Retroactive assessment for DNA mismatch repair deficiency in women with a history of endometrial cancer
Main Article Content
Abstract
Objectives. Beginning in 2014, the Society of Gynecologic Oncology and the American College of Obstetricians and Gynecologists have recommended universal tumor testing for mismatch repair deficiency in endometrial cancer. Mismatch repair testing can triage patients who may benefit from genetic testing for Lynch syndrome. Many women previously diagnosed with endometrial cancer have not undergone mismatch repair tumor testing. We sought to determine the feasibility of retroactive assessment for mismatch repair deficiency among women with diagnosed with endometrial cancer prior to 2014.
Methods. Between 2016 and 2018, we identified 36 patients presenting for gynecologic oncology follow-up visits who were previously diagnosed with endometrial cancer. The endometrial pathology underwent tumor assessment for loss of expression of mismatch repair proteins by immunohistochemistry. Patients with abnormal mismatch repair testing were referred to genetic counseling and, if indicated, for germline genetic testing.
Results. Thirty-six patients underwent retroactive tumor immunohistochemistry, yielding 10 (28%) abnormal results, including nine (25%) with loss of one or more mismatch repair proteins and one with inconclusive staining (2.8%). All ten patients with abnormal immunohistochemistry were referred to genetic counseling; 9 (90%) accepted the referral and proceeded with genetic testing. One pathogenic mutation was identified in CHEK2 (11%). Five patients (56%) were found to have a variant of unknown significance.
Conclusions. Implementation of universal retroactive tumor testing for mismatch repair deficiency in patients previously diagnosed with endometrial cancer is feasible. With the growing use of new molecular classification protocols for endometrial tumors, identification of mismatch repair deficiency may have significant clinicopathologic implications.

BACKGROUND
Endometrial cancer is the most common gynecologic cancer in developed countries. In the United States, the incidence of endometrial cancer in 2019 was 61,880, with the incidence and mortality of endometrial cancer increasing by 0.7% and 1.1% respectively per year from 1999-2015 (1). As with many other solid tumors, researchers have turned to genetics and molecular tumor analysis in management of endometrial cancer (2).
Lynch syndrome is a highly penetrant autosomal dominant hereditary cancer syndrome and the most common cause of hereditary endometrial and colon cancers. Approximately 2-5% of endometrial cancers are attributable to Lynch syndrome (3). Identification of Lynch-associated mutations is critical as it allows for optimization of cancer surveillance and disease prevention in patients and their at-risk family members. Historically, referral for Lynch syndrome was based on the Amsterdam and Bethesda criteria, however, these methods have limited sensitivity (4). Tumor screening with immunohistochemistry is another option to identify women with mismatch repair deficient tumors, which can then triage patients for Lynch syndrome genetic testing. It is estimated that 98% of individuals with Lynch syndrome are not aware of their underlying mutation (5). A recent meta-analysis demonstrated that 43% of patients with endometrial cancer who were diagnosed with Lynch syndrome via tumor assessment would have been missed by family-history based screening alone (6).
There is a growing body of literature demonstrating clinicopathologic implications of mismatch repair deficiency in endometrial cancer (7–13). In 2014, the Society for Gynecologic Oncology released a clinical practice statement, recommending universal molecular tumor screening for mismatch repair deficiency for all endometrial cancers (14). Later that year, the American College of Obstetricians and Gynecologists reaffirmed this recommendation (15). At this time, no studies have been conducted to assess the feasibility and yield of retroactive universal genetic screening for mismatch repair deficiency in patients with a history of endometrial cancer. In accordance with the statement released by the Society of Gynecologic Oncology and the American College of Obstetricians and Gynecologists, our institution implemented a policy in 2016 whereby all newly and previously diagnosed endometrial tumors undergo screening for mismatch repair deficiency. Here we report our experience with retroactive tumor screening in all women with a history of endometrial cancer presenting for follow-up gynecologic oncology visits.
METHODS
This quality improvement study was approved by the Weill Cornell Medicine Institutional Review Board (#1711018743). The requirement for written informed consent was waived by the Institutional Review Board. All women with previously diagnosed endometrial cancer presenting for a follow-up visits to the gynecologic oncology clinic between September 1, 2016 and May 31, 2018 had tumor assessment for mismatch repair deficiency. Date of diagnosis of previously diagnosed endometrial cancer ranged from December 8, 2009 to September 14, 2017. Patients were followed until January 1, 2020 to identify outcomes up to 40 months after tumor assessment of mismatch repair and subsequent referral to genetic testing. Patients were excluded from analysis if they did not have pathologic tissue available for analysis and/or they had previously undergone genetic or tumor testing. Tumor assessment was performed via immunohistochemistry analysis of MLH1, MSH2, MSH6 and PMS2 from paraffin embedded blocks. If MLH1 protein expression was absent, the sample was reflexively tested for MLH1 promoter methylation. Patients were referred for genetic counseling and testing if any mismatch repair proteins were found to have absent staining, and in the case of MLH1 absence, if the MLH1 promoter was negative for methylation.
Data extracted from the medical record included age at endometrial cancer diagnosis, histology, stage, grade, treatment history (surgery, chemotherapy, radiation), personal medical history, family oncologic history, molecular testing results, genetic testing results, and additional cancer screening (colonoscopy/ endoscopy). Univariable tests were applied on the basis of whether the variable of interest was distributed normally (ie, t test, analysis of variance) or not normally (ie, Mann-Whitney U test). All P-values are two-sided with statistical significance evaluated at the 0.05 alpha level. All analyses were performed in Statistical Product and Service Solutions (SPSS) Version 24 (IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.).
RESULTS
We identified 36 patients previously diagnosed with endometrial cancer without prior tumor or germline analysis during the study period. The median age at diagnosis was 56 years (range 38-77 years). Tumor histology included endometrioid (26 patients, 72.2%), serous (3 patients, 8.3%), carcinosarcoma (1, 2.8%), and mixed (6, 16.7%). Stage distribution was as follows: Stage IA (23, 63.9%), IB (8, 22.2%), II (1, 2.8%), IIIC (1, 2.8%), IVB (1, 2.8%), and incomplete staging (2, 5.6%). International Federation of Gynecology and Obstetrics (FIGO) grade included 15 with FIGO Grade I disease (41.7%), 10 with FIGO grade II disease (27.8%), eight with FIGO grade III disease (22.2%) and three patients who were unable to be classified (8.3%). Eight patients (22.2%) had lymphovascular invasion of the tumor. Nine patients (25%) had recurrence of their disease with a median time to recurrence of 24 months (range 11-84) (Table 1). The follow-up period ranged from 19 to 40 months after retroactive tumor testing.
Table 1- Characteristics of study population
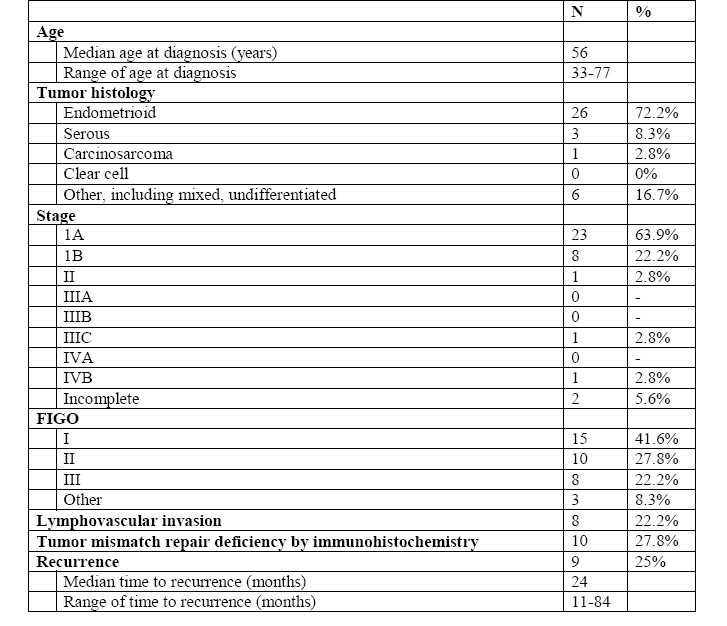
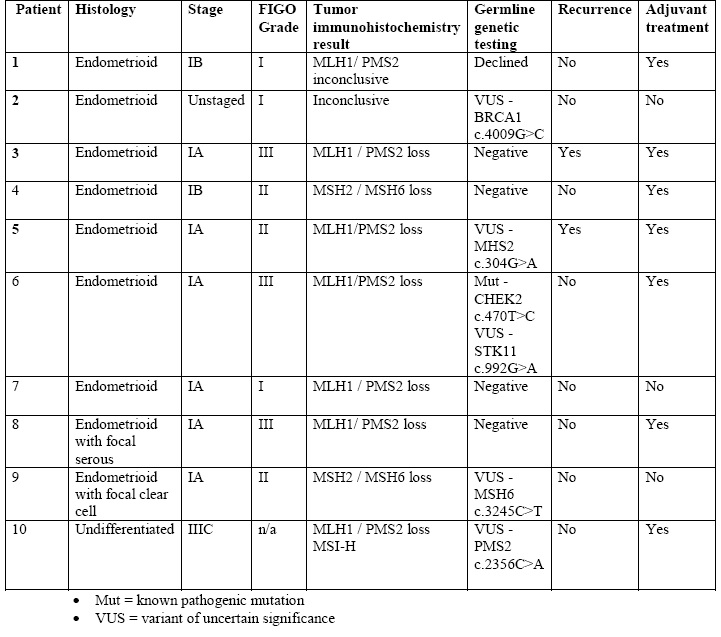
All 36 identified patients underwent retroactive tumor immunohistochemistry assessment. Ten patients (27.8%) had abnormal results on tumor immunohistochemistry assessment (Table 2). Abnormal immunohistochemistry results included loss of MLH1/PMS2 (6 of 10, 60%), loss of MSH2/MSH6 (2 of 10, 20%), inconclusive staining of MLH1/PMS2 (1 of 10, 10%), and inconclusive staining (1 of 10, 10%). Tumor histology of those patients with abnormal tumor immunohistochemistry included endometrioid (7 of 10, 70%), endometrioid with focal serous (1 of 10, 10%), endometrioid with focal clear cell (1 of 10, 10%), and undifferentiated (1 of 10, 10%). The stage distribution for patients with abnormal immunohistochemistry was Stage IA (6 of 10, 60%), IB (2 of 10, 20%), IIIC (1 of 10, 10%), and unstaged (1 of 10, 10%).
The patient who was unstaged underwent a total laparoscopic hysterectomy and left salpingoophorectomy for a preoperative diagnosis of endometrial hyperplasia and left ovarian cyst. She was found to have endometrial carcinoma on final pathology and decision was made to not pursue surgical staging given FIGO grade 1 disease without evidence of lymphovascular invasion.
Three patients (30%) had FIGO grade 1 disease, three patients (30%) had FIGO grade 2, three patients (30%) had FIGO grade 3, and one patient (10%) had undifferentiated endometrial cancer. Seven patients received adjuvant therapy; six received vaginal brachytherapy (60%), and one received chemotherapy (10%). Two of 10 patients with abnormal mismatch repair (20%) had recurrence of disease. One patient with recurrence was treated with radiation therapy (1 of 10, 10%) and one was treated with surgical resection and chemotherapy (1 of 10, 10%). The median time from diagnosis to recurrence was 16 months between the two patients who had recurrence of disease (range 12-20). None of the patients with abnormal immunohistochemistry testing received immunotherapy based on their testing results.
All ten patients with abnormal immunohistochemistry were referred to genetic counseling. Nine (90%) patients accepted the referral and proceeded with genetic counseling and testing. Germline testing revealed one patient with a pathogenic mutation in CHEK2 (11%), and five patients (56%) were found to have a variant of uncertain significance. For three patients, the variant of unknown significance was located in genes coding for mismatch repair proteins (33%). For two patients, the variant of unknown significance was located in the gene with absent protein expression on mismatch repair assessment. One patient was found to have a variant of unknown significance in BRCA1 (11%), and one patient was found to have a variant of unknown significance in STK11 (Table 2).
Table 2- Clinical and genetic data for patients with abnormal endometrial tumor testing
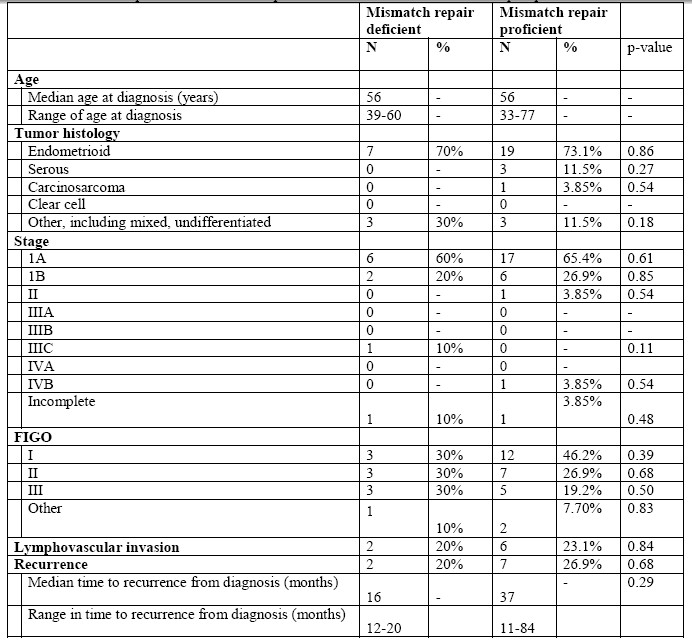
A comparison of the clinicopathologic features among patients with and without mismatch repair deficient tumors based on immunohistochemistry testing demonstrated no significant differences. Additionally, there was no statistical difference between median time to recurrence between the two groups (Table 3).
Table 3- Comparison of mismatch repair deficient tumors with mismatch repair proficient tumors
DISCUSSION
All 36 patients underwent retrospective tumor mismatch repair assessment and 10 (28%) were found to have mismatch repair deficiency. Our patient population resembles national statistics with respect to tumor histology, stage, grade, and age at diagnosis (1,16). One hundred percent of patients with tumor MMR deficiency were referred for germline testing and 90% of patients accepted the referral and completed genetic counseling and testing. One patient was found to have a pathogenic mutation in CHEK2, a cell cycle regulator and putative tumor suppressor. One patient was found to have inconclusive tumor IHC staining and germline testing identified a VUS in the BRCA1 gene. In total, five VUS were identified. No statistical differences were noted between the groups of patients with and without MMR-deficient tumors.
Over a quarter of our study patients (28%) had tumor mismatch repair deficiency which is similar to rates reported in the literature (6). While none were found to have a pathogenic mutation in MMR proteins associated with Lynch syndrome, several VUS were discovered in MMR proteins. While identification of these VUS are not as informative as pathogenic mutations, variants must be followed as many will be reclassified as benign or pathogenic over time, informing care for the patient and at-risk relatives.
Information about mismatch repair deficiency even in the absence of a germline mutation has clinical implications including eligibility for targeted therapy for recurrent mismatch repair-deficient tumors. While mismatch repair status was initially found to predict clinical benefit of a PD-1 blockade in patients with colorectal cancer, subsequent studies then demonstrated response in other solid tumors as well, including endometrial cancer, where mismatch repair deficiency is found in up to 30% of tumors (6,17,18). Since the Food and Drug Administration (FDA) approval in 2017, PD-1 blockade with pembrolizumab has been used in the treatment of mismatch repair-deficient recurrent endometrial cancer. While none of the patients in our study received immunotherapy for recurrence, this now remains an option for treatment in the future for these patients with mismatch repair deficiency. Adjuvant radiotherapy recently has been shown to be associated with improved disease-specific survival in a cohort of patients with mismatch repair-deficient endometrioid endometrial cancers, suggesting that mismatch repair status could be used as a predictive biomarker to select patients who may benefit most from adjuvant radiotherapy (7).
A tumor’s molecular profile, including MMR deficiency, has specific disease implications. Some studies suggest that mismatch repair-deficient endometrial cancers have worse overall survival, higher disease recurrence rate, and decreased recurrence-free survival compared to other molecular profiles (8,9). In particular, MLH1 promoter hypermethylation is associated with larger tumor burden and more frequent lymphovascular invasion (13). This study did not find that mismatch repair-deficient endometrial cancers had overall worse survival or higher disease recurrence rate, although among the women with mismatch repair deficient tumors, the median time to recurrence was shorter (16 vs 37 months, not significant). These findings are likely attributable to the small sample size.
The strengths of this study include the novel addition of retroactive tumor testing for patients with a history of endometrial cancer. While we did not identify any patients with germline Lynch syndrome mutations, this is not surprising given our sample size and meta-analysis data suggesting that 28% of endometrial tumors will exhibit mismatch repair tumor deficiency by immunohistochemistry, and within this group, only 15% of patients with abnormal immunohistochemistry tumors will harbor a Lynch syndrome mutation (6). However, given that there were approximately 772,245 women living with endometrial cancer in the United States in 2016, we can predict that 216,228 women will have abnormal immunohistochemistry tumor testing, and among those, approximately 32,434 women will have a Lynch syndrome mutation (1,6). Therefore, Lynch syndrome screening may be indicated for hundreds of thousands of unaware women.
This study suggests that while retroactive tumor testing for women previously diagnosed with endometrial cancer is feasible, there are limitations. The small sample size of 36 patients precludes the study from making strong conclusions regarding the large-scale feasibility of universal tumor testing and its implications. This study lacks follow-up data beyond three years evaluating how mismatch repair information impacts treatment, survival, and tumor surveillance or its effect on family members of those with abnormal immunohistochemistry testing. Additionally, because this study is examining retroactive tumor testing, we are unable to perform prospective testing. Finally, while universal tumor testing irrespective of age at diagnosis has the greatest sensitivity for detecting Lynch syndrome, it comes at a cost of requiring tumor testing on three to four times as many patients compared to selective tumor testing, a cost which many patients and medical centers may not be able to justify (21). In this study, retroactive tumor immunohistochemistry was covered by patient insurance, but it remains unclear if this will be the case for all patients moving forward, and cost implications will be imperative to consider as well.
Universal screening for tumor mismatch repair deficiency with immunohistochemistry or microsatellite instability has also been recommended for patients newly diagnosed with colorectal cancer and has proven to be feasible and with cost-effectiveness similar to other preventative health strategies (22–24). Screening strategies within endometrial cancer have not been as well established, however tumor testing by immunohistochemistry has been increasingly shown to be sensitive, as well as cost-effective compared to other methods (25,26).
CONCLUSIONS
This study has implications for women with endometrial cancer who were diagnosed prior to the recommendation of universal tumor mismatch repair assessment. Despite the new Society of Gynecologic Oncology and American College of Obstetricians and Gynecologists recommendations for universal screening of endometrial cancer, not all centers have incorporated tumor assessment into routine practice. Unlike in ovarian cancer, genetic testing is not recommended for all women with a new diagnosis of endometrial cancer (27). Many centers continue to rely on the Amsterdam or Bethesda criteria or age of diagnosis as a means of triaging those who should be screened for Lynch syndrome (15,28). While not diagnostic of Lynch syndrome, molecular screening of tumors for presence of mismatch repair proteins using immunohistochemistry or microsatellite instability has demonstrated greater sensitivity than family-history driven strategies, ranging between 80 and 100% (29).
As clinicians and patients become more accustomed to tumor immunohistochemistry and microsatellite instability as part of standard of care for endometrial cancer pathologic analysis, there may be more women who seek out retroactive tumor testing. Furthermore, if there is growing evidence that tumor molecular profiling has actionable clinical implications, tumor assessment—even for patients previously diagnosed—may prove valuable. While we demonstrate that retroactive tumor mismatch repair deficiency can be offered, it remains unclear if this process is reasonable from a financial and clinical standpoint. Future studies are necessary to determine the clinical significance of this finding and the cost-effectiveness of strategies of tumor assessment and triage for germline genetic testing.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Funding declaration
Melissa K. Frey is supported by the following grant: NIH/NCATS Grant # KL2-TR-002385.
Authors’ contributions
Y. Stefanie Chen, M.D.: project development; data collection or management; data analysis; manuscript writing/editing.
Sushmita Gordhandas, M.D.: project development; data collection or management; data analysis; manuscript writing/editing.
Kelsey Musselman, M.D.: project development; data collection or management; data analysis; manuscript writing/editing.
Zhen Ni Zhou, M.D./Ph.D: project development; data collection or management; data analysis; manuscript writing/editing.
Brandon P. Maddy, M.D.: project development; data collection or management; data analysis; manuscript writing/editing.
Ryan M Kahn M.D., M.H.S: project development; data collection or management; data analysis; manuscript writing/editing.
Nora Badiner, M.D.: project development; data collection or management; data analysis; manuscript writing/editing.
Lora H. Ellenson, M.D.: project development; data collection or management; data analysis; manuscript writing/editing.
Kevin Holcomb: project development; data collection or management; data analysis; manuscript writing/editing.
Eloise Chapman-Davis M.D.: project development; data collection or management; data analysis; manuscript writing/editing.
Melissa K Frey M.D.: project development; data collection or management; data analysis; manuscript writing/editing.
Article Details
The Medical Research Archives grants authors the right to publish and reproduce the unrevised contribution in whole or in part at any time and in any form for any scholarly non-commercial purpose with the condition that all publications of the contribution include a full citation to the journal as published by the Medical Research Archives.
References
2. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;67–73.
3. Koornstra JJ, Mourits MJ, Sijmons RH, Leliveld AM, Hollema H, Kleibeuker JH. Management of extracolonic tumours in patients with Lynch syndrome. Lancet Oncol [Internet]. 2009;10(4):400–8. Available from: http://dx.doi.org/10.1016/S1470-2045(09)70041-5
4. Giardiello FM, Allen JI, Axilbund JE, Boland CR, Burke CA, Burt RW, et al. Guidelines on Genetic Evaluation and Management of Lynch Syndrome: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Dis colon rectum. 2014;57(8):1025–48.
5. Hampel H, de la Chapelle A. The search for unaffected individuals with Lynch syndrome: do the ends justify the means? Cancer Prev Res (Phila) [Internet]. 2011 Jan;4(1):1–5. Available from: https://www.ncbi.nlm.nih.gov/pubmed/21205737
6. Kahn RM, Gordhandas S, Maddy BP, Baltich-Nelson B, Askin G, Christos PJ, et al. Universal endometrial cancer tumor typing: How much has immunohistochemistry, microsatellite instability, and MLH1 methylation improved the diagnosis of Lynch syndrome across the population? Cancer. 2019;125(3):3172–83.
7. Reijnen C, Kusters-Vandevelde HVN, Prinsen CF, Massuger LFAG, Snijders MPML, Kommoss S, et al. Mismatch repair deficiency as a predictive marker for response to adjuvant radiotherapy in endometrial cancer. Gynecol Oncol. 2019 Jul;154(1):124–30.
8. Talhouk A, McConechy MK, Leung S, Yang W, Lum A, Senz J, et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer. 2017 Mar;123(5):802–13.
9. Kommoss S, McConechy MK, Kommoss F, Leung S, Bunz A, Magrill J, et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann Oncol Off J Eur Soc Med Oncol. 2018 May;29(5):1180–8.
10. Proctor L, Pradhan M, Leung S, Cheng A, Lee CH, Soslow RA, et al. Assessment of DNA Ploidy in the ProMisE molecular subgroups of endometrial cancer. Gynecol Oncol. 2017 Sep;146(3):596–602.
11. Stelloo E, Bosse T, Nout RA, MacKay HJ, Church DN, Nijman HW, et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod Pathol an Off J United States Can Acad Pathol Inc. 2015 Jun;28(6):836–44.
12. Britton H, Huang L, Lum A, Leung S, Shum K, Kale M, et al. Molecular classification defines outcomes and opportunities in young women with endometrial carcinoma. Gynecol Oncol. 2019 Jun;153(3):487–95.
13. Cosgrove CM, Cohn DE, Hampel H, Frankel WL, Jones D, McElroy JP, et al. Epigenetic silencing of MLH1 in endometrial cancers is associated with larger tumor volume, increased rate of lymph node positivity and reduced recurrence-free survival. Gynecol Oncol [Internet]. 2017/07/11. 2017 Sep;146(3):588–95. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28709704
14. Lancaster JM, Powell CB, Chen LM, Richardson DL SCPC. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2015;136(1):3–7.
15. Lynch Syndrome. Practice Bulletin No. 147. Obstet Gynecol [Internet]. 2014;124:1042–54. Available from: https://www.acog.org/-/media/Practice-Bulletins/Committee-on-Practice-Bulletins----Gynecology/pb147.pdf?dmc=1&ts=20190627T2300323617
16. Sorosky JI. Endometrial cancer. Obstet Gynecol. 2012 Aug;120(2 Pt 1):383–97.
17. Hause RJ, Pritchard CC, Shendure J, Salipante SJ. Classification and characterization of microsatellite instability across 18 cancer types. Nat Med. 2016 Nov;22(11):1342–50.
18. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015 Jun;372(26):2509–20.
19. Eskander RN. Mismatch repair in endometrioid endometrial cancer: Increasing our therapeutic proficiency by capitalizing on molecular deficiency. Vol. 125, Cancer. United States; 2019. p. 337–9.
20. Backes FJ, Haag J, Cosgrove CM, Suarez A, Cohn DE, Goodfellow PJ. Mismatch repair deficiency identifies patients with high-intermediate-risk (HIR) endometrioid endometrial cancer at the highest risk of recurrence: A prognostic biomarker. Cancer. 2019 Feb;125(3):398–405.
21. Moreira L, Balaguer F, Lindor N, de la Chapelle A, Hampel H, Aaltonen LA, et al. Identification of Lynch syndrome among patients with colorectal cancer. JAMA. 2012 Oct;308(15):1555–65.
22. Hampel, Heather; Frankel W. Screening for the Lynch Syndrome. N Engl J Med. 2005;352(18):1851–60.
23. Ladabaum U, Wang G, Terdiman J, Blanco A, Kuppermann M, Richard Boland C, et al. Strategies to identify the Lynch syndrome among patients with colorectal cancer. Ann Intern Med. 2011;155(2):69–79.
24. Mvundura M, Grosse SD, Hampel H, Palomaki GE. The cost-effectiveness of genetic testing strategies for Lynch syndrome among newly diagnosed patients with colorectal cancer. Genet Med. 2010;12(2):93–104.
25. Frolova AI, Babb SA, Zantow E, Hagemann AR, Powell MA, Thaker PH, et al. Impact of an immunohistochemistry-based universal screening protocol for Lynch syndrome in endometrial cancer on genetic counseling and testing. Gynecol Oncol [Internet]. 2015;137(1):7–13. Available from: http://dx.doi.org/10.1016/j.ygyno.2015.01.535
26. Kwon JS, Scott JL, Gilks CB, Daniels MS, Sun CC, Lu KH. Testing women with endometrial cancer to detect lynch syndrome. J Clin Oncol. 2011;29(16):2247–52.
27. Randall LM, Pothuri B, Swisher EM, Diaz JP, Buchanan A, Witkop CT, et al. Multi-disciplinary summit on genetics services for women with gynecologic cancers: A Society of Gynecologic Oncology White Paper. Gynecol Oncol [Internet]. 2017 Aug 1;146(2):217–24. Available from: https://doi.org/10.1016/j.ygyno.2017.06.002
28. Endometrial Cancer. Practice Bulletin No. 149. Obstet Gynecol. 2015;125:1006–26.
29. Stewart A. Genetic Testing Strategies in Newly Diagnosed Endometrial Cancer Patients Aimed at Reducing Morbidity or Mortality from Lynch Syndrome in the Index Case or Her Relatives. PLoS Curr. 2016;5:1–41.