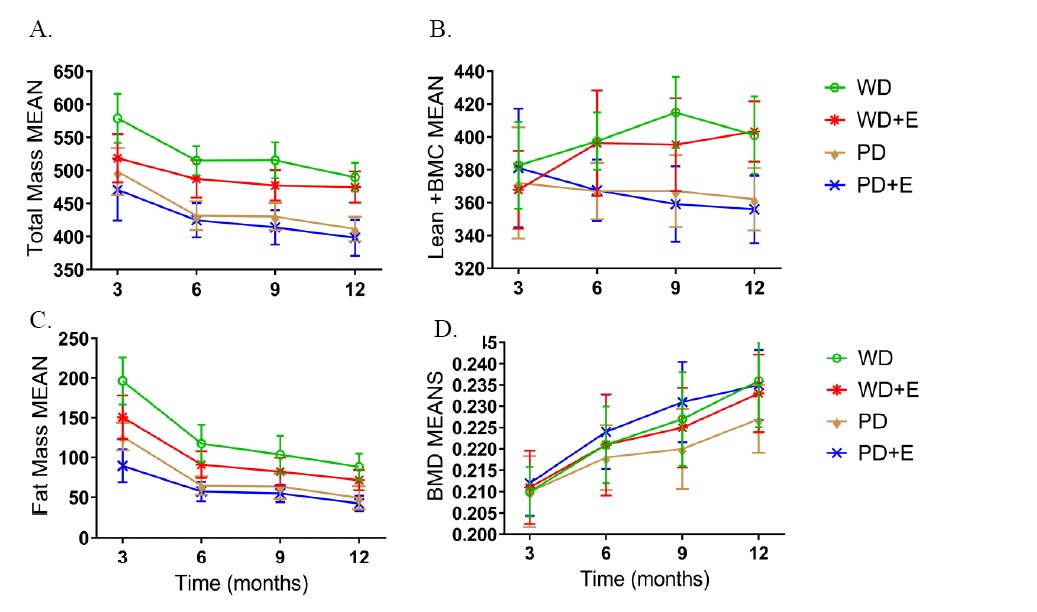
Figure 2. Total mass in grams (A), lean mass + bone mineral content in grams (B), fat mass in grams (C), and bone density (D) of rats assigned to a Western Diet (WD) or a Purified Diet (PD) for 12 months
Group (p<0.0001) and time (p<0.0001) effects for total body mass (A).
Group effect (p<0.0001) for lean body mass (B).
Group (p<0.0001) and time (p<0.0001) effects for fat mass (C); significant interaction (p<0.007) was also observed, indicating that the PD animals had sustained reduced fat mass over time, from month 6-12.
Group (p<0.05) and time (p<0.0001) effects for bone mineral density (D).
4. Discussion
This is the first study to our knowledge to investigate the influences of both dietary restriction and exercise, inclusive of both ad libitum feeding and caloric restriction, on outcome measures of physical performance and body composition. The key findings from this study are as follows: 1) Treadmill run time to exhaustion increased in both exercise groups, with a slightly greater increase in the PD+E group compared to the WD+E during ad libitum feeding; 2) Exercise performance was maintained to a similar extent for all groups from months 3-12; 3) During ad libitum feeding, body mass was greater in the WD group compared to all other groups; 4) With caloric restriction, body mass declined in all groups, more for PD rats compared to WD rats, and remained greater in both WD groups compared to the PD groups; 5) During both ad libitum feeding and caloric restriction, fat mass and percent body fat was greater in the WD groups compared to the PD groups; 6) Lean body mass did not differ significantly between groups but was slightly higher in the WD animals; 7) The caloric restriction resulted in a slight increase in lean body mass for animals assigned to the WD but a small decrease in lean body mass was recorded for animals assigned to the PD diet; 8) The exercise training decreased body mass and fat mass during the periods of ad libitum feeding and caloric restriction, as compared to no exercise. Yet, the exercise training did not have an effect on lean body mass during either feeding period; 9) All animals experienced a linear increase in bone mineral density from months 3-12.
4.1 Physical Performance Findings
Studies have demonstrated that exercise training increases endurance performance time9,10,11 and our results support this, with overall run times increasing approximately 50-60% for animals in both diet groups that were exercised. We believed that the WD with and without exercise would impair endurance performance due to the relatively high fat content, which over time could lead to increased fat mass and adiposity. High fat diets have also been linked to impaired glucose metabolism by skeletal muscle, which is important for muscle oxidation during exercise.12 That said, diet composition did not have much of an influence on run time, as can be viewed in Figure 1. Rather, exercise training was the main variable to influence run time on the treadmill. Although run times during months 6-12 remained higher than baseline for animals in the PD group, they were slightly lower than those observed at the end of month 3. It is possible that due to the purified nature of the PD, in particular the high fiber content, the animals in the PD groups were not receiving adequate calories to fuel long duration running.13 Specifically, the caloric content of the PD diet was lower than for the WD diet. Since the diets were fed based on body mass, animals in the PD group may have consumed fewer overall calories (as well as "digestible" calories in the form of fiber) during months 3-12 of the study, which may have negatively influenced running performance.
4.2 Anthropometric Findings
During ad libitum feeding, body mass was greater in the WD group compared to all other groups. We anticipated that the exercise groups would have a lower overall body mass due to the increased caloric expenditure from the thrice weekly exercise bouts. However, it was interesting that PD animals, despite being allowed to freely feed throughout the three month period, were slightly lower in body mass as compared to animals in the WD groups. This is consistent with a previous report that rats consume similar amounts of food, despite differences in caloric density.14 Although the amounts of food were not monitored during the period of ad libitum feeding, this would explain the body mass results at the end of month 3. Following the three month period of caloric restriction, body mass was greater in both WD groups compared to the PD groups, with slightly lower masses displayed by animals in both exercise groups as compared to those in the no exercise groups. It should be noted that animals in all groups experienced a similar percentage decrease in body mass from month 3 to month 12 of the intervention.
As expected, body mass and fat mass were higher for WD than PD rats during both ad libitum feeding and caloric restriction. Those animals assigned to the exercise groups realized a slightly lower fat mass as compared to those animals not performing exercise. As with body mass, following caloric restriction, values for fat mass were significantly lower than with ad libitum feeding (e.g., reduction of approximately 30-45%). What is interesting and related to the above, despite the dramatic decrease in body mass during caloric restriction, very little or no lean body mass was lost during that period. In fact, animals in the WD groups actually gained a small amount of lean body mass during caloric restriction. These findings highlight the idea that increased calories and protein may not be necessary for maintenance of, or increase in, lean body mass. Related to the protein intake, it should be noted that despite the plant-based protein source used in the PD, all animals grew as expected over the course of the 12-month period, while maintaining a reasonable amount of lean body mass despite having an extremely low percentage of body fat (14-15%). That said, animals assigned to the PD groups did experience a slight decrease in lean body mass during the caloric restriction period. It is possible that due to the purified nature of this feeding plan, additional calories are needed to better maintain lean mass over time.
While regular exercise training results in a lower body mass and fat mass during periods of ad libitum feeding and caloric restriction, as compared to no exercise, exercise as performed in the present study has little influence on lean body mass during either feeding period. Prior studies have noted a gain in lean mass when animals exercise vigorously.15-16 This exercise mimics the same response that humans have to exercise, which result in increased lean mass. However, the exercise in the present study may have been of two low of an intensity or volume to stimulate lean mass gain. Future studies involving dietary manipulation and exercise may utilize a more intensive exercise protocol in an attempt to more favorably influence lean body mass, with the possible inclusion of biochemical markers that may be associated with lean and fat mass. Although no biochemical data are presented in the present paper, it should be noted that we have previously reported a significant lowering in blood triglycerides, cholesterol, malondialdehyde, and advanced oxidation protein products in rats following three months of consuming the PD, as compared to the WD.17
5. Conclusion
To our knowledge, this is the first study to investigate the impact of both dietary restriction and exercise, inclusive of both ad libitum feeding and caloric restriction, on measures of physical performance and body composition in animals. The findings suggest that macronutrient composition and not simply calorie intake has an influence on the degree of adiposity, with or without exercise. This study extends our prior work in human subjects partaking in the Daniel Fast dietary plan. Our findings may be attributed to the macronutrient mix (low glycemic carbohydrate, high polyunsaturated fat) of the PD, as compared to the higher glycemic and saturated fatty acid rich WD. Future investigations should focus on the specific mechanisms responsible for the noted effects, both related to enhanced physical performance and improved body composition. This may involve the use of animal models, while human clinical trials may be designed to further investigate the value of the PD dietary plan, with and without exercise, to improve aspects of human health and physical performance.
6. Acknowledgements
Funding for this work was provided by the University of Memphis. Appreciation is extended to Drs. Sang-Rok Lee, Randy Buddington, and Karyl Buddington for assistance with data collection.
7. Author Contributions
RJB was responsible for the study design, statistical analyses, and manuscript preparation. TAG and JMS were responsible for data collection, database management, and manuscript preparation. All authors read and approved of the manuscript.
8. Conflicts of Interest
The authors declare no conflicts of interest.
Article Details
The Medical Research Archives grants authors the right to publish and reproduce the unrevised contribution in whole or in part at any time and in any form for any scholarly non-commercial purpose with the condition that all publications of the contribution include a full citation to the journal as published by the Medical Research Archives.
References
2. Golbidi S, Daiber A, Korac B, Li H, Essop MF, Laher I. Health Benefits of Fasting and Caloric Restriction. Curr Diab Rep. 2017, Oct 23;17(12):123.
3. Bloomer, RJ, Kabir, MM, Canale, RE, Trepanowski, JF, Marshall, KE, Farney, TM, and Hammond, KG. Effect of a 21 day Daniel Fast on metabolic and cardiovascular disease risk factors in men and women. Lipids in health and disease 2010, 9: 94.
4. Bloomer, RJ, Kabir, MM, Trepanowski, JF, Canale, RE, and Farney, TM. A 21 day Daniel Fast improves selected biomarkers of antioxidant status and oxidative stress in men and women. Nutr. Metab. (Lond) 2011, 8: 17.
5. Trepanowski, JF, Kabir, MM, Alleman, RJ,Jr, and Bloomer, RJ. A 21-day Daniel fast with or without krill oil supplementation improves anthropometric parameters and the cardiometabolic profile in men and women. Nutr. Metab. (Lond) 2012, 9: 82.
6. National Research Council. Guide for the care and use of laboratory animals. 8th edition. Washington, DC: The National Academies Press. 2011.
7. Copp, SW, Davis, RT, Poole, DC, and Musch, TI. Reproducibility of endurance capacity and VO2peak in male Sprague-Dawley rats. J. Appl. Physiol. 2009, 106: 1072-1078.
8. Koch, LG, Meredith, TA, Fraker, TD, Metting, PJ, and Britton, SL. Heritability of treadmill running endurance in rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 1998, 275: R1455-R1460.
9. Huang, T, Chang, F, Lin, S, Liu, S, Hsieh, SS, and Yang, R. Endurance treadmill running training benefits the biomaterial quality of bone in growing male Wistar rats. J. Bone Miner. Metab. 2008, 26: 350-357.
10. Dolinsky, VW, Jones, KE, Sidhu, RS, Haykowsky, M, Czubryt, MP, Gordon, T, and Dyck, JR. Improvements in skeletal muscle strength and cardiac function induced by resveratrol during exercise training contribute to enhanced exercise performance in rats. J. Physiol. (Lond) 2012, 590: 2783-2799.
11. Lee, JS, Bruce, CR, Spriet, LL, and Hawley, JA. Interaction of diet and training on endurance performance in rats. Exp. Physiol. 2001, 86: 499-508.
12. Tanaka, S, Hayashi, T, Toyoda, T, Hamada, T, Shimizu, Y, Hirata, M, Ebihara, K, Masuzaki, H, Hosoda, K, and Fushiki, T. High-fat diet impairs the effects of a single bout of endurance exercise on glucose transport and insulin sensitivity in rat skeletal muscle. Metab. Clin. Exp. 2007, 56: 1719-1728.
13. Guezennec, C, Satabin, P, Legrand, H, and Bigard, A. Physical performance and metabolic changes induced by combined prolonged exercise and different energy intakes in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 68: 525-530.
14. Yao, M, and Roberts, SB. Dietary energy density and weight regulation. Nutr. Rev. 2001, 59: 247-258.
15. Bhattacharya, A, Rahman, MM, Sun, D, Lawrence, R, Mejia, W, McCarter, R, O'Shea, M, and Fernandes, G. The combination of dietary conjugated linoleic acid and treadmill exercise lowers gain in body fat mass and enhances lean body mass in high fat-fed male Balb/C mice. J. Nutr. 2005, 135: 1124-1130.
16. Kemi, OJ, Loennechen, JP, Wisloff, U, and Ellingsen, O. Intensity-controlled treadmill running in mice: cardiac and skeletal muscle hypertrophy. J. Appl. Physiol. 2002, (1985) 93: 1301-1309.
17. Bloomer RJ, Schriefer JHM, Gunnels TA, Lee SR, Sable HJ, van der Merwe M, Buddington RK, Buddington KK. Nutrient Intake and Physical Exercise Significantly Impact Physical Performance, Body Composition, Blood Lipids, Oxidative Stress, and Inflammation in Male Rats. Nutrients. 2018 Aug 17;10(8):1109.