Enhancing HIV Prevention Services: Exploring Psychosocial Associations of HIV Antibody Testing Frequency among Sexually Active Men who have Sex with Men
Main Article Content
Abstract
Despite recommendations of annual HIV testing for high-risk individuals, one-third of infected men who have sex with men (MSM) are unaware of their serostatus. In an effort to improve HIV prevention services and subsequently decrease prevalence, this study aimed to examine the HIV testing patterns and factors associated with testing frequency among MSM. Utilizing eight reliable and validated instruments and a sample of 374 sexually active MSM, this study examined a series of demographic, behavioral and psychosocial associations of antibody testing frequency, including substance use/abuse, depression levels, internalized homophobia, unprotected anal intercourse, sexual regulation and attribution, and HIV knowledge, . MSM who tested for HIV frequently were more likely to be older, have higher levels of educational attainment, and self-identify as gay. Respondents who reported never having been tested and irregularly tested had higher levels of internalized homophobia, depression, and alcohol use and abuse patterns. Respondents who had never been tested or infrequently tested engaged in lower levels of sexual risk, particularly unprotected receptive anal intercourse. Those with no or irregular histories of testing also exhibited greater external sexual loci of control, and were significantly more likely to attribute life events to external, unstable, and pessimistic causes.

Introduction
Undiagnosed HIV is a significant driver of the ongoing HIV epidemic in the United States, with up to 14% of infected individuals unaware of their status.1,2 Those with undiagnosed HIV have the highest transmission rates, and are estimated to contribute to nearly one-third of new cases, with men who have sex with men (MSM) as the primary source of transmission.3 Seventy percent of new HIV diagnoses occur among MSM.4
HIV infection is hyperendemic among MSM in many areas of the United States, particularly in the South. Of the 25 metropolitan statistical areas with the highest diagnosed prevalence rates among MSM, 21 are in the South.5 Georgia – whose statewide rate of infection among MSM is more than forty-four times higher than that of heterosexual men – is the only state that ranks in the top five for both percentage of MSM and absolute number of MSM living with a diagnosis of HIV.6 More than sixty percent of new diagnoses in Atlanta are among MSM.7 The disproportionate burden of HIV among MSM necessitates interventions to improve HIV screening, to increase diagnoses and access to preventative services including pre-exposure prophylaxis.
HIV testing plays a pivotal role in preventing viral transmission and identifying at-risk individuals.8 Increasing testing uptake and frequency among at-risk populations such as MSM is critical to reaching the first UNAIDS target of 90% of all persons living with HIV becoming aware of their status.9,10 Frequent HIV testing has been associated with a reduction in HIV risk and is an effective strategy for reducing infection rates among MSM. Current guidelines recommend annual HIV screenings for all MSM, with more frequent testing for those at greater risk of exposure.11,12 High prevalence of risk behavior coupled with a low perception of risk of infection pose a significant risk of transmission. Behavioral data suggest that high levels of unprotected anal intercourse among MSM may persist after HIV voluntary testing, and many young MSM acquire HIV within one year of their most previous antibody test.9 These challenges suggest that regular testing to detect HIV positive status is needed to better control secondary transmission among MSM.
Defining human behavior as triadic, dynamic, and a reciprocal interaction of personal factors, behaviors, and the environment, the Social Cognitive Theory is one of the most commonly used models exploring HIV-related risk and protective behaviors.13-16 This theory suggests that individuals engage in cognitive processes which allow them to weigh both positive and negative elements of preventative behaviors, thus influencing self-efficacy and decision making.16,17 The application of Social Cognitive Theory has documented both individual- and structural-level barriers and facilitators of HIV-related risk behavior and testing services among MSM. Individual level factors include limited HIV knowledge, internalized homophobia, low perception of risk, binge alcohol consumption, and injection drug use. Structural elements include costs associated with testing, social norms, stigma, and discrimination.9 Anecdotal data indicate that MSM who are more likely to engage in repeated HIV testing are younger, have lower HIV risk perception, engage in sexual behavior with a greater number of partners, and inject drugs.18 These data have assisted policymakers and practitioners in developing strategies for increased testing.
Despite substantial evidence of the impact of frequent testing on HIV risk behavior, less is known about factors associated with frequency of HIV testing among MSM. Though focused public health efforts have been made to slow the surge of the epidemic, nearly 30% of MSM have never received an antibody test. National HIV Behavior Surveillance data also suggest that the frequency of HIV may be inadequate, even among MSM who are tested.1 Given the burden of HIV among MSM, particularly in the southern United States, interventions are needed to enhance HIV screening and diagnosis, and access to treatment as prevention (TasP) strategies including pre- and post-exposure prophylaxis. The purpose of this study, in an effort to improve HIV prevention services and subsequently decrease prevalence, was to examine the HIV testing patterns and factors associated with testing frequency among MSM. The results of this study may inform future interventions to expand antibody testing programs to include at-risk MSM who exhibit low testing frequency.
Methods
Sampling and Recruitment
Participants were recruited from self-identified MSM attending an annual fundraising event promoting unity and visibility across the LGBTQ community in Atlanta in 2017. Consistent with CDC surveillance and reporting methods, MSM were identified as having either a) had sex only with men, or b) having had sex with both men and women in their lifetimes, per their response to a demographic item addressing sexual behaviors.19
A trained researcher was responsible for face-to-face survey procedures. After briefly introducing the study for participants, those consenting to participate were subjected to eligibility screenings. Surveys were self-administered via electronic tablets, and collected data pertaining to sociodemographic characteristics and psychosocial determinants. The questionnaire took approximately 30 minutes to complete.
Electronic consent was obtained from each participant via a prompt on the tablet, with the consent script appearing on the initial screen. Participants offering consent were then directed to the questionnaires. All study procedures were approved by the University of North Dakota Institutional Review Board.
Measures
Psychosocial and behavioral determinants measured in this study included sexual behavior with men and women, HIV-related knowledge, substance use and abuse, depression, attributional style, sexual regulation, and internalized homophobia. Sexual risk taking was assessed via the University of California San Francisco Center for AIDS Prevention Studies’ Sexual Behavior Questionnaire.20 This 20-item scale (α =.82) asked respondents to report the number of individuals with whom they had engaged in sexual activity in the prior ninety days. Respondents were similarly asked to respond dichotomously (yes/no) to inquiries regarding condom use and insertive and receptive anal, oral, and vaginal intercourse. Summary scores could range from 0 to 22, with higher scores indicating greater HIV-related sexual risk taking.
Knowledge of HIV/AIDS was assessed via the Brief HIV Knowledge Questionnaire. The 18-item true/false questionnaire reliably (α=.82) assesses HIV knowledge among high-risk populations, spanning common information deficits and misconceptions about sexual risk behavior, informed decisions, and behavior change, as well as misconceptions about risk associated with close contact with individuals suspected or known to be living with HIV.21 In accordance with prior measures, summary scores, ranging from 0 to 18, were obtained by summing the number of items answered correctly.22
Substance use and abuse was assessed via the Brief Michigan Alcoholism Screening Test (BMAST), as well as the Drug Abuse Screening Test (DAST-20).23,24 The 10-item BMAST (α=.84) examined both behaviors and consequences associated with alcohol abuse and dependence. Aggregate scores, ranging from 0 to 29 are generated by summing weighted scores to each item, with higher scores suggesting greater behavioral risks for alcoholism. The 20-item DAST (α=.79) examined the use and consequences of both illegal and prescription drugs. Aggregate scores range from 0 to 20, with higher scores reflecting increased drug use problems.24
Research has reported associations between depression and riskier sexual behaviors, as depression may impact the ability to negotiate safer sex, initiate harm reduction strategies, or engage in risky sex as a form of self-harming behavior.25 The Center for Epidemiological Studies Depression Scale was thus used in an effort to examine depressive symptomology among respondents.26 The eight-item instrument asked respondents to indicate their frequency of experiencing significant characteristics of depression (e.g., depressed mood, feelings of hopelessness, etc.) within the prior seven days. Aggregate scores could range from 0 to 24, with higher scores reflecting greater depressive symptomatology.26
Individuals are predisposed to explain the causes of events in a habitual manner, which has been shown to influence emotions, cognition, motivation, and future behavior.27,28 These explanatory or attributional styles have been linked to health-related issues, including depression and illness.29,30 The Attributional Style Questionnaire (α=.83) examined the degree to which respondents attributed life events to internal, stable, and global causes (i.e., optimistic), as opposed to external, unstable, and specific (i.e., pessimistic) causes.31 The instrument asked participants to generate causes for four hypothetical events, while rating themselves on a 7-point scale.32 Each of the three attributional elements (internality, stability and globality) were measured, producing a total of 12 items. Summary scores could range from 0 to 84, with higher scores indicating greater degrees of internal, stable, and global attribution.19
According to prior research, individuals with an internal locus of control report greater HIV-related preventative behaviors, including increased condom use.33,34 To address this possibility, the Dyadic Sexual Regulation Scale (α=.79) was used to examine the degree to which respondents perceived their sexual activities as being regulated from internal or external loci of control.35 The 11-item instrument asked subjects to respond on a 7-point scale to statements addressing personal control in dyadic sexual relations.36 Summary scores could range from 11 to 77, with higher scores indicating greater external locus of control.
Internalized homophobia, which has been associated with high-risk sexual behavior, was examined via the 9-item Internalized Homophobia Questionnaire (α=.81).37,38 Items with a 5-point response scale ranging from strong disagreement to strong agreement were derived from the ego-dystonic homosexuality diagnostic criteria, found in the 3rd edition of the Diagnostic and Statistical Manual of the American Psychiatric Association, which has since been removed.39,40 Summary scores could range from 0 to 45.
Frequency of HIV Testing
Three items were used to measure HIV testing frequency. Participants were first asked if they had ever been tested for HIV. Those who indicated a history of testing were then asked how many antibody tests they had received in the past four years. Participants receiving more than one test were further asked about their testing frequency within the past four years. Response options included every three months, every 6 months, annually, and irregularly. HIV testing frequency was then categorized into three groups based on participants’ self-reported history and frequency: never tested, irregularly tested, and frequently tested. Individuals reporting no history of antibody testing were categorized as never tested MSM. Irregularly tested MSM included those who had a history of antibody testing, yet whose tests were less frequent than twice annually or reported irregular testing in the past four years. Those MSM who engaged in antibody testing at least twice annually, or reporting receiving tests every three-to-six months, were classified as frequent testers.
Data Analyses
All data were entered into IBM SPSS v. 24, and then screened to identify missing information and/or outliers. Frequency counts were generated for each scale to examine missing data. A series of two-tailed t-tests revealed no significant univariate outliers. All missing data were excluded from analysis.
Means and standard deviations (for normal continuous data), and the frequencies and percentages were calculated for each of the three groups of differential HIV testing frequency categories. Sociodemographic and psychosocial measurements were then compared among MSM who never tested, irregularly tested, and frequently tested. One-way ANOVA was used for continuous variables including scale scores assessing sexual risk, HIV-related knowledge, substance use and abuse, depression, attributional style, sexual regulation, and internalized homophobia.
Results
An a priori statistical power analysis was performed for sample size estimation, based on Mirandola et al.’s examination of sociodemographic predictors of HIV test seeking behavior among MSM.41 With an alpha = .05 and power = 0.80, the projected sample size needed for a moderate effect was approximately 273. A total of 374 sexually active MSM participated in the study.
Table 1 presents a comparison of sociodemographic characteristics between the three HIV testing participant classes. Among the 374 participants, 139 (37.2%) were never tested, 100 (26.7%) were tested irregularly, and 135 (36.1%) were tested frequently in the past four years. Significant differences existed between the three groups of MSM in age, educational attainment, and self-reported sexual orientation. Participants who reported never having received an HIV antibody test were significantly younger than their peers (84.2% were between the ages of 18 and 29, compared to 71% and 75.6% of those who were irregularly and frequently tested, respectively, χ2 = 21.24, p = 0.026). Likewise, respondents who were never tested were significantly more likely to have lower levels of educational attainment (28.1% had a high school diploma or less, compared to 17% and 11.1% of irregular and frequent testers, χ2 = 19.68, p = 0.014). Between-group differences were also found with regard to self-identified sexual orientation, with a greater proportion of those never tested identifying as bisexual (27.3%, compared to 21.0% and 16.3% of those irregularly and frequently tested, respectively, χ2 = 3.97, p = 0.048).
Table 1. Sociodemographic Characteristics of Sexually Active Men Who Have Sex with Men Reporting Never Testing for HIV, Irregularly Testing for HIV, and Frequently Testing for HIV (N = 374)
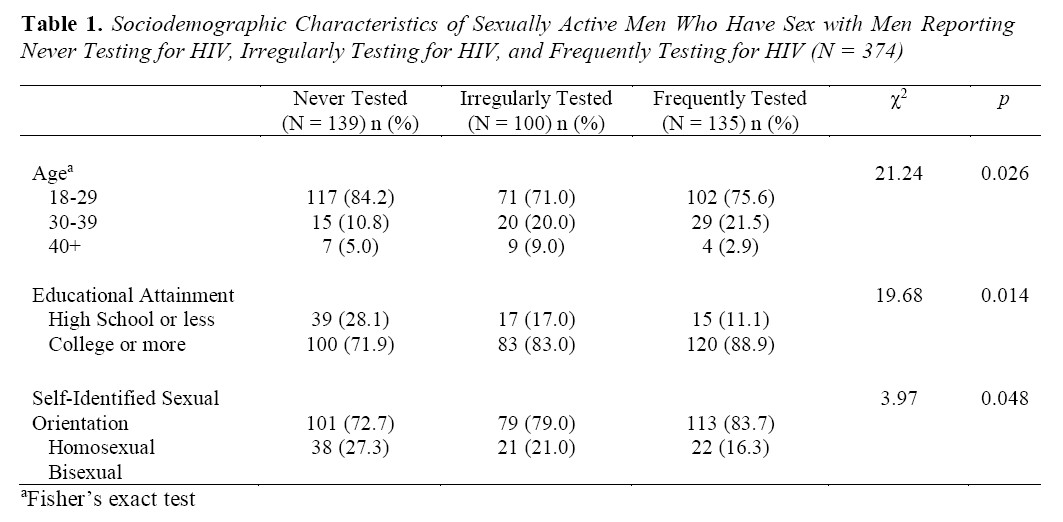
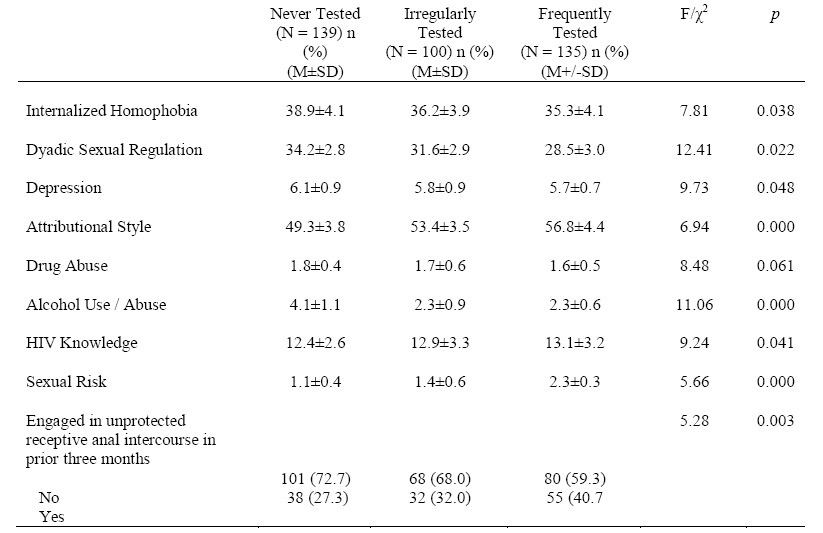
Table 2 presents comparisons of psychosocial and behavioral factors between MSM respondents who were never tested for HIV, tested irregularly, and those indicating frequent testing. Compared to those who were frequently tested for HIV, those reporting never having been tested and irregularly tested had higher levels of internalized homophobia (F = 7.81, p = 0.038), depression (F = 9.73, p = .048), and alcohol use and abuse patterns (F = 11.06, p = 0.000). Exhibiting lower levels of HIV transmission and prevention knowledge (F = 9.24, p = 0.041), respondents who had never been tested or infrequently tested engaged in lower levels of sexual risk (F = 5.66, p = 0.000), particularly unprotected receptive anal intercourse (χ2 = 5.28, p = 0.003). Those with no or irregular histories of testing also exhibited greater external sexual loci of control (F = 12.41, p = 0.022) and were significantly more likely to attribute life events to external, unstable, and specific (e.g., pessimistic) causes (F = 6.94, p = 0.000).
Table 2. Psychosocial and Behavioral Factors Among Men Who Have Sex with Men Reporting Never Testing for HIV, Irregularly Testing for HIV, and Frequently Testing for HIV (N = 374)
Discussion
The present study examined the impact of sexual regulation, depression, internalized homophobia, attributional style, drug and alcohol use, knowledge of HIV transmission and prevention, and sexual risk on self-identified MSM’s choices to seek voluntary HIV testing. With the exception of drug abuse, results of the study support the existence of independent associations between each of these factors and the frequency of HIV testing. Significant differences in testing frequency were further found by age, educational attainment, and self-reported sexual orientation.
The finding that MSM reporting more frequent antibody testing exhibited lower internalized homophobia is supported by research, which found an inverse correlation between internalized homophobia and HIV and other STI testing rates among MSM in Europe and Asia.42 In their research among Chinese MSM, Wei et al. similarly found an indirect relationship between experienced homophobia and recent testing.43 Greater levels of self-acceptance have also been associated with the likelihood and frequency of testing, suggesting that stigma and subjective norms associated with sexual orientation may serve as a barrier to participation in HIV testing and other health-promoting behaviors.42,44
Findings suggest that an internal locus of control, related to sexual behaviors, enhanced the likelihood and frequency of testing among MSM. Research supports the beneficial impact of an internal locus of control on self-rated health, as well as healthy behaviors.45,46 An internal health locus of control has further been found to facilitate a reduction in HIV and other STI risk behaviors.47-49 A pessimistic attributional style, as found among non-testers in the current study, may likewise serve as an impediment to prevention efforts, as it may interfere with logical reasoning and information-seeking behaviors. Those with pessimistic attributes may have low self-confidence, or may not effectively use existing skills to address immediate health concerns.50
Self-reported levels of depressive symptomatology and alcohol use further impacted antibody testing status and frequency. Not only do mental health impairments such as depression and alcohol or other substance abuse or dependence play a critical role in HIV transmission – increasing the risk of transmission by four- to ten-fold – but they may also interfere with prevention efforts.51 Mangurian et al. found disparate rates of regular antibody testing among adults with severe mental illness, echoing findings of the current research.52 Research exploring the implementation and efficacy of PrEP to prevent HIV transmission among MSM similarly has found that individuals exhibiting higher depression scores had lower levels of emtricitabine and tenofovir, as well as higher levels of condomless receptive anal intercourse.53
The finding that greater amounts of alcohol use impact antibody testing is not groundless. Inquiries about substance use during routine testing procedures may dissuade those with abuse patterns from participating. Prior research has demonstrated that alcohol use is a mediator of HIV risk taking behaviors such as unprotected sexual activities.54-56 Often used to temporarily relieved symptoms of depression, alcohol use among MSM may similarly mediate viral transmission by providing greater opportunities for cognitive escape.57 Despite enhancing the risk of transmission by greater than 200%, research has consistently found a negative relationship between levels of drinking and the likelihood of ever testing for HIV.58-61
For people who may have been exposed to HIV, knowledge is critical to making informed decisions. An antibody test is a serious event with potentially serious outcomes. The current findings suggest that greater HIV knowledge is associated with greater likelihood and frequency of antibody testing. Research in both developing and developed countries has illuminated the value of HIV-related awareness and knowledge as consistent contributors to voluntary counseling and testing.62-64 Despite a link between perceived knowledge and testing status, much of the literature suggests that knowledge of one’s HIV-negative status does not impact HIV risk-related sexual behaviors.65
The CDC has aggressively invested in both proven technologies and approaches to ensure that HIV-infected MSM are aware of their status, and that all MSM have both knowledge and tools to protect themselves from infection.66 Thus, the present study’s finding that MSM engaging in greater risk-taking behaviors are more frequently tested is not unfounded, and may be mediated by enhanced knowledge levels. Research has demonstrated that men with prior testing experience are significantly more knowledgeable about HIV transmission, prevention, and treatment.22
Despite such knowledge, research has similarly found a positive correlation between knowledge and HIV risk among MSM – suggesting that, while MSM have enhanced knowledge on the topic, their risk behaviors may not change.63 Since awareness is associated with decreased concern about HIV, and subsequently less concern about consistent condom use, it has been hypothesized that a greater depth of knowledge about HIV may in fact heighten the risk of transmission, and consequently increase the likelihood of antibody testing.67
Study Limitations
While the present study incorporated findings from a sample of sexually active MSM, several limitations exist. A convenience sample of self-identified MSM attending an LGBTQ unity event leaves the study open to a number of sampling biases, and limits the ability to generalize results to the larger population of sexual minority males. Similarly, the homogenous nature of the sample, with regard to ethnicity and sexual orientation, limits applicability.
The self-reporting nature of the instruments may similarly limit the accuracy of responses. Owing to sexual bragging, social norms, memory recall, and a refusal to respond to sexuality-based questions, self-reported sexual behaviors may be also be biased.68 Items were presented in a manner that limited the directionality of responses, compromising the ability to assess whether risk behaviors occurred before or after testing among those who were tested. Despite such limitations, including the impact of sample size on statistical power and effect, this study appropriately highlights the associations between psychosocial attributes, behavior, and the likelihood of HIV antibody testing among MSM, and can significantly inform prevention-based interventions.
Conclusion
Mounting evidence suggests that MSM in the United States continue to be disproportionately affected by HIV and AIDS due to their involvement in higher risk behaviors.69 The present study highlights the need to increase HIV testing and frequency among subpopulations of MSM. Traditional prevention efforts, which target specific risk groups, may not be effective at reaching populations susceptible to infrequent or no testing histories.70,71 It is imperative that, in addition to concerted efforts focusing on knowledge as a prevention tool, a combination of feasible and effective strategies, such as condom use, medical male circumcision, antiretroviral therapy (ART) for treatment as prevention, pre- and post-exposure prophylaxis, and continued antibody testing, be implemented.72 Medical professionals, when engaging patients, should address not only behavioral predictors of HIV risk, but similarly the impact of mental health (e.g., depression, attribution, and locus of control) on both risk and protective behaviors. Outreach efforts should consider alternative methods of reaching those MSM who experience internalized homophobia and/or engage in substance use or abuse patterns, which may limit regular access to medical care and testing services. Future research and prevention efforts should explore developing, implementing and evaluating HIV testing initiatives and campaigns that will be acceptable to those to those with little or no testing history. Such MSM may be less aware of the need for frequent HIV testing or may fear stigmatization associated with mental health or prior risk behaviors.
Article Details
The Medical Research Archives grants authors the right to publish and reproduce the unrevised contribution in whole or in part at any time and in any form for any scholarly non-commercial purpose with the condition that all publications of the contribution include a full citation to the journal as published by the Medical Research Archives.
References
doi: 10.1080/.9540121.2018.1533228
2. Hall HI, An Q, Tang T, Song R, Chen M, Green T, Kang J. Prevalence of diagnosed and undiagnosed HIV infection – United States, 2008-2012. MMWR Morb Mortal Wkly Rep. 2015;64(24):657-662.
3. Skarbinski J, Rosenberg E, Paz-Bailey G, Hall HI, Rose CE, Viall AH, Fagan JL, Lansky A, Mermin, J.H. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med. 2015;175(4):588-596. doi: 10.1001/ jamainternmed.2014.8180
4. Centers for Disease Control and Prevention. HIV infection risk, prevention, and testing behaviors among men who have sex with men – National HIV behavioral surveillance, 20 U.S. cities, 2014. Centers for Disease Control and Prevention. https://www.cdc.gov/ hiv/pdf/ library/reports/surveillance/cdc-hiv-surveillance-special-report-number-15.pdf. Published January, 2016. Accessed December 23, 2020.
5. Emory University Rollins School of Public Health. Local data: Atlanta. AIDSVu. https://aidsvu.org/local-data/united-states/south/georgia/atlanta/. Accessed December 23, 2020.
6. Goodreau SM, Rosenberg ES, Jenness SM, Luisi N, Stansfield SE, Millett GA, Sullivan PS. Sources of racial disparities in HIV prevalence in men who have sex with men in Atlanta, GA, USA: A modelling study. Lancet HIV. 2017;4(7):E311-E320. doi: 10.1016/ S2352- 3018(17) 30067-X
7. Georgia Department of Public Health. Georgia Integrated HIV Care and Prevention Plan. Georgia HIV Surveillance Data. https://dph.georgia.gov/epidemiology/ georgias-hivaids-epidemiology-section/georgia-hiv-surveillance-data. Published September 23, 2020. Accessed December 23, 2020.
8. World Health Organization. Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations, 2016 update. https://www.who.int/hiv/pub/ guidelines/ keypopulations-2016/en/. Published July, 2016. Accessed January 19, 2021.
9. Cheng W, Egan JE, Liu Q, Xu H, Stall R, Friedman MR. Psychosocial correlates of HIV testing frequency among men who have sex with men in Guangzhou, China. AIDS Behav. 2020;24:363-372. doi: 10.1007/s10461-019-02431-w
10. UNAIDS. 90-90-90: An ambitious treatment target to help end the AIDS epidemic. UNAIDS; 2014.
11. McDaid LM, Hart GJ. Increased HIV testing and reduced undiagnosed infection among gay men in Scotland, 2005-8: Support for the opt-out testing policy? Sex Transm Infect. 2011;87 (3):221-224. doi: 10.1136/sti.2010.044560
12. Centers for Disease Control and Prevention. HIV testing and risk behaviors among gay, bisexual, and other men who have sex with men – United States. MMWR Morb Mortal Wkly Rep. 2013;62(47):958-962.
13. Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191-215.
14. Bandura A. Social foundations of thought and action: A social cognitive theory. Englewood Cliffs, NJ: Prentice Hall; 1986.
15. Bandura A. Social cognitive theory. In: Vasta R, ed. Annals of child development. Greenwich, CT: Jai Press; 1989;1-60.
16. Safren SA, Traeger L, Skeer M, O’Cleirigh C, Meade CS, Covahey C, Mayer KH. Testing a social-cognitive model of HIV transmission risk behaviors in HIV-infected MSM with and without depression. Health Psychol. 2010;29(2):215-221. doi: 10.1037/ a0017859
17. Li X, Zhang L, Mao R, Zhao Q, Stanton B. Effect of social cognitive theory-based HIV education prevention program among high school students in Nanjing, China. Health Educ Res. 2011;26(3):419-431. doi: 10.1093/her/cyr001
18. Puljic VM, Licina MLK, Javic M, Blazic TN. Repeat HIV testing at voluntary testing and counseling centers in Croatia: Successful HIV prevention or failure to modify risk behaviors? PLoS One. 2014;9(4):e93734. doi: 10.1371/journal.pone.0093734
19. Sabato TM, Burnett AJ, Kerr DL, Wagner L. Examining behavioral and psychosocial predictors of antibody testing among college youth: Implications for HIV prevention education and testing. Am J Sex Educ. 2013;8(1-2):56-72. doi: 10.1080/15546128. 2012.740893
20. Chesney M, Folkman S, Chambers D. Coping effectiveness training for men living with HIV: Preliminary findings. Int J STD AIDS. 1996;7(2):75-82.
21. Carey MP, Schroder KE. Development and psychometric evaluation of the brief HIV knowledge questionnaire (HIV-KQ-18). AIDS Educ Prev. 2002;14:174-184.
22. Pando MAM, Balan I, Marone R, Dolezal C, Barreda V, Carballo Dieguez A, Avila MM. HIV knowledge and beliefs among men who have sex with men (MSM) in Buenos Aires, Argentina. AIDS Behav. 2013;17(4):1305-1312.
doi: 10.1007/s10461-012-0404-x
23. Pokorny AD, Miller BA, Kaplan HB. The Brief Michigan Alcoholism Screening Test. Am J Psychiatry. 1972;129:342-354.
24. Skinner HA. Drug Use Questionnaire: DAST-20. Addiction Research Foundation; 1982.
25. DeCamp W, Bakken NW. Self-injury, suicide ideation, and sexual orientation: Differences in in causes and correlates among high school students. J Inj Violence Res. 2016;8(1):15-24.
doi: 10.5249/jivr.v8i1.545
26. Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385-401.
27. Rubenstein LM, Freed RD, Shapero BG, Fauber RL, Alloy LB. Cognitive attributions in depression: Bridging the gap between research and clinical practice. J Psychother Integr. 2016;26(2):103-115. doi: 10.1037/int0000030
28. Shapcott KM., Carron AV. Development and validation of a team attributional style questionnaire. Group Dyn. 2010;14(2):93-113. doi: 10.1037/a0018252
29. Fresco DM, Alloy LB, Reilly-Harrington N. Association of attribution style for negative and positive events and the occurrence of life events with depression and anxiety. J Soc Clin Psychol. 2006;25(10):1140-1160.
30. Peterson C, Park N, Pole N, D’Andrea W, Seligman ME. Strengths of character and posttraumatic growth. J Trauma Stress Disord Treat. 2008;21(2):214-217. doi: 10.1002/ jts.20332
31. Peterson C, Semmel A, von Bayer C, Abramson LY, Metalsky GI, Seligman ME. The attributional style questionnaire. Cognit Ther Res. 1982;6:287-299.
32. Kleim B, Gonzalo D, Ehlers A. The Depressive Attributions Questionnaire (DAQ): Development of a short self-report measure of depressogenic attributions. J Psychopathol Behav Assess. 2011;33:375-385. doi: 10.1007/s10862-011-9234-9
33. Dixon D, Saul J, Peters M. Psychosocial correlates of HIV sexual protective behavior among Puerto Rican women residing in the Bronx, New York. Health Care Women Int. 2010;31(3):274-293. doi: 10.1080/07399330903171416
34. Gwandure C, Mayekiso T. Predicting HIV risk using a loc of control-based model among university students. J Child Adolesc Ment Health. 2010;22(2):119-129. doi: 10.2989/ 17580583.2010.528579
35. Catania JA, McDermott LJ, Wood JA. Assessment of locus of control: Situational specificity in the sexual context. J Sex Res. 1984;20:310-324.
36. Segrin C, Hanzal A, Donnerstein C, Taylor M, Domschke TJ. Social skills, psychological well-being, and the mediating role of perceived stress. Anxiety Stress Coping. 2007;20(3): 321-329. doi: 10.1080/10615800701282252
37. Martin JL, Dean L. The impact of AIDS on gay men: A research instrument. New York, NY: Columbia University; 1988
38. Meyer I. Minority stress and mental health in gay men. J Health Sex Behav. 1995;36:38-56.
39. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994.
40. Herek GM, Gillis, JR, Cogan JC. Internalized stigma among sexual minority adults: Insights from a social psychological perspective. J Couns Psychol. 2009;56(1):32-43. doi: 10.1037/ a0014672
41. Mirandola M, Gios L, Davis RJ, Furegato M, Breveglieri M, Folch C, Stanekova D, Nita I, Stehlikova D. Socio-demographic factors predicting HIV test seeking behavior among MSM in 6 EU cities. Eur J Public Health. 2017;27(2):313-318. doi: 10.1093/eurpub/ ckw144
42. GayPress.eu. New research: gays in Belarus don’t accept themselves. Dangers of internalized homophobia. GayPress.eu. https://gaypress.eu/2017/11/19/new-research-gays-in-belarus-don-t-accept-themselves-dangers-of-internalized-homophobia/. Published November 19, 2017. Accessed December 23, 2020.
43. Wei C, Cheung DH, Yan H, Jianjun L, Shi L, Raymond HF. The impact of homophobia and HIV stigma on HIV testing uptake among Chinese men who have sex with men: A mediation analysis. J Acquir Immune Defic Syndr. 2016;71(1):87-93.
doi: QAI.0000000000000815
44. Pyun T, Santos G, Arreola S, Do T, Hebert P, Beck J, Makofane K, Wilson P, Ayala G. Internalized homophobia and reduced HIV testing among men who have sex with men in China. Asia Pac J Public Health. 2014;26(2):118-125.
doi: 10.1177/1010539514524434
45. Zhang A, Jang Y. The role of internal health locus of control in relation to self-rated health in older adults. J Gerontol Soc Work. 2017;60(1):68-78. doi: 10.1080/ 01634372.2016.1267672
46. Sandhu D, Shafiq H. To study physical activity and locus of control among adolescents. adolescents. Indian J Educ Stud. 2015;(1):115-121.
47. Victor EA, Haruna K. Relationship between health locus of control and sexual risk behavior. Retrovirology. 2012;9(1):62.
48. Ogunleye AJ, Omojola G, Abikoye GE, Oke OS. Health locus of control, death anxiety and risky sexual behavior among undergraduate students in Nigeria. Psychol Behav Sci. 2015;4(2): 51-57. doi: 10.11648/j.pbs.20150402.13
49. Pharr J, Enejoh V, Mavegam BO, Olutola A, Karick H, Ezeanolue E. Relationships between health locus of control and risky sexual behaviors among Nigerian adolescents. J AIDS Clin Res. 2015;6(6):104. doi: 10.4172/2155-6113.1000471
50. Gwandure C. Dissociative fugue: Diagnosis, presentation and treatment among the traditional Shona people. Open Anthropol J. 2008;1(1):1-10. doi: 10.2147/ 1874912700801010001
51. Remien RH, Stirratt MJ, Nguyen N, Robbins RN, Pala AN, Mellins CA. Mental health and HIV/AIDS: The need for an integrated response. AIDS. 2019;33(9):1411-1420. doi: 10.1097/ QAD.0000000000002227
52. Mangurian C, Cournos F, Schillinger D, Vittinghoff E, Creasman JM, Lee B, Knapp P, Fuentes-Afflick E, Dilley J. Low rates of HIV testing among adults with severe mental illness receiving care in community mental health settings. Psychiatr Serv. 2017;68 (5): 443-448. doi: 10.1176/appi.pd.201600248
53. Mehrotra ML, Glidden DV, McMahan V, Amico KR, Hosek S, Defechereux P, Mayer KH, Veloso VG, Bekker LG, Avelino-Silva VI, Schechter M, Grant RM. The effect of depressive symptoms on adherence to daily oral PrEP in men who have sex with men and transgender women: A marginal structural model analysis of the iPrEx OLE study. AIDS Behav. 2016;20: 1527-1534.
54. Meredith SE, Rash CJ, Petry NM. Alcohol use disorders are associated with increased HIV risk behaviors in cocaine-dependent methadone patients. J Subst Abuse Treat. 2017;83:10-14.
doi: 10.1016/j.jsat.2017.09.016
55. Tan D, Holloway IW, Gildner J, Jauregui JC, Alvarez RG, Guilamo-Ramos V. Alcohol use and HIV risk within social networks of MSM sex workers in the Dominican Republic. AIDS Behav. 2017;21:216-227. doi: 10.1007/s10461-017-1896-1
56. Okafor CN, Christodoulou J, Bantjes J, Qondela T, Stewart J, Shoptaw S, Tomlinson M., Rotherman-Borus MJ. Understanding HIV risk behaviors among young men in South Africa: A syndemic approach. AIDS Behav. 2018;22:3962-3970. doi: 10.1007/s10461-018-2227-x
57. Melendez-Torres GJ, Bourne A. Illicit drug use and its association with sexual risk behavior among MSM: More questions than answers? Curr Opin Infect Dis. 2016;29(1): 58-63. doi: 10.1097/QCO.0000000000000234
58. Walter AW, Lundgren L, Umez-Eronini A, Ritter GA. Alcohol use and HIV testing in a national sample of women. AIDS Behav. 2016;20(1):84-96. doi: 10.1007/ s10461-015-1144-5
59. Bengston AM, L’Engle K, Mwarogo P, King’ola N. Levels of alcohol use and history of HIV testing among female sex workers in Mombasa, Kenya. AIDS Care. 2014;26(12): 1619-1624. doi: 10.1080/.9540121.2014.938013
60. Kyle TL, Horigian VE, Tross S, Grober VA, Pereyra M, Mandler RN, Feaster DJ, Metsch LR. Uptake of HIV testing in substance use disorder treatment programs that offer on-site testing. AIDS Behav. 2015;19(3):536-542. doi: 10.1007/s10461-014-0864-2
61. Baliunas D, Rehm J, Irving H, Shuper P. Alcohol consumption and risk of incident human immunodeficiency virus infection: A meta-analysis. Int J Public Health. 2010;55(3):159-166. doi: 10.1007/s00038-009-0095-x
62. Chimoyi L, Tshuma N, Muloongo K, Setswe G, Sarfo B, Nyasulu PS. HIV-related knowledge, perceptions, attitudes, and utilization of HIV counseling and testing: A venue-based intercept commuter population survey in the inner city of Johannesburg, South Africa. Glob Health Action. 2015;8(1):26950. doi: 10.3402/gha.v8.26950
63. Scott-Sheldon LAJ, Carey MP, Carey KB, Cain D, Simbayi LC, Mehlomakhulu V, Kalichman SC. HIV testing is associated with increased knowledge and reductions in sexual risk behaviors among men in Cape Town, South Africa. Afr J AIDS Res. 2013;12(4):195-201. doi: 10.2989/ 16085906.2013.863219
64. Kuehne A, Koschollek C, Santos-Hovener C, Thorlie A, Mullerschon J, Tshibadi CM, Mayamba P, Batemona-Abeke H, Amoah S, Greiner VW, Bursi TD, Bremer V. Impact of HIV knowledge and stigma on the uptake of HIV testing – Results from a community-based participatory research survey among migrants from sub-Saharan Africa in Germany. PLoS One. 2018;13(4):e019424.
doi: 10.1371/journal.pone.0194244
65. Ortblad KF, Chanda MM, Mwale M, Haberer JE, McConnell M, Oldenburg CE, Barnighausen T. Perceived knowledge of HIV-negative status increases condom use among female sex workers in Zambian transit towns. AIDS Patient Care STDS. 2020;34(4):184-192. doi: 10.1089/ apc.2019.0266
66. Centers for Disease Control and Prevention. CDC fact sheet: HIV testing in the United States. Centers for Disease Control and Prevention. https://www.cdc.gov/nchhstp/ newsroom/ docs/factsheets/hiv-testing-us-508.pdf. Published August, 2016. Accessed December 23, 2020.
67. Lewis JE, Miguez-Burbano MJ, Malow RM. HIV risk behavior among college students in the United States. Coll Stud J. 2009;43(2):475-491.
68. Latkin CA, Mai NVT, Ha TV, Sripaipan T, Zelaya C, Minh NL, Morales G, Go VF. Social desirability response bias and other factors that may influence self-reports of substance use and HIV risk factors: A qualitative study of drug users in Vietnam. AIDS Educ Prev. 2016;28(5): 417-425. doi: 10.1521/aeap.2016.28.5.417
69. Bruce D, Harper GW, Suleta K. Sexual risk behavior and risk reduction beliefs among HIV-positive young men who have sex with men. AIDS Behav. 2013;17(4):1515-1523. doi: 10.1007/ s10461-012-0155-8
70. Reilly KH, Neaigus A, Jennes SM, Wendel T, Marshall DM, Hagan H. Factors associated with recent HIV testing among men who have sex with men in New York City. AIDS Behav. 2014;18(3):297-304. doi: 10.1007/s10461-013-0483-3
71. Braunstein S, Shepard C. The potential pitfalls of targeted screening for acute HIV infection: The view from New York City. J Infect Dis. 2011;204(3):487-488.
72. Centers for Disease Control and Prevention. Effectiveness of prevention strategies to reduce the risk of acquiring or transmitting HIV.
https://www.cdc.gov/hiv/risk/estimates/ preventionstrategies.html. Updated November 12, 2019. Accessed December 23, 2020.