Neuro Deficit Caused by Vertebral Hemangioma Enneking Stage 3: Incidence, Treatment Options and One Extraordinary Serious Case
Main Article Content
Abstract
Objective
Aggressive Vertebral Hemangiomas (VH) are very rare lesions that may present with compression fractures or bony expansion and erosion into the epidural space resulting in neurological symptoms. Especially in these patients a surgical treatment is essential. We illustrate our institutional experience and discuss the relevant literature.
Method
Our database was searched for cases with vertebral hemangioma and the appearance of a neurological deficit between 2015 and 2020. We were able to identify one very extraordinary case which showed a very aggressive nature and resulted in paraparesis twice in the patient. Furthermore a Medline analysis was performed to identify data of this rare illness (aggressive VH, Enneking stage 3, S3) to assess the incidence and therapeutic options for this so called benign vascular tumor.
Results
One patient was identified in the database with an extraordinary course which resulted in paraparesis twice. A 54-year-old man presented with an acute onset of paraparesis of the legs. Contrast-enhanced magnetic resonance imaging (MRI) revealed a hypervascular tumor of the entire L3 vertebral body and the surrounding tissue with subtotal compression of the spinal canal (Enneking stage 3). After decompression and spinal stabilization a complete recovery of the paraparesis was seen. Two weeks after the initial presentation a recurrence of the paraparesis was noted. The subsequent MRI demonstrated a recurrent increase in the tumor size with spinal canal compression. After endovascular embolization, a gross total tumor resection with a vertebral replacement was performed. The patient was noted to have a complete recovery.
Conclusion
The number of cases with aggressive vertebral hemangiomas (Enneking Stage 3) is limited. However, despite limited treatment algorithms for these rare cases due to the lack of data, surgical treatment should be recommended, especially in the presence of neurological symptoms. That can be underlined by our institutional experience.

In 1863 the first case of vertebral hemangiomas (VH) was described by Virchow in Germany. He found “a tumor composed of dilated blood channels in the center of the 10th thoracic and 3rd lumbar vertebra” in a patient suffering from acute paraplegia during the autopsy1. Some years later, in 1895 Mr. Gerhardt made a connection between vertebral hemangiomas and the appearance of neurological symptoms. And the first clear association between an epidural expansion of a vertebral hemangioma which resulted in a spinal canal stenosis and neurological deficits, was described by Makrykostas in 1927. 2
VH are classified as tumors. However, because of the lack of neoplasm criteria they are also considered to be hamartomas or vascular malformations. Even genetic rearrangements, like the EWSR1-NFATC1 were discussed as a fundament for neoplasm growth. 3
VH are common and usually benign lesions, which are usually an incidental finding by magnetic resonance imaging (MRI), computed tomography (CT) or radiography.4,5 Overall, the incidence of VH is 10 to12 %. Of these, solely 3.7% are classed as active and/or symptomatic. Only 1% expand over the vertebral body into the paravertebral tissue or into the spinal canal. 1
Rare cases of aggressive hemangiomas are reported with compression fractures or expansion into the epidural space causing neurological deficits. Literature shows few cases with an aggressive development and neurological deficits2,6. Surgical treatment, particularly in this patient subgroup, seems to be essential even if it is very challenging7 .
Nowadays, there is a wide array of therapy options. Besides the above mentioned surgical treatment there is radiation therapy, endovascular embolization, alcohol injection and combined methods available. Especially with regard to the rare aggressive vertebral hemangioma cases and those cases with a neurological deficits the question often arises as to which action should be taken.
We illustrate our institutional experience and discuss the relevant literature especially with regards to incidence and treatment options.
Method
Database from our institution was searched for cases with vertebral hemangioma presenting with neurological deficits between 2014 and 2020. Medical records, radiological data (computed tomography, magnetic resonance imaging, X-Ray) were reviewed as well as follow up data. The surgical records with pre-, peri- and postoperative data were analyzed. Furthermore a Medline analysis was performed to identify data of this rare illness. To assess in particular the incidence and therapeutic options for this so called benign vascular tumor.
Case Presentation
A 54-year-old man with a 2 month history of lower back pain, which he attributed to sciatica presented to our hospital. . Within 5 days, he presented with a proximal marked paraparesis of his legs (flexion of the hip with grade of muscle strength 3/5 on both sides on the Janda rating scale). The X-ray showed a unilateral fracture of the third lumbar vertebra on the right side. The contrast-enhanced MRI of the lumbar spine revealed a homogeneously contrast-enhanced tumor of L3 with an extension of 65 mm, subtotal stenosis of the spinal canal but no infiltration of the paravertebral soft tissue (Figure 1). Appropriate to the regularly used Enneking classification, the tumor is graded as an aggressive lesion (Enneking S3) 8,9.
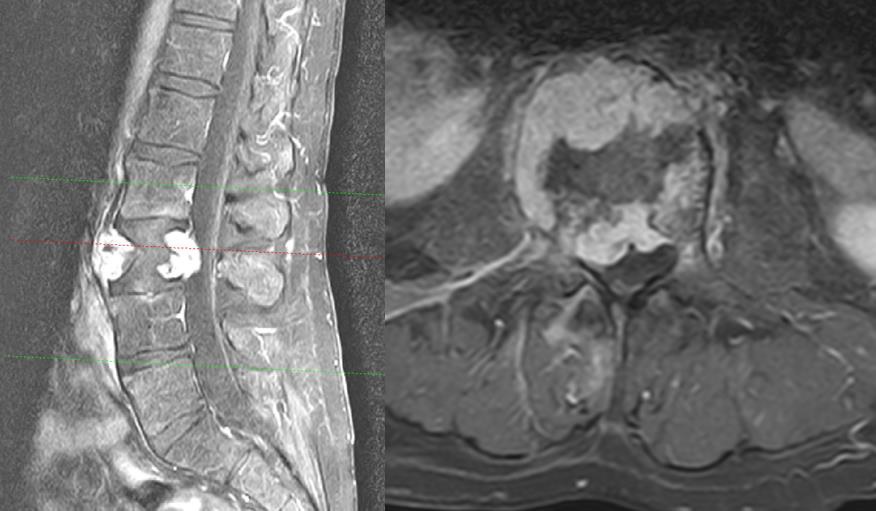
Figure 1: T2-weighted MRI, sagittal and axial view, showing a tumor of the whole vertebra L3 with a pathologic fracture, spinal stenosis, and no tumor infiltration of the adjacent vertebras and muscles
Surgery and process
In this emergency situation we performed a dorsal stabilization L2-4 using a carbon screw-rod system. In addition, a decompression of the spinal canal via hemilaminectomy L3 on the right side and transpedicular biopsy was performed The paraparesis was fully reversible within 2 weeks postoperative. After surgery, a tumor staging was performed which revealed no further evidence of additional tumors or metastases of the spinal column, thorax, abdomen, and the pelvis.
A few days later the patient showed progressive lumbago with sciatica on the left side. He complained of a paresthesia along dermatomes L3 and L4 and a weakness of hip flexion with grade of muscle strength 4/5 on the left side. The MRI revealed tumor progression with almost complete occlusion of the spinal canal (Figure 2). Hence, interventional tumor embolization was performed immediately and then, a ventral (lateral, retroperitoneal approach from left side) tumor resection and vertebral body replacement L3 were carried out. A small embolized fragment in the contralateral pedicle (right side) was left in situ (R2 resection) (Figure 3). After this operation the muscle weakness recovered completely. The patient could be discharged on day nine without any neurological deficit.
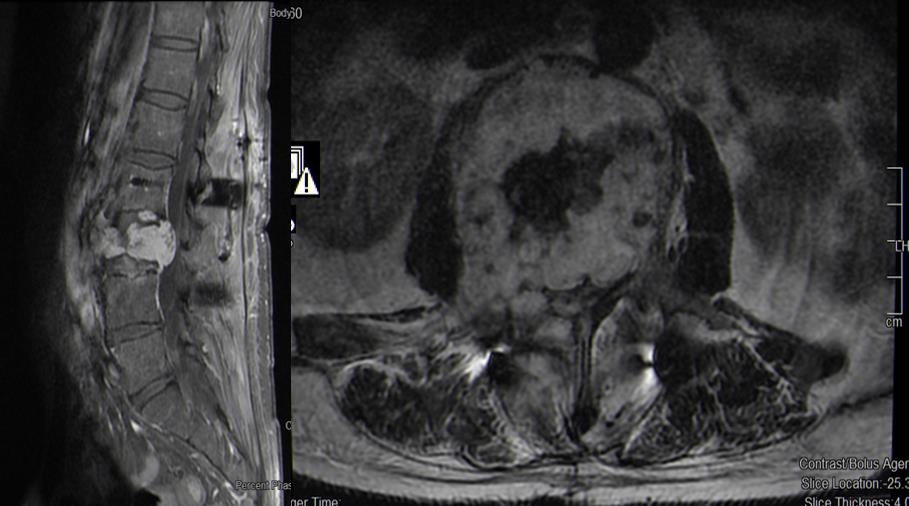
Figure 2: T2-weighted MRI postoperative, sagittal and axial view, showing an enlarged tumor of the vertebra L3 compared to Fig1 (preoperative) and a recurrent absolute spinal stenosis
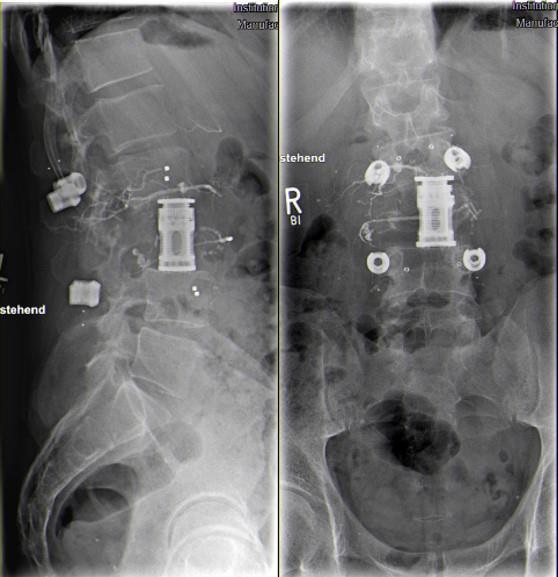
Figure 3: X-Ray (lateral and ap) of the lumbar spine showing the screw rod system (carbon) and vertebra replacement L3. Residual intravascular contrast agent after embolization in vessels girdling the vertebra L3.
Follow up
The last follow-up was 24 months after the operation with no evidence of recurrence. The neurologic status had fully recovered. No adjuvant radiotherapy was required because of proof of embolization of the afferent vessels to the right pedicle and the entire tumor resection of the vertebra L3.
Histopathology
Pathological examination showed a neoplasia with fine capillary lumen. The preparation was immunohistochemically positive for CD34, factor VIII, and vimentin and also negative for CD10, PAX8, PsAP, and pan-cytokeratin. There was no indication of malignant cells. The final histologic results depicted an intraosseous hemangioma of L3 (Figure 4).
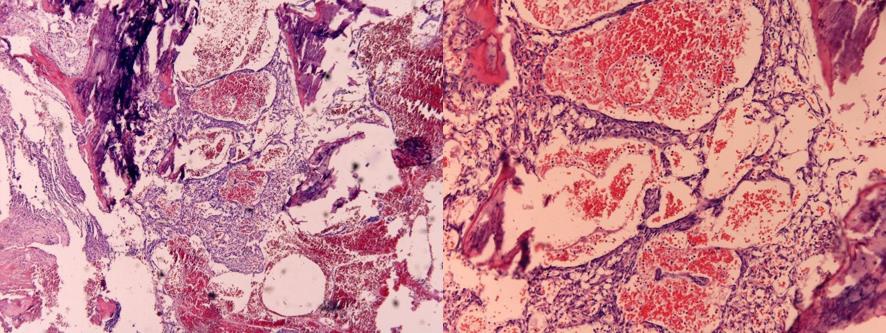
Figure 4: Hemangioma of the lumbar vertebra 3: fractured parts of trabecular bone and a neoplasia with fine capillary lumen. Hematoxylin-eosin staining, 10× und 100× magnification.
Discussion
Hemangioma are classified as benign vascular tumors or benign vascular dysplasias. About 10 % of hemangiomas occur in the spinal column. 10 Vertebral hemangiomas are frequently seen as incidental findings in computed tomography and magnetic resonance imaging. Its incidence in the general population is 10-12%1,11. Most vertebral hemangiomas are asymptomatic and more often seen in females7. About 0.9 % to 1.2 % of spinal hemangiomas are symptomatic. Of these symptomatic cases approximately 45 % present with neurologic deficits due to paravertebral or intraspinal invasion with nerve root or spinal cord compression. Symptoms typically develop gradually and are progressive12,13.
Classification
Based to their pathophysiology and histopathology, VH can be divided into three subtypes with capillary, cavernous and mixed appearance.7
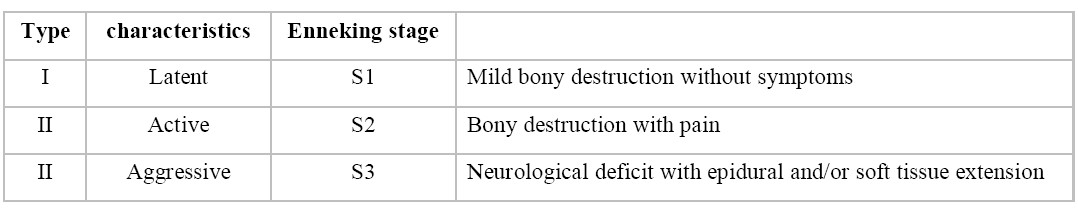
The classification and grading concerning clinical aspects is often done using the “Enneking Stages”. 8,9 Table 1 shows the three types7,14.
Table 1: Clinical classification of vertebral hemangioma (Enneking Stage)
Therapy
Taking into account the above mentioned grading, type I (Enneking stage S1) VHs do not need any specific therapy. Type II (Enneking stage S2) tends to make it more difficult to assess whether there is the need for specific treatment and if necessary, how to treat. Several options can be considered like observation, radiation therapy, percutaneous treatment, endovascular embolization, surgery and combined methods. Surgical treatment is mostly mandatory for Type III (Enneking stage S3) due to the neurological deficits and it often affects the anterior and posterior elements of the vertebral column which influences the stability that results in a circumferential spinal cord or nerve root compression. Often combined approaches with a dorsal and ventral decompression and stabilization of the spine are necessary.
Patients with vertebral hemangiomas, which lead to neurologic deficits due to spinal cord or nerve compression, instability of the spinal column, or immense pain, should undergo surgery15. The different surgical methods are controversially discussed. The aim of surgical treatment should be best local control and recurrence-free survival.
The surgical methods vary. The options range from decompression, cement augmentation, and tumor reduction16-18, intralesional vertebrectomy 19 to gross-total resection and en bloc spondylectomy. 2,20,21
Due to the rapid progression of the paraparesis and the bony destruction in this case, dorsal Instrumentation and decompression of the neuronal structures was necessary. Soon after the index operation and before embolization could be performed a rapid tumor growth was seen leading to neurological deterioration once more. Immediately, the endovascular embolization and the gross total resection and vertebra replacement of L3 from the ventral side were done. Fortunately, the patient benefited from this therapy with complete recovery of the neurologic deficits.
The least complicated surgical technique is spinal decompression surgery with or without posterior stabilization12,16, but only with a moderate local tumor control because of incomplete tumor resection. 4,16 To reduce the risk of recurrence, radiotherapy is often recommended. The rate of recurrence after radiotherapy varies between 2.9 % 19 and 25 % in formerly data. 12 Goldstein et al. in their work reported two patients with local recurrence after 4.4 and 5.3 years after intralesional excision with and without adjuvant radiotherapy, respectively. 19 In a short period of time, tumor progression was seen in our patient with numbness and muscle weakness in the lower extremities. No further therapy such as radiotherapy or embolization was done in this short period.
Therefore, radical surgical methods in case of aggressive VHs (Enneking stage 3 lesions) should be preferred. Intraoperative ethanol injection, preoperative transarterial embolization, or vertebroplasty in addition to decompression and reconstruction are state of the art. It has two advantages. It leads to less intraoperative blood loss and reduces the risk of recurrence. 4,8,22 Nevertheless, severe blood loss up to 2.4 L is possible23, if an intralesional or en bloc tumor resection is done. Wang et al. reported lower blood loss during decompressions when combined with vertebroplasty 8. In the first operation carried out, there was severe blood loss, about 1.5 L. The blood loss in the second operation after embolization, however, was notably lower.
Some authors noted total en bloc spondylectomy of the hemangiomas to be preferential2,20. Decisive superiority could not be proved when compared with intralesional vertebrectomy and reconstruction, because surgery was technically more demanding and perioperative morbidity was greater2. Goldstein et al.19 , contrary to Ji et al7, described that en bloc spondylectomy is not necessary. They observed similar and distinguished results in local control and long-time survival after aggressive intralesional resection.19
Radiotherapy was not initiated after the third intervention despite R2 resection. The remaining tumor in the right pedicle was certainly embolized and the corpus L3 was completely resected. So, we assessed the postoperative situation as “fully resected tumor” and scheduled a short-term follow up. Some studies showed that there is no adjuvant radiation necessary if the hemangioma was totally removed. 4,19 Many authors recommend radiation, when total resection of the tumor is not feasible. 16
In cases of slowly progressing VH, radiotherapy is seen as treatment of choice. There is data which proves that there is a local control of disease, halt of progression and a resolution of symptoms.24 However, the lack of a radiographically effect on the surrounding tissue in aggressive VH means that this method is mostly utilised as an adjuvant therapy to decompression surgery.2
Cement implantation in the affected vertebral body can lead to a hemostatic embolization of VH and optimizes the stability of the vertebral body. But in cases with a neurological deficit and an underlying aggressive VH, the vertebroplasty alone is not adequate most of the time. At the very least it should be combined with a decompression.18
Endovascular embolization serves a treatment of aggressive VH in combination with surgery and as a single treatment.25 Good clinical results are documented for both variations and there is the big advantage of a reduced bleeding during surgery after prior embolization.2
Recommendation
Aggressive vertebral hemangioma, subtype Enneking S3, is extremely rare. That is the reason why there are solely case series with cohorts less than 30 cases and a low evidence level. Because of the inhomogeneity, one can hardly summarize the data for treatment algorithms, outcomes, recurrence rates and clear recommendations.
It can be postulated that on the basis of the available literature, there is a good effect of the current practiced heterogeneous treatment methods with surgery and radiation. Clinical improvements are usually seen and the recurrence rates are low. In 2015 Goldstein et al could demonstrate a recurrence rate less than 3% and a good clinical outcome with improved symptoms in their 68 surgically treated patients (Enneking Stage 1 to 3).19 Jiang et al in 2014 (n=29) and Li et al in 2020 (n= 23) could prove, in the largest retrospective series with aggressive VH (S3) to date, the results from Goldstein et al after surgery.7,16
Conclusion
The number of cases with aggressive vertebral hemangiomas (Enneking Stage 3) is limited. Therefore, effective recommendations for treatment are still lacking. If there is an aggressive tumor as presented in the case above, urgent surgical intervention is required. If only a subtotal tumor resection is possible in the index surgery, the definitive treatment should be performed without delay after acquiring all the needed information (i.e., histopathologic findings, additional imaging, preoperative embolization), because decompression alone would be insufficient. Therefore, gross total resection of aggressive hemangiomas is often needed and possible with good clinical results.
Article Details
The Medical Research Archives grants authors the right to publish and reproduce the unrevised contribution in whole or in part at any time and in any form for any scholarly non-commercial purpose with the condition that all publications of the contribution include a full citation to the journal as published by the Medical Research Archives.
References
2. Vasudeva VS, Chi JH, Groff MW. Surgical treatment of aggressive vertebral hemangiomas. Neurosurgical focus. 2016;41(2):E7.
3. Arbajian E, Magnusson L, Brosjo O, et al. A benign vascular tumor with a new fusion gene: EWSR1-NFATC1 in hemangioma of the bone. The American journal of surgical pathology. 2013;37(4):613-616.
4. Acosta FL, Jr., Sanai N, Cloyd J, Deviren V, Chou D, Ames CP. Treatment of Enneking stage 3 aggressive vertebral hemangiomas with intralesional spondylectomy: report of 10 cases and review of the literature. Journal of spinal disorders & techniques. 2011;24(4):268-275.
5. Dang L, Liu C, Yang SM, et al. Aggressive vertebral hemangioma of the thoracic spine without typical radiological appearance. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2012;21(10):1994-1999.
6. Huang Y, Xu W, Chen Q, Lan Z. Treatment of Typical Enneking Stage 3 Thoracic Aggressive Vertebral Hemangiomas with Pain and Neurologic Deficits: Results After at Least 36 Months of Follow-Up. World neurosurgery. 2020;134:e642-e648.
7. Ji X, Wang S, Oner FC, Bird JE, Lu N. Surgical Management of Enneking Stage 3 Aggressive Vertebral Hemangiomas With Neurological Deficit by One-stage Posterior Total En Bloc Spondylectomy: A Review of 23 Cases. Spine. 2020;45(2):E67-E75.
8. Wang B, Han SB, Jiang L, et al. Intraoperative vertebroplasty during surgical decompression and instrumentation for aggressive vertebral hemangiomas: a retrospective study of 39 patients and review of the literature. The spine journal : official journal of the North American Spine Society. 2018;18(7):1128-1135.
9. Tonn JC, Westphal M, Rutka JT. Oncology of CNS Tumors. Springer Berlin Heidelberg; 2010.
10. Babu R, Owens TR, Karikari IO, et al. Spinal cavernous and capillary hemangiomas in adults. Spine. 2013;38(7):E423-430.
11. Ciftdemir M, Kaya M, Selcuk E, Yalniz E. Tumors of the spine. World journal of orthopedics. 2016;7(2):109-116.
12. Pastushyn AI, Slin'ko EI, Mirzoyeva GM. Vertebral hemangiomas: diagnosis, management, natural history and clinicopathological correlates in 86 patients. Surgical neurology. 1998;50(6):535-547.
13. Prabhakar H, Singh GP. Absolute alcohol embolization of symptomatic vertebral hemangiomas may not be absolutely safe during intraoperative period! Neurosurgery. 2011;69(2):E502; author reply E502.
14. Boriani S, Weinstein JN, Biagini R. Primary bone tumors of the spine. Terminology and surgical staging. Spine. 1997;22(9):1036-1044.
15. Wang B, Meng N, Zhuang H, et al. The Role of Radiotherapy and Surgery in the Management of Aggressive Vertebral Hemangioma: A Retrospective Study of 20 Patients. Medical science monitor : international medical journal of experimental and clinical research. 2018;24:6840-6850.
16. Jiang L, Liu XG, Yuan HS, et al. Diagnosis and treatment of vertebral hemangiomas with neurologic deficit: a report of 29 cases and literature review. The spine journal : official journal of the North American Spine Society. 2014;14(6):944-954.
17. Templin CR, Stambough JB, Stambough JL. Acute spinal cord compression caused by vertebral hemangioma. The spine journal : official journal of the North American Spine Society. 2004;4(5):595-600.
18. Acosta FL, Jr., Dowd CF, Chin C, Tihan T, Ames CP, Weinstein PR. Current treatment strategies and outcomes in the management of symptomatic vertebral hemangiomas. Neurosurgery. 2006;58(2):287-295; discussion 287-295.
19. Goldstein CL, Varga PP, Gokaslan ZL, et al. Spinal hemangiomas: results of surgical management for local recurrence and mortality in a multicenter study. Spine. 2015;40(9):656-664.
20. Ogawa R, Hikata T, Mikami S, et al. Total en bloc spondylectomy for locally aggressive vertebral hemangioma causing neurological deficits. Case reports in orthopedics. 2015;2015:724364.
21. Okacha N, Chrif E, Brahim E, et al. Extraosseous epidural multiple myeloma presenting with thoracic spine compression. Joint Bone Spine. 2008;75(1):70-72.
22. Hadjipavlou A, Tosounidis T, Gaitanis I, Kakavelakis K, Katonis P. Balloon kyphoplasty as a single or as an adjunct procedure for the management of symptomatic vertebral haemangiomas. The Journal of bone and joint surgery British volume. 2007;89(4):495-502.
23. Kato S, Kawahara N, Murakami H, et al. Surgical management of aggressive vertebral hemangiomas causing spinal cord compression: long-term clinical follow-up of five cases. Journal of orthopaedic science : official journal of the Japanese Orthopaedic Association. 2010;15(3):350-356.
24. Rades D, Bajrovic A, Alberti W, Rudat V. Is there a dose-effect relationship for the treatment of symptomatic vertebral hemangioma? International journal of radiation oncology, biology, physics. 2003;55(1):178-181.
25. Raco A, Ciappetta P, Artico M, Salvati M, Guidetti G, Guglielmi G. Vertebral hemangiomas with cord compression: the role of embolization in five cases. Surgical neurology. 1990;34(3):164-168.